4. Ferro JM, Falcao I, Rodrigues G et al. Diagnosis of transient ischemic attack by the nonneurologist: a validation study. Stroke 1996; 27: 2225–9.
5. Geddes JML, Fear J, Tennant A, Pickering A. Prevalence of self-reported stroke in a population in northern England. J Epidemiol Community Health 1996; 50: 140–3.
6. Wade DT. Stroke. In Stevens A, Raftery J, eds. Health Care Needs Assessment. Oxford: Radcliffe Medical Press, 1994. 7. Wilkinson WE, Heyman A, Burch JG, Ostfield A, Lambarth DR,
Leviton A. Use of a self-administered questionnaire for detection of transient cerebral ischaemic attacks: survey of elderly persons living in retirement facilities. Ann Neurol 1979; 6: 40–6. 8. O’Mahony PG, Dobson R, Rodgers H, James OFW, Thomson
RG. Validation of a population screening questionnaire to assess prevalence of stroke. Stroke 1995; 26: 1334–7.
9. Albers GW, Caplan LR, Easton JD et al. Transient ischemic attack – proposal for a new definition. N Engl J Med 2002; 347: 21. 10.Jones WJ, Williams LS, Meschia JF. Validating the
Question-naire for Verifying Stroke-Free Status (QVSFS) by neurolog-ical history and examination. Stroke 2001; 32: 2232–6. 11.Landis JR, Koch GG. The measurement of observer
agree-ment for categorical data. Biometrics 1977; 33: 159–74. 12.Koudstaal PJ, van Gijn J, Staal A, Duivenvoorden HJ. Diagnosis
of transient ischemic attacks: improvement of interobserver agreement by a check-list in ordinary language. Stroke 1986; 17: 723–8.
Received 12 May 2004; accepted 13 July 2004
Age and Ageing 2005; 34: 35–40 Age and Ageing Vol. 34 No. 1 British Geriatrics Society 2005; all rights reserved doi:10.1093/ageing/afi004
Efficacy of anticoagulation for secondary
stroke prevention in older people with
non-valvular atrial fibrillation:
a prospective case series study
G
EORGIOST
SIVGOULIS1, K
ONSTANTINOSS
PENGOS1, N
IKOLAOSZ
AKOPOULOS2, E
FSTATHIOSM
ANIOS2,
V
ASSILIOSP
EPPES2, K
ONSTANTINOSV
EMMOS21Department of Neurology, Eginition Hospital, University of Athens, Greece
2Department of Clinical Therapeutics, Alexandra Hospital, University of Athens, Greece
Address correspondence to: K. Spengos, Vas. Sofias 82, 11528 Athens, Greece. Fax: (+30) 210 7216474. Email: spengos@hol.gr
Abstract
Background and purpose: despite large randomised trials that demonstrated the efficacy of oral anticoagulants in the
primary and secondary prevention of stroke in patients with non-valvular atrial fibrillation (AF), anticoagulation therapy remains largely under-used in older patients, who are at risk of first ever or recurrent stroke. The aim of the present study was to assess the influence of anticoagulation therapy on long-term prognosis in the oldest old stroke patients with AF after adjusting for baseline risk factors.
Methods: we evaluated prospectively a consecutive series of 207 older people (>75 years) with AF and first ever ischaemic
stroke. During the follow-up period (mean 88.4 months, range 3–120), the study population was under either oral anticoagu-lants (n=72) or aspirin (n=135). Death and recurrent vascular events (stroke and systemic embolism) were documented.
Statistical analyses were performed by means of the Kaplan–Meier product limit method and the Cox proportional hazards model.
Results: the cumulative 10 year mortality and recurrence rate were 92.5% (95% CI 85.7–99.3) and 66.1% (95% CI 43.1–
89.1), respectively. Cox regression analysis revealed increasing age, functional dependency at hospital discharge and antiplate-let versus anticoagulation therapy as independent determinants of mortality. Antiplateantiplate-let versus anticoagulation therapy was the sole determinant of vascular recurrence. Anticoagulation was associated with decreased risk of death (hazards ratio (HR) 0.47, 95% CI 0.31–0.72, P=0.001)) and recurrent thromboembolism (HR 0.31, 95% CI 0.16–0.62, P=0.002).
by guest on May 14, 2011
ageing.oxfordjournals.org
Conclusions: our results suggest that the benefits of anticoagulation for secondary stroke prevention in AF patients extend to the oldest old. Prospective randomised clinical trials are needed to verify the potential benefit of anticoagulation in such patients.
Keywords: ischaemic stroke, atrial fibrillation, anticoagulants, elderly, prognosis
Introduction
Non-valvular atrial fibrillation (AF) is a common cardiac arrhythmia that predisposes to left atrial thrombus forma-tion and carries an increased risk for stroke [1]. Older people are particularly at high risk because the prevalence of AF and the risk of AF-associated stroke increase with age [2, 3]. In people over 75 years, AF is the most important sin-gle cause of ischaemic stroke (IS) [4].
Meta-analyses of randomised clinical trials have demon-strated the efficacy of anticoagulation therapy for the primary prevention of stroke in AF patients [5, 6]. In addition, the European Atrial Fibrillation Trial (EAFT) has established the value of anticoagulation for the secondary prevention of thromboembolic events [7]. Despite the preventive potential of oral anticoagulants (OA), several studies have reported that less than 50% of older patients who have clear-cut indi-cations and no contraindiindi-cations receive OA [8–12].
This low use of warfarin is driven by many factors, but physicians’ fear of haemorrhage is among the most important [12–14]. Moreover, older people were signifi-cantly under-represented in clinical trials with only 20% of patients being over 75 [5], although more than 50% of AF patients were in this age group in population-based trials [1]. Additional trials that focus on this specific subgroup of AF patients are warranted to define better the risks and benefits of antithrombotic therapies [4]. The aim of the present study was to assess the influence of anticoagula-tion therapy on long-term prognosis in stroke patients over the age of 75 and with AF after adjusting for baseline risk factors.
Subjects and methods
Baseline assessment
This study was based on 1736 first ever stroke patients consecutively included in the ‘Athens Stroke Registry’ between June 1992 and December 2003. This is a comput-erised prospective observational databank, gathered in a university teaching hospital, which provides tertiary care services to the urban population of Athens. Details of the stroke population evaluated over the first 5 year period and exclusion criteria have been previously described [15].
All patients were hospitalised in a five bed acute stroke unit or a general medical ward. On admission, a physician specialising in stroke examined all patients. The initial evalu-ation consisted of a non-contrast brain CT scan, standard blood tests, a chest X-ray and a 12-lead electrocardiogram. A second CT scan or brain MRI was performed in most cases (4–14 days later). During hospitalisation the following
risk factors were documented: hypertension, diabetes melli-tus, atrial fibrillation, hypercholesterolaemia, smoking, coro-nary artery disease, congestive heart failure and history of transient ischaemic attacks (TIA) [15]. Atrial fibrillation was classified as intermittent if electrocardiographic evidence of sinus rhythm between at least two episodes of AF during the preceding 12 months was obtained and as constant if electrocardiographic evidence of sustained AF for more than 3 weeks without intervening sinus rhythm in the preceding 12 months was obtained [16].
All patients with no evidence of intracerebral haemor-rhage on the first CT scan received aspirin (100–325 mg) on admission. According to the treating physician’s decision both in the stroke unit and the general medical wards anti-coagulation was initiated 1–4 weeks after ictus in medically and neurologically stable AF patients after radiographic exclusion of haemorrhagic infarct transformation. The remaining patients were treated with aspirin (100–325 mg daily). The selection of patients for anticoagulation therapy relied heavily on their compliance, the presence of a moti-vated family environment and their access to anticoagula-tion services. The target range for anticoagulaanticoagula-tion was an international normalised ratio (INR) of 2.0–3.0 using adjusted OA doses according to current recommendations [17]. We used the modified Rankin Scale adapted by the Oxfordshire Community Stroke Project to classify the func-tional deficit at hospital discharge [18]. Disabling strokes were defined as those with Rankin Scores of 2–5 [14, 18].
Study population
We prospectively evaluated the cases fulfilling all the follow-ing criteria: (i) age over 75 years; (ii) electrocardiographically proven AF in the 12 months preceding the index event; (iii) first ever IS (347 cases fulfilled the above-mentioned three criteria); (iv) survival during hospitalisation (78 patients excluded on the basis of this criterion); (v) no echocardio-graphic evidence of valvular disease (eight patients excluded on the basis of this criterion); (vi) no other sources of cardiac emboli: prosthetic valves, cardiac aneurysm, myocardial infarction in the preceding 3 months, atrial myxoma, cardiothoracic ratio >0.65 (11 patients excluded on the basis of this criterion) [7]; (vii) no contraindications for OA: repeated falls, chronic alcohol habituation, recur-rent syncope, uncontrolled seizure disorder, uncontrolled hypertension, gastrointestinal or genitourinary bleeding in the preceding 6 months and daily use of non-steroidal anti-inflammatory drugs (21 patients excluded on the basis of this criterion) [16]. Patients who were transferred to rehabilitation centres (n=17) or lost (n=5) during follow-up were excluded from further evaluation. The final study population consisted of 207 patients over 75 years with stroke and AF.
by guest on May 14, 2011
ageing.oxfordjournals.org
Follow-up
All survivors were followed up prospectively at month 1, 3 and 6, and every 6 months thereafter, up to 10 years after stroke by a study investigator and a trained nurse. Follow-up was routinely performed in our outpatient clinic or in the patient’s place of residence in cases with severe handicap. The outcome events of interest were death and recurrent vascular event (stroke and systemic embolism). To deter-mine recurrent vascular events and causes of death we evaluated all the available information obtained from death certificates, hospital records, physicians’ notes in private practice, necropsy findings and the patients’ clinical presen-tation at the regular follow-up assessments.
Recurrent stroke was defined as a cerebrovascular event of sudden onset and lasting for more than 24 hours subse-quent to the initial stroke that clearly resulted in a new or an increase in an existing neurological deficit [7, 19]. The diagnosis of systemic embolism was clinically defined as abrupt vascular insufficiency of limbs or internal organs associated with clinical or radiological evidence of arterial occlusion, in the absence of other likely mechanisms (atherosclerosis) [19]. The causes of death were divided into the following subgroups: (i) death due to infections (pulmonary infections, urinary infections and septic shock); (ii) death due to vascular causes (myocardial infarction, car-diac arrhythmia, acute pulmonary oedema or heart failure, systemic embolism, recurrent stroke); (iii) death due to other cause (e.g. renal failure, cancer); (iv) undetermined cause of death.
Statistical analyses
Statistical analysis was performed to compare patients on OA (group A) and on aspirin (group B) in terms of demo-graphics, pre-existing conditions and functional outcome at hospital discharge. Dichotomous or categorical variables were compared with the chi-squared test and continuous variables were compared with the unpaired t-test or Mann–
Whitney U test as indicated. Continuous data are presented
as mean (SD) and non-continuous data as percentages. The Kaplan–Meier product limit method was used to estimate the probability of survival and recurrent vascular events after 10 years from the index event. To evaluate which factors contribute to long-term mortality and vascular recurrence, a univariate Cox’s proportional hazards model was used. Those factors that contributed to the outcome in the univariate analyses at P values <0.1 (because of the risk
of type II error due to low statistical power in such an analysis) were included in the multivariate model as candidate variables. In the final multivariate analyses, statistical signifi-cance was achieved if P< 0.05. Associations are presented
as hazards ratios (HR) with their corresponding 95% confidence intervals (95% CI). The Statistical Package for Social Science (SPSS Inc., version 10.0 for Windows) was used for statistical analyses.
Results
The present cohort consisted of 97 men and 110 women (mean age 80.4 ± 3.0 and 80.5 ± 2.9 years, respectively). The
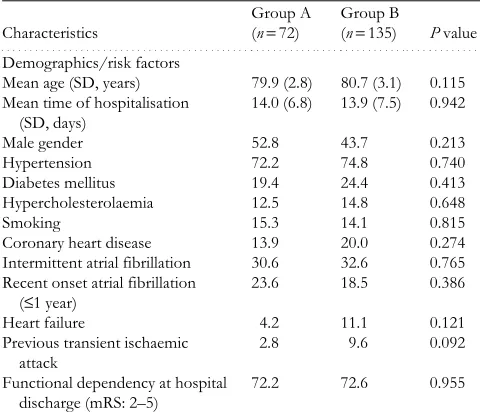
detailed characteristics of the entire collective are presented in Table 1. Hypertension was the most common risk factor (73.9%), while intermittent AF was highly prevalent (31.9%). Most patients (72.5%) were dependent at hospital discharge. Only 20 patients (9.7%) were on OA before the index event, while 56 subjects (27.1%) were receiving antiplatelets. Anticoagulation was provided in 72 patients (34.8%, group A) at discharge and during follow-up, while in the remaining 135 subjects (65.2%, group B) aspirin ther-apy was administered. No statistically significant differences were documented between the two groups in terms of base-line characteristics (Table 1).
During mean follow-up of 88.4 months (range 3–120), 132 (63.8%) deaths and 58 (28.0%) recurrent vascular events (52 strokes and 6 cases of systemic embolism) were documented. Intracranial haemorrhage was identified by means of CT in two patients on OA and in one on aspirin. Vascular disease was the major underlying cause of mortality, accounting for 46.9% of deaths (22.7% secondary to stroke and 24.2% from cardiovascular diseases). Infections were the second cause of mortality (21.2%), while the cause of death remained undetermined in 20.5%. The cumulative 10 year mortality and recurrence rate were 92.5% (95% CI 85.7–97.3) and 66.1% (95% CI 43.1–89.1), respectively. The annual event rate of vascular recurrence was estimated at 5.5% in group A and 16.3% in group B, while the annual rate of intracranial haemorrhage was 1.1% and 0.3%, respectively.
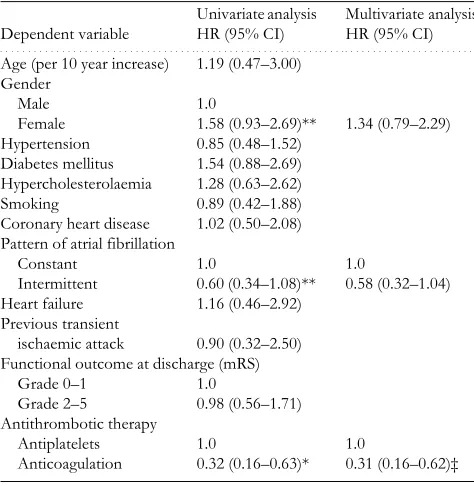
In order to identify independent predictors of long-term mortality and recurrence we performed Cox proportional hazard analyses (Tables 2 and 3). In the initial univariate anal-yses, age, sex, AF pattern, functional outcome at discharge and antithrombotic therapy were related to mortality. The
Table 1. Baseline characteristics of the study population
(n=207)
Continuous data are presented as mean (SD) and non-continuous data as percentages. Mean time of hospitalisation
(SD, days)
14.0 (6.8) 13.9 (7.5) 0.942
Male gender 52.8 43.7 0.213
Hypertension 72.2 74.8 0.740
Diabetes mellitus 19.4 24.4 0.413 Hypercholesterolaemia 12.5 14.8 0.648
Smoking 15.3 14.1 0.815
Coronary heart disease 13.9 20.0 0.274 Intermittent atrial fibrillation 30.6 32.6 0.765 Recent onset atrial fibrillation
multivariate model revealed only increasing age, depend-ency and anticoagulation therapy as significantly independ-ent outcome predictors. AF pattern, female sex and
antithrombotic therapy were associated with vascular recur-rence on univariate analysis, but only antithrombotic ther-apy was retained as independent determinant of recurrence in the multivariate model. The HR associated with OA ver-sus aspirin for long-term mortality and recurrence was 0.47 (95% CI 0.31–0.72, P=0.001) and 0.31 (95% CI 0.16–0.62, P=0.002), respectively.
Discussion
Efficacy and safety of OA for secondary prevention in AF patients have been established by the EAFT [7]. However, in that study, age was the main reason for ineligibility, accounting for 55% of the exclusions [7]. Subgroup analysis of a review of five primary prevention trials suggested that warfarin did reduce stroke in patients over age 75, but it was based on only 24 events [5]. As a result, the evidence for the effectiveness of anticoagulation in older AF patients is less impressive. Furthermore, several surveys have demon-strated that older age is a deterrent of anticoagulation inde-pendent of severity of stroke and bleeding risk [8–12], suggesting that many physicians remain unconvinced of the net benefit of OA to older people [20]. Hence, our data provide additional information about the value of antico-agulation in secondary prevention in this specific age subgroup.
The principal finding of this work is that after adjusting for known risk factors, anticoagulation therapy independ-ently decreases the risk of recurrent vascular events by more than two-thirds and halves the risk of long-term mortality in AF stroke survivors over age 75. It should be noted though that our assessment of the effect of OA on long-term prog-nosis is observational and raises the issue of randomised clinical trials. The BAFTA study is currently being conducted in the UK and will provide further evidence of the risks and benefits of warfarin versus aspirin for stroke prevention among older people in a primary care setting [21].
We observed an annual event rate of vascular recurrence of 5.5% in the coumadin-treated group, which is higher than reported by the EAFT (4%) [7]. On the other hand, our results are in keeping with the annual recurrence rate (5.1%) reported in a prospective cohort study which evalu-ated the efficacy of anticoagulation for secondary prevention in 288 elderly AF patients (mean age 76 years) [22]. The rate of recurrent vascular events in the aspirin-treated group of our cohort (16.3% per year) was higher than that in the EAFT (10%) [7], but closer to that (20%) observed among high-risk older patients in the SPAF I–III trials [23].
The under-use of OA in older AF patients is mainly caused by the fear of intracerebral haemorrhage [12–14]. In the SPAF II trial, the only primary prevention trial especially focusing on 385 older (>75 years) AF patients, the high rate of intracranial haemorrhage in the anticoagulant-treated group (1.8% per year) offset the reduction in IS [24]. In contrast, a meta-analysis of pooled data (223 cases) from other primary prevention trials found a substantially lower rate of intracranial haemorrhage in this age subgroup (0.3% per year) [25]. The documented rate (1.1% per year) in our cohort lies in the middle of the above-mentioned values.
Table 2. Univariate and multivariate Cox proportional
hazard analyses determining the effect of different factors on mortality
(per 10 year increase) 2.72 (1.50–4.91)* 2.03 (1.09–3.79)† Gender
Male 1.0
Female 1.61 (1.13–2.29)‡ 1.27 (0.89–1.83) Hypertension 1.14 (0.76–1.71)
Diabetes mellitus 1.09 (0.73–1.62) Hypercholesterolaemia 0.98 (0.58–1.66)
Smoking 0.69 (0.41–1.17)
Coronary heart disease 1.35 (0.87–2.11) Pattern of atrial fibrillation
Constant 1.0 1.0
Intermittent 0.70 (0.49–1.05)** 0.72 (0.47–1.06) Heart failure 1.21 (0.65–2.25)
Previous transient ischaemic attack
0.94 (0.47–1.85)
Functional outcome at discharge (mRS)
Grade 0–1 1.0 1.0
Grade 2–5 2.00 (1.33–3.01)* 2.12 (1.35–3.08)* Antithrombotic therapy
Antiplatelets 1.0 1.0
Anticoagulation 0.45 (0.29–0.70)* 0.47 (0.31–0.72)‡
Table 3. Univariate and multivariate Cox proportional
hazard analyses determining the effect of different factors on recurrence
Age (per 10 year increase) 1.19 (0.47–3.00)
Gender Male 1.0
Female 1.58 (0.93–2.69)** 1.34 (0.79–2.29) Hypertension 0.85 (0.48–1.52) Diabetes mellitus 1.54 (0.88–2.69)
Hypercholesterolaemia 1.28 (0.63–2.62)
Smoking 0.89 (0.42–1.88)
Coronary heart disease 1.02 (0.50–2.08) Pattern of atrial fibrillation
Constant 1.0 1.0 Intermittent 0.60 (0.34–1.08)** 0.58 (0.32–1.04) Heart failure 1.16 (0.46–2.92)
Previous transient
ischaemic attack 0.90 (0.32–2.50) Functional outcome at discharge (mRS)
Grade 0–1 1.0
Grade 2–5 0.98 (0.56–1.71) Antithrombotic therapy
Antiplatelets 1.0 1.0
Anticoagulation 0.32 (0.16–0.63)* 0.31 (0.16–0.62)‡
by guest on May 14, 2011
ageing.oxfordjournals.org
This difference could be attributed to the significantly wider range for the intensity of anticoagulation in SPAF II (upper INR range 4.5) in comparison with other trials [20, 24].
Our results also indicate that anticoagulation therapy is independently inversely associated with long-term mortality, in contrast to the findings of the EAFT which found no significant benefit of OA on mortality [7]. Hospital-based studies have established cardiac disease and recurrent stroke as the major causes of death in stroke survivors with AF [19, 26]. An autopsy study verified that AF is an important cause of fatal first ever or recurrent IS in older people [27]. The efficacy of anticoagulants in preventing recurrent stroke and systemic embolism may therefore translate into lower long-term mortality rates in this specific age subgroup of stroke survivors.
Apart from anticoagulation therapy, our data identified age and the degree of post-stroke handicap as independent predictors of mortality. These results are in agreement with previous reports supporting age as the most robust predic-tor of death within 1–5 years after IS [28, 29]. Functional status at discharge has been acknowledged as a predictor of long-term mortality [29]. The pattern of AF has not emerged as an independent outcome predictor in multivariate ana-lyses of our study population, despite its association with decreased stroke risk and mortality in the initial univariate analyses. This finding is in line with previous reports suggesting that intermittency of rhythm does not appear independently to influence stroke risk when other risk factors are considered [5, 30].
Certain limitations of the present report need to be addressed. First, our assessment of the effect of OA on long-term recurrence and mortality is observational and therefore subject to selection bias. Even though functional depend-ency at discharge was similar in the two groups, it is likely that patients selected to receive coumadin were more able, motivated and generally fitter. Another limitation is related to the potential observer’s bias during follow-up, affecting the documentation of recurrent vascular events, although these were mostly devastating or fatal and in most cases confirmed by means of hospital records and autopsy findings. Furthermore, we cannot provide detailed data concerning the patients’ compliance with OA, the intensity of anticoagu-lation and possible complications. On the other hand, our dataset has a number of strengths. The observation period was longer than in the EAFT and SPAF trials. Although randomisation was not performed, no significant differ-ences concerning baseline factors were documented between both groups. Finally, the influence of anticoagula-tion on long-term prognosis was assessed after controlling for age, sex, major vascular risk factors, dependency and comorbidities.
In conclusion, our results suggest that anticoagulation therapy is an independent predictor of decreased long-term recurrence and mortality even after taking into account all known prognostic factors in older people with AF and recent IS. Although the risk of intracranial haemorrhage while receiving OA increases with age [24], so does the stroke risk [23]. People over 75 with AF and IS are consid-ered as high risk by all the risk stratification schemes [1, 23].
These patients consequently sustain the largest proportion of cardioembolic strokes and have the largest relative and absolute risk reduction in stroke by warfarin compared with aspirin [1,4]. Our findings indicate that in older patients, the risk of stroke when not receiving OA appears to outweigh the risk of intracranial haemorrhage when receiving OA. The case of wider but judicious use of anticoagulation in older people is strengthened by this study, which shows that anticoagulation for secondary stroke prevention is feasible and effective in such patients.
Key points
• Antiplatelets versus oral anticoagulants are independent determinants of mortality and vascular recurrence in older patients with atrial fibrillation. Anticoagulation is associated with decreased risk of death.
• The benefits of anticoagulation for secondary stroke prevention in patients with atrial fibrillation extend to the oldest old.
• Prospective randomised clinical trials are needed to verify the potential benefit of anticoagulation in older patients.
Acknowledgements
The authors wish to thank Ms Mary Batsara and Mrs Athina Peppa for their assistance in data collection. There is no conflict of interests.
References
1. Hart RG, Halperin JL. Atrial fibrillation and thromboembolism: a decade of progress in stroke prevention. Ann Intern Med 1999; 131: 688–95.
2. Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 1995; 155: 469–73.
3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22: 983–8.
4. Hart RG, Halperin JL. Atrial fibrillation and stroke: concepts and controversies. Stroke 2001; 32: 803–8.
5. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five rand-omized controlled trials. Arch Intern Med 1994; 154: 1449–57. 6. Hart RG, Benavente O, McBride R, Pearce LA. Antithrom-botic therapy to prevent stroke in patients with atrial fibrilla-tion: a meta-analysis. Ann Intern Med 1999; 131: 492–501. 7. Secondary prevention in non-rheumatic atrial fibrillation after
transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet 1993; 342: 1255–62.
8. Sudlow M, Thomson R, Thwaites B, Rodgers H, Kenny RA. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet 1998; 352: 1167–71.
9. Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk
by guest on May 14, 2011
ageing.oxfordjournals.org
40
factors in atrial fibrillation (ATRIA) study. Ann Intern Med 1999; 131: 927–34.
10.Deplanque D, Corea F, Arquizan C et al. Stroke and atrial
fibrillation: is stroke prevention treatment appropriate before-hand? SAFE I Study Investigators. Heart 1999; 82: 563–9. 11.Lamassa M, Di Carlo A, Pracucci G et al. Characteristics,
out-come, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project). Stroke 2001; 32: 392–8.
12.McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155: 277–81.
13.Chatap G, Giraud K, Vincent JP. Atrial fibrillation in the eld-erly: facts and management. Drugs Aging 2002; 19: 819–46. 14.York M, Agarwal A, Ezekowitz M. Physicians’ attitudes and
the use of oral anticoagulants: surveying the present and envi-sioning future. J Thromb Thrombolysis 2003; 16: 33–7. 15.Vemmos KN, Takis CE, Georgilis K et al. The Athens stroke
registry: results of a five-year hospital-based study. Cerebro-vasc Dis 2000; 10: 133–41.
16.Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation 1991; 84: 527–39.
17.Laupacis A, Albers G, Dunn M, Feinberg W. Antithrombotic therapy in atrial fibrillation. Chest 1992; 102: 426S–33S. 18.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A
prospective study of acute cerebrovascular disease in the com-munity: the Oxfordshire Community Stroke Project–1981–86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990; 53: 16–22. 19.Sandercock P, Bamford J, Dennis M et al. Atrial fibrillation
and stroke: prevalence in different types of stroke and influ-ence on early and long term prognosis (Oxfordshire com-munity stroke project). BMJ 1992; 305: 1460–5.
20.Marine JE, Goldhaber SZ. Controversies surrounding long-term anticoagulation of very elderly patients in atrial fibrilla-tion. Chest 1998; 113: 1115–18.
21.Mant JW, Richards SH, Hobbs FD et al. Midlands Research
Consortium of General Practice. Protocol for Birmingham
Atrial Fibrillation Treatment of the Aged study (BAFTA): a randomised controlled trial of warfarin versus aspirin for stroke prevention in the management of atrial fibrillation in an elderly primary care population [ISRCTN89345269]. BMC Cardiovasc Disord 2003; 26: 3–9.
22.Evans A, Perez I, Yu G, Kalra L. Secondary stroke prevention in atrial fibrillation: lessons from clinical practice. Stroke 2000; 31: 2106–11.
23.Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke 1999; 30: 1223–9.
24.Bleeding during antithrombotic therapy in patients with atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Inves-tigators. Arch Intern Med 1996; 156: 409–16.
25.Connolly S. Stroke Prevention in Atrial Fibrillation II Study. Lancet 1994; 343: 1509.
26.Candelise L, Pinardi G, Morabito A. Mortality in acute stroke with atrial fibrillation. The Italian Acute Stroke Study Group. Stroke 1991; 22: 169–74.
27.Yamanouchi H, Tomonaga M, Shimada H, Matsushita S, Kuramoto K, Toyokura Y. Nonvalvular atrial fibrillation as a cause of fatal massive cerebral infarction in the elderly. Stroke 1989; 20: 1653–6.
28.Kammersgaard LP, Jorgensen HS, Reith J, Nakayama H, Pedersen PM, Olsen TS. Short- and long-term prognosis for very old stroke patients. The Copenhagen Stroke Study. Age Ageing 2004; 33: 149–54.
29.Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke 2000; 31: 1062–8.
30.Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Pre-vention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000; 35: 183–7.
Received 20 May 2004; accepted 22 September 2004
by guest on May 14, 2011
ageing.oxfordjournals.org