www.elsevier.com / locate / bres
Short communication
Different astroglial reaction between the vagal dorsal motor nucleus
and nucleus ambiguus following vagal–hypoglossal nerve anastomosis
in cats
a ,
*
b c dChyn-Tair Lan
, Jiang Chuan Liu , Jee-Ching Hsu , Eng-Ang Ling
a
Department of Anatomy, Chung-Shan Medical and Dental College, No. 110, Sec. 1, Chien Kuo North Road, Taichung, Taiwan
b
Department of Biology and Anatomy, National Defense Medical Center, Taipei, Taiwan
c
Department of Anesthesiology, Chang-Gung Memorial Hospital, Taipei, Taiwan
d
Department of Anatomy, Faculty of Medicine, National University of Singapore, Singapore, Singapore
Accepted 8 August 2000
Abstract
The dorsal motor nucleus of the vagus (DMV) and nucleus ambiguus (NA) were both traced with horseradish peroxidase (HRP) retrograde labelling technique after vagal–hypoglossal nerve anastomosis (VHA). By light microscopy, reinnervation of the new target, viz. tongue skeletal musculature, by DMV and NA was established at 22 days postoperation (dpo) as shown by the neuronal labelling with HRP. Ultrastructurally, signs of retrograde degeneration occurred in some DMV and NA neurons between 3 and 25 days after VHA. The incidence of darkened dendrites, an early sign of dendritic loss, was more common in the DMV compared to the NA. Accompanying the neuronal alteration were drastic astrocytic reactions in the DMV, but not in the NA. Between 3 and 7 dpo, the astrocytes in the DMV showed extensively hypertrophied processes and by 22 dpo, the somata and dendrites of HRP-labelled DMV neurons, but not NA’s, appeared to be delineated by the increased lamellar astrocytic processes. Such a feature was sustained throughout the remaining postoperative intervals up to 500 dpo. It is concluded that the DMV motoneurons being autonomic in nature are probably not conducive to the newly acquired target organ. Hence, the insulation of the regenerating DMV motoneurons by the astroglial ensheathment would be vital in the neuronal remodelling and reconstruction of the vagal–hypoglossal pathway. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Vagus nerve; Hypoglossal nerve; Nerve heteroconnection; Astrocyte
It is well documented that astrocytes and microglia Studies have shown that the varying degrees of glial respond in varying degrees to different types of neuronal reaction following the peripheral nerve lesion seem to damage. For example, transection of the rat hypoglossal depend on neuronal conditions [2,3,9–11,20]. In this nerve is known to elicit microglial proliferation and connection, one of the factors influencing the neuron–glia phagocytosis of degenerating hypoglossal neurons. The interaction is the neurotrophic agents released from the synaptic boutons contacting the axotomized neurons were severed axon tip, distal stump, peripheral target cells and denuded which was accompanied by growth of astrocytic ambient environment [4]. So far, information concerning sheaths over the plasmalemmal surface vacated by the retrograde signal control has been based on experi-boutons; other features affecting the lesioned neurons ments using axotomy. This study was aimed to determine included retraction of dendrites and loss of subsurface how glial cells might react when two different nerves are cisterns [18]. If the transected nerve regenerated sponta- sectioned and then anastomosed heterogenously, a con-neously, the above features returned to normal by about 84 dition termed ‘nerve heteroconnection’. In this connection, days postoperation (dpo) and failing which, the only experiments of vagal–hypoglossal nerve anastomosis feature recovered was the subsurface cisterns [19]. (VHA) have been carried out in rats by Flumerfelt et al. [5] and McWilliam et al. [14] and, more recently, in cats by us [1]. We sought to examine the glial reaction associated with the neuronal changes in the dorsal motor nucleus of
*Corresponding author. Fax:1886-4-473-9030.
E-mail address: [email protected] (C.-T. Lan). the vagus (DMV) and nucleus ambiguus (NA), following
VHA in an attempt to better understand the roles of glial that, all tissue sections were postfixed for 1 h in 1% OsO4 cells in neuroplasticity after the nerve heteroconnection. in 0.1 M PB (pH 6.0), dehydrated through an ascending Twenty-seven adult cats of both sexes and weighing series of ethanol, embedded in Epon-Araldite and ex-2.0–3.0 kg were used. In the handling of all animals, the amined.
NIH regulations along with the guidance of the committee In agreement with previous study [1], the DMV and NA for Animal Research of Chung-Shan Medical and Dental neurons on the VHA side were first labelled at 22 dpo College were followed. Unilateral VHA was performed following HRP injections. Beyond this time point, the microsurgically in sterile conditions and under deep anaes- HRP-labelled neurons in the NA on the VHA side dis-thesia achieved with an intraperitoneal (i.p.) injection of played a profound dendritic sprouting, while those in the a-chloralose and urethane at 36 and 315 mg / kg, respec- DMV exhibited signs of dendritic withdrawal. By electron tively. The surgical procedure followed that described microscopy, the HRP-labelled neurons in the two vagal
previously [1]. nuclei on both of the intact / control and VHA (Figs. 1–4)
After VHA, all animals were closely monitored and then sides were identified by the presence of HRP reaction subjected to simultaneous injections of horseradish per- products as rod-like electron-dense crystals in the cyto-oxidase (HRP, type VI; Sigma, USA) into the left intact plasm. The ultrastructural features of the HRP-labelled vagus nerve and intrinsic tongue muscles at 1 (n51), 5 DMV and NA neurons on the intact side were consistent (n51), 12 (n51), 16 (n52), 20 (n52), 23 (n54), 44 with those described by McLean and Hopkins [12,13] in (n52), 65 (n52), 121 (n54), 270 (n51), 313 (n54), 363 the same species. At 3 days after VHA, many neurons in (n51) or 498 (n52) dpo. A total of 60 ml of 30% HRP the ipsilateral DMV underwent chromatolytic changes with dissolved in 2% DMSO was given at 12 discrete points (5 characteristic degenerating features. A notable feature was ml for each point) about 3 mm deep to the ventral surface the enhanced electron density in some neurons affecting of the tongue to ensure a sufficient deposition of the tracer the somata and dendrites. Between 7 and 25 dpo, some of throughout the intrinsic tongue muscles. This was followed the DMV neurons showed an unusual increase in lipofuscin by an intraneural injection of 10ml HRP into the left intact granules, but by 67 dpo, the cells appeared reasonably vagus nerve at the level of ansa cervicalis with a glass
micropipette connected to a 10-ml Hamilton syringe by a polyethylene tube filled with liquid paraffin. After 2 days, all cats were reanaesthetized with sodium pentobarbital (40 mg / kg, i.p.) and killed by transcardiac perfusion, i.e. sacrificed at 3, 7, 14, 18, 22, 25, 46, 67, 123, 272, 315, 365, or 500 dpo. Perfusion began with a prewash of 1000 ml of Ringer’s solution and then with a 2000–3000 ml mixture of 1% paraformaldehyde and 1.25% glutaral-dehyde in 0.1 M phosphate buffer (PB, pH 7.4). Ten minutes prior to perfusion-fixation, the animals were given an injection of heparin (1000 unit / kg) and sodium nitrite (20 mg / kg) through the femoral vein. The entire fixation procedure lasted 30 min. After that, the brainstem was removed. Eight brainstems derived from cats killed at 18, 22, 25, 46, 67, 123, 272 and 315 dpo were rinsed in 0.1 M PB (pH 7.4) containing 10% sucrose and then transferred into 30% sucrose buffer, while the remaining 19 brain-stems for ultrastructural study were post-fixed for 2 h in the same fixative and kept in 10% sucrose buffer in the refrigerator overnight at 48C.
For light microscopic study, the 8 brainstems stored in 30% sucrose buffer mentioned above were cut transversely at 40-mm thickness on the following day with a freezing microtome. All serial brainstem sections collected were then processed for HRP histochemistry according to the method of Mesulam [15]. Finally, the treated sections were counterstained in 1% neutral red and examined under bright-field illumination. The brainstems for ultrastructural
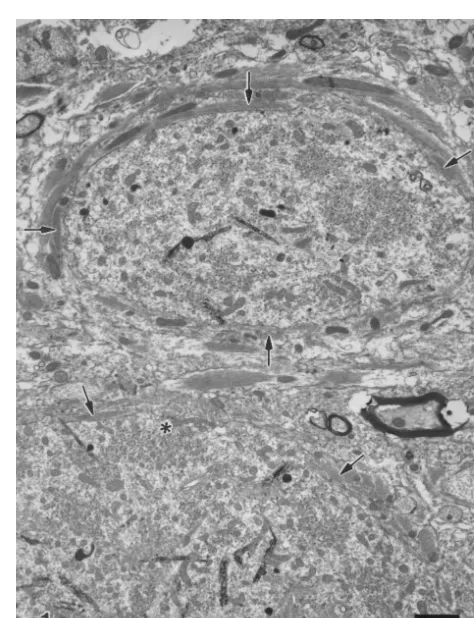
Fig. 1. HRP-labelled dendrite (upper) and soma (lower) of DMV neurons
study were cut transversely at 80-mm thickness with a are ensheathed by lamellae of astrocytic processes (arrows), 123 days vibratome and then reacted in the tetramethyl benzidine / after VHA. The astroglial ensheathment is so extensive that most areas of
Fig. 3. HRP-labelled dendrite of an NA neuron at 315 day after VHA. Astroglial ensheathment is not evident. Filamentous hyperplasia (f) is Fig. 2. An HRP-labelled DMV motoneuron is incompletely ensheathed obvious in dendrite. Many axon terminals make synaptic contacts with the by multilayered astrocytic processes (arrow), 365 days after VHA. *, HRP-labelled neuronal profiles. *, synapse.
synapse.
DMV, astrocytes in the NA responded mildly to VHA. In normal in ultrastructure. In longer surviving animals, i.e. fact, in long-term VHA the plasmalemmal surface of most beyond 67 dpo, there was no noticeable difference in the of the HRP-labelled dendrites (Fig. 3) and somata (Fig. 4) DMV neurons between the operated (Figs. 1 and 2) and of NA neurons remained to be contacted by numerous intact sides. As in the DMV, the NA motoneurons under- synaptic boutons. In contrast to the drastic response of went chromatolytic changes after VHA. Beyond 25 dpo, astrocytes to VHA, there was only mild microglial reaction however, the ultrastructure of most of the HRP-labelled in both the DMV and NA at all postoperative intervals. NA neurons appeared normal (Fig. 4), except for the There was no evidence of phagocytosis.
evidence of filamentous hyperplasia in some dendrites This study has demonstrated the ultrastructural changes
(Fig. 3). of astrocytes during retrograde axon reaction along with
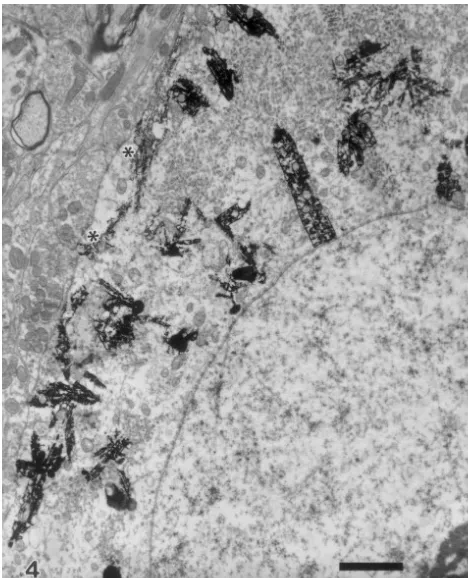
Fig. 4. HRP-labelled soma of an NA neuron at 500 day after VHA.
Astroglial ensheathment is not evident. Many axon terminals make Fig. 5. Showing a marked hypertrophic astrocyte (A) and numerous synaptic contacts with the HRP-labelled neuronal profiles. *, synapse. astrocytic processes in the neuropil of DMV, 3 days after VHA; both are
filled with bundles of glial filaments (f).
may explain the subsequent dendritic withdrawal or
denu-dation of the HRP-labelled DMV neurons [1]. Present astrocytes were necessitated for local needs such as results suggest that the vagal motoneurons of DMV are neuronal remodelling. The astrocytic reaction could not probably more responsive to VHA compared with those of have been induced by direct retrograde degeneration or NA and that the dendritic withdrawal in the DMV may be else it would also have occurred in the NA.
reflective of part of the neuronal remodelling process. Following VHA, the DMV motoneurons now giving rise Along with the moderate neuronal degeneration in the to the vagal–hypoglossal heteroconnected nerve are proba-DMV and NA was the mild microglial response to VHA. bly incompatible with the newly acquired target. In view of This is contrary to the more vigorous microglial reaction in their autonomic function in nature, they may not be axotomy [2,9,11,20] or toxin-induced neuronal suicide appropriate for the tongue skeletal musculature for vol-[10]. Since microglial cells are known to function as untary movement. It is speculated that some modifications potential phagocytes, their mild reactive changes after in neural regulation of the regenerating DMV motoneurons VHA would be consistent with the lower degrees of would be necessary to facilitate a successful functional
neuronal degeneration in both the DMV and NA. recovery. The increased astrocytic processes in the DMV
[2] D.H. Chen, Qualitative and quantitative study of synaptic displace-ment in chromatolyzed spinal motoneurons of the cat, J. Comp. Neurol. 177 (1978) 635–664.
[3] J.L. Cova, H. Aldskogius, A morphological study of glial cells in the hypoglossal nucleus of the cat during nerve regeneration, J. Comp. Neurol. 233 (1985) 421–428.
[4] J.W. Fawcett, R.J. Keynes, Peripheral nerve regeneration, Annu. Rev. Neurosci. 13 (1990) 43–60.
[5] B.A. Flumerfelt, J.A. Kiernan, J.P. Krcek, J. Sholdice, Reinnervation of skeletal muscle in the tongue by preganglionic parasympathetic vagal neurons, J. Anat. 146 (1986) 117–130.
[6] M.B. Graeber, G.W. Kreutzberg, Astrocytes increase in glial fibril-lary acidic protein during retrograde changes of facial motor neurons, J. Neurocytol. 15 (1986) 363–373.
[7] M.B. Graeber, G.W. Kreutzberg, Delayed astrocyte reaction follow-ing facial nerve axotomy, J. Anat. 17 (1988) 209–220.
[8] C.T. Lan, C.Y. Wen, C.K. Tan, E.A. Ling, J.Y. Shieh, Ultrastructural study of external cuneothalamic neurons and their synaptic relation-ships with primary afferents in the gerbil, J. Comp. Neurol. 366 (1996) 406–415.
[9] E.A. Ling, W.C. Wong, T.Y. Yick, S.K. Leong, Ultrastructural changes in the dorsal motor nucleus of monkey following bilateral cervical vagotomy, J. Neurocytol. 15 (1986) 1–15.
[10] E.A. Ling, S.K. Leong, Effects of intraneural injection of ricinus communis agglutinin-60 into the rat vagus nerve, J. Neurocytol. 16 (1987) 373–387.
[11] E.A. Ling, J.Y. Shieh, C.Y. Wen, T.Y. Yick, W.C. Wong, The dorsal motor nucleus of the vagus of the hamster: ultrastructure of vagal neurons and their responses to vagotomy, J. Anat. 152 (1987) 161–172.
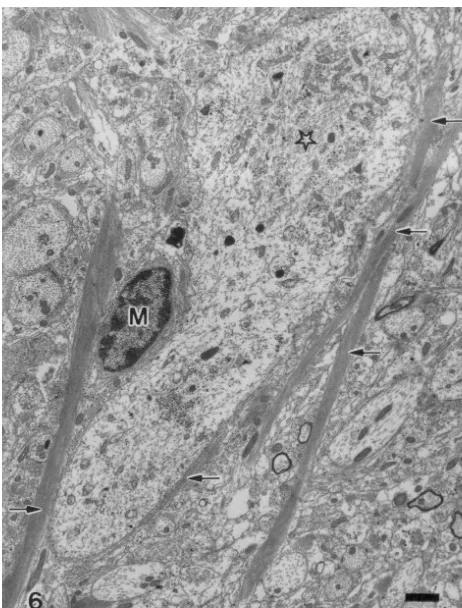
[12] J.H. McLean, D.A. Hopkins, Ultrastructural identification of labeled neurons in the dorsal motor nucleus of the vagus nerve following Fig. 6. Several elongated astrocytic processes (arrows) containing
bun-injections of horseradish peroxidase into the vagus nerve and dles of glial filaments are in close apposition to a dendrite (star) in the
brainstem, J. Comp. Neurol. 206 (1982) 243–252. DMV, 22 days after VHA. M, microglial cell.
[13] J.H. McLean, D.A. Hopkins, Ultrastructural studies of the nucleus ambiguus in cat and monkey following injection of HRP into the
pivotal role in the central visceromotor system [16]. By the vagus nerve, J. Neurocytol. 14 (1985) 961–979.
[14] P.N. McWilliam, A. Maqbool, T.F.C. Batten, J.C. Kaye, Influence of
astroglial ensheathment induced by VHA, the regenerating
peripheral targets on the expression of calcitonin gene-related
DMV motoneurons could physically displace most, if not
peptide immunoreactivity in rat cranial motoneurones, J. Neurobiol.
all, of the synaptic boutons originally conveying visceral 28 (1995) 506–514.
influences and hence, preventing the tongue muscles from [15] M.M. Mesulam, Tetramethylbenzidine for horseradish peroxidase the involuntary autonomic influence. neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents, J. Histochem. Cytochem. 26 (1978) 106–117.
[16] P.E. Sawchenko, Central connections of the sensory and motor
Acknowledgements nuclei of the vagus nerve, J. Autonom. Nerv. Syst. 9 (1983) 13–26.
[17] R.S. Sloviter, E. Dean, A.L. Sollas, J.H. Goodman, Apoptosis and
This study was supported by research grant (No. necrosis induced in different hippocampal neuron populations by repetitive perforant path stimulation in the rat, J. Comp. Neurol. 366
NSC88-2314-B-040-014) from the National Science
Coun-(1996) 516–533.
cil, Taiwan.
[18] B.E.H. Sumner, A quantitative analysis of the response of presynap-tic boutons to postsynappresynap-tic motor neuron axotomy, Exp. Neurol. 46 (1975) 605–615.
References [19] B.E.H. Sumner, Ultrastructural observations on the inhibited
re-covery of the hypoglossal nucleus from the axotomy response when regeneration of the hypoglossal nerve is prevented, Exp. Brain Res. [1] S.T. Chang, F.K. Lieu, S.D. Wang, J.C. Liu, Neuronal
supernumer-26 (1976) 141–150. ary and dendritic sprouting of the nucleus ambiguus after chronic
[20] A. Torvik, A.J. Søreide, The perineuronal glial reaction after alteration of peripheral targets in cats, Brain Res. 805 (1998)