www.elsevier.com / locate / bres
Short communication
Transfection of the plasma-type platelet-activating factor
acetylhydrolase gene attenuates glutamate-induced apoptosis in
cultured rat cortical neurons
a ,
*
b c c cYutaka Hirashima
, Hikaru Ueno , Ken Karasawa , Kazuaki Yokoyama , Morio Setaka ,
a
Akira Takaku
a
Department of Neurosurgery, Toyama Medical and Pharmaceutical University, Toyama-shi, Toyama 930-0194, Japan
b
Department of Cardiology, Graduate School of Medical Sciences, Kyushu University, Fukuoka-shi, Fukuoka 812-8582, Japan
c
Faculty of Pharmaceutical Sciences, Teikyo University, Sagamiko-cho, Kanagawa 199-0106, Japan
Accepted 15 August 2000
Abstract
Using an adenoviral vector, we induced overexpression of the plasma type of platelet-activating factor acetylhydrolase in cultured rat neurons. Neurons overexpressing this enzyme showed a decrease in glutamate-induced injury, mainly, apparent as decreased apoptosis. Reduction of lipid peroxidation by this enzyme and protection of mitochondrial function were demonstrated, and these may be the basis of the resistance to glutamate-induced neuronal injury that we observed. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Neuronal death
Keywords: Neurons; Apoptosis; Platelet-activating factor (PAF) acetylhydrolase; Glutamate; Mitochondrial damage
Platelet-activating factor (PAF) acetylhydrolase Plasma PAFAH also has the ability to catabolize oxidized (PAFAH) removes the acetyl group at the sn-2 position of phospholipids [11,21,22]. Although plasma PAFAH is PAF. Forms of mammalian PAFAH include an intracellular secreted in vitro from various cells such as macrophages (tissue) type and an extracellular (plasma) type. In turn, [4] and hepatocytes [23], the exact cellular source(s) of this tissue cytosol includes at least two types of tissue PAFAH, enzyme in vivo remains unclear. Recently, Asano et al. isoforms Ib and II [7]. Isoform Ib is a tertiary G protein reported that most of the PAFAH activity in human plasma complex-like heterotrimeric enzyme, while isoform II is a originates from hematopoietic lineage cells [2]
40-kDa monomer. Plasma PAFAH is a 45-kDa monomeric Neurotoxicity initiated by overstimulation of glutamate enzyme in humans and a 63-kDa monomeric enzyme in receptors and subsequent depolarization of mitochondria guinea pigs [5,12,24]. Isoform II has an amino acid has been suggested to contribute to oxidative stress in sequence with 41% identity to that of human plasma neuronal injury [6,26]. Previous investigation has indicated PAFAH [8,9,24]. Although human and guinea pig plasma that two types of neuronal death, apoptosis and necrosis, PAFAH show some differences in physical properties such can result from glutamate receptor-mediated excitotoxicity as association of lipoproteins or molecular mass, they [1]. Mild insults lead to transient mitochondial depolariza-share many similar biochemical properties [11]. Isoform II tion, reversible energy failure, and apoptosis, while intense preferentially hydrolyzes oxidized phospholipids as well as insults produce irreversible mitochondrial depolarization, PAF [8], and therefore may function as an antioxidant [15]. permanent energy collapse, and ionic imbalances resulting in cellular swelling and necrosis [1]. Overexpression of isoform II in Chinese hamster ovary K1 cells has been
*Corresponding author. Tel.: 181-76-434-2281; fax: 1
81-76-434-reported to suppress tert-butylhydroperoxide
(t-BuOOH)-5034.
E-mail address: [email protected] (Y. Hirashima). induced apoptotic cell death, most likely by its antioxidant
effects [15]. In the present study, we determined whether performed essentially as described by Musser et al. [16]. glutamate-induced neuronal death was reduced in neuronal MTT reagent was dissolved at 5 mg / ml in PBS, filter-cultures by overexpression of the plasma PAFAH gene sterilized using 0.22 mm disposable filters (Millex-GV; transfected with an adenovirus vector in place of tissue- Millipore, Bedford, MA), and stored at 48C. MTT stock type PADAH gene and estimated whether oxidative stress solution (25ml) was added to each well and incubated at due to impairment of mitochondrial function contributed to 378C for 4 h to allow mitochondria to form a colored,
neuronal apoptosis. insoluble formazan product. After the supernatant was
We purchased 5-bromo-4-chloro-3-indolyl-b-D-galacto- removed by aspiration, 100ml of dimethylsulphoxide was pyranoside (X-gal) from Wako (Osaka, Japan). Thiobar- added to each well to solubilize the formazan crystals. The bituric acid (TBA) reagent as well as 1,1,3,3-tetraethox- plate was agitated to fully dissolve the crystals, and ypropane, 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyl tetra- absorbance at 550 nm was measured by a model 340 zolium bromide (MTT reagent), and Hoechst 33258 was ATTC microplate reader (SLT, Saltzburg, Austria). obtained from Sigma (St. Louis, MO). A protein assay kit Cells on glass slides were stained with Hoechst 33258 was obtained from Bio-Rad (Hercules, CA). (10mM) for 5 min and analyzed morphologically under a Replication-defective E1- and E3-adenoviral vectors nonconfocal fluorescence microscope (Olympus BX50, without an inserted gene (Ad1W) or alternatively con- Tokyo, Japan). A photomicrograph was taken immediately taining either guinea pig plasma PAFAH gene with color reversal film (Fujichrome Provia 400, Fuji, (AdPAFAH)[12] or b-galactosidase gene (AdLacZ)[14] Tokyo, Japan). Apoptotic cells identifiable by nuclear under control of cytomegalovirus enhancer and a chicken fragmentation were counted from the color print.
b-actin promoter together constituting a CA promoter [17] Data are presented as the mean6S.D. For statistical were prepared as described previously [25]. comparisons, one-way analyses of variance followed by Cerebral cortical neuronal cells were prepared from Fisher’s protected least-squares difference test were used 17-day-old rat embryos according to a previously reported for multiple comparisons. P-values,0.05 were considered procedure [18,19]. The dissociated cells were plated in to indicate significant differences.
polyethylenimine-coated 96-well and 24-well culture plates We first examined the expression of LacZ by X-Gal and 2-well glass slides. Incubation with recombinant staining in neurons exposed to AdLacZ at various m.o.i. adenovirus (10–50 multiplicity of infection (m.o.i.)) was for 72 h. Neurons showed deep blue coloration with carried out for 6 h using 4-day-old cultures. Cells next intensity dependent on vector quantity from 10 to 50 m.o.i. were exposed to 1 mM glutamate for 60 min on day 7 in (data not shown). The percentage of neurons with positive culture, and the medium was replaced. Cultures then were X-gal staining at 50 m.o.i. was 70.8610.9% (n54). Next, maintained for 24 h before neuronal death was assessed. we measured PAFAH activity in neurons infected with PAFAH activity was assayed as described by Stafforini et AdPAFAH. Like LacZ, the activity of PAFAH increased
al. [20]. with increasing m.o.i. (Fig. 1). PAFAH activity in culture
X-gal staining was performed to demonstrate gene expression for b-galactosidase, as previously described in detail [25]. Briefly, cells on glass slides were prefixed with 2% formaldehyde and 0.2% glutaraldehyde in phosphate-buffered saline (PBS), incubated in 5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside, and postfixed with 2% formaldehyde and 0.2% glutaraldehyde in PBS, with a thorough rinse with PBS before each step.
The TBA test protocol of Lee and Csallany [13] was used with the modification that phosphoric acid was replaced with 10% trichloroacetic acid (TCA) in 1% HCl [3]. After addition of the TCA solution samples, 0.5 ml of TBA reagent (1% w / v TBA in 50 mM NaOH) was added, and the mixture was heated for 15 min at 1008C. After cooling, the chromophore was extracted into 1.5 ml butan-1-ol, and absorbance was measured at 532 nm. Since the
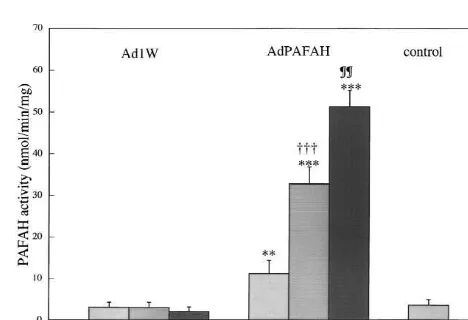
TBA test measures malondialdehyde (MDA), we prepared Fig. 1. Platelet-activating factor acetylhydrolase (PAFAH) activity in
a standard plot for MDA in the TBA test system, which neurons transfected with AdPAFAH (recombinant adenovirus vector containing the guinea pig plasma PAFAH gene). Values are expressed as
was linear up to 20 nmol MDA per sample [3]. The plot
the mean6S.D. (n54). **, P,0.01 and ***, P,0.001 compared with
was used to convert absorbance values to nmol of MDA.
controls. †††, P,0.001 compared with AdPAFAH at 10 m.o.i.; ¶¶,
MDA content was normalized to protein concentration. P,0.01 compared with AdPAFAH at 50 m.o.i. (Significance testing: Mitochondrial function was assessed by the MTT assay. one-way analysis of variance followed by Fisher’s protected least-squares
medium showed no difference between media from neu-rons without infection (control), neuneu-rons infected with Ad1W, and neurons infected with AdPAFAH. Activity in medium was negligible compared with activity in cell suspensions (data not shown). Plasma type of PAFAH should be produced and secreted from some type of cells [2,4,23] and some enzyme activity was expected in the culture medium infected with AdPAFAH. The discrepancy may be explained by the pooling system of the enzyme in neurons. The result suggested that transfection at m.o.i. 50 for 72 h achieves sufficient cellular expression of plasma PAFAH.
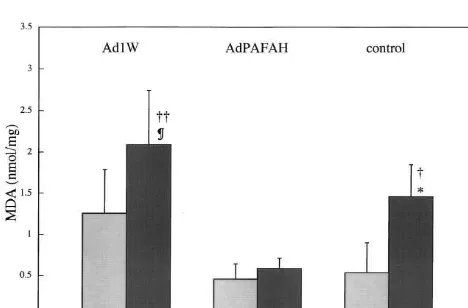
Oxidation of lipids, (lipid peroxidation) was assayed with TBA. In experiments without glutamate, neurons
infected with Ad1W were associated with more reaction Fig. 3 . Tetraepoxypropane dimethylthiazolediphenyl tetrazolium (MMT)
product (MDA) than neurons without infection (control) or product in neurons with and without exposure to 1 mM glutamate (Glu).Values are expressed as the mean6S.D. (n54). AdPAFAH
repre-those infected with AdPAFAH. Infection with adenovirus
sents neurons transfected with recombinant adenovirus vector containing
then, may induce oxidation of lipids (Fig. 2) [11,15].
the guinea pig plasma platelet-activating factor acetylhydrolase gene;
Although glutamate treatment resulted in increased MDA Ad1W, neurons infected with adenoviral vectors without an inserted gene; in neurons without infection (control) or infection with and control, neurons without infection.; transfected with recombinant
Ad1W, MDA content was not significantly increased by adenovirus vector containing the guinea pig plasma PAFAH gene, Ad1W; infected with only adenoviral vector, and control; without infection. **,
glutamate loading in neurons infected with AdPAFAH
P,0.01 compared with Ad1W/ Glu(2). §§§, P,0.001 compared with
(Fig. 2). PAFAH may attenuate increased membrane lipid
Ad1W/ Glu(2). ¶¶¶, P,0.001 compared with control / Glu(2). ††, P, peroxidation resulting from both viral infection and gluta- 0.01 and †††, P,0.001 compared with control / Glu(1) and Ad1W/
mate treatment. Glu(1). (Significance testing:one-way analysis of variance followed by
Extent of neuronal injury was assayed in terms of Fisher’s protected least-squares difference test.). OD550, optical density at 550 nm.
mitochondrial function in the MTT assay [16]. Neurons infected with AdPAFAH and those without infection
(control) showed significantly higher amounts of MTT neuronal mitochondrial function from this insult in addi-product than neurons infected with Ad1W (Fig. 3). These tion to attenuating oxidation of membrane phospholipids results suggest that without glutamate loading neuronal [11,15].
mitochondrial function is impaired by viral infection, while the antioxidant action of plasma PAFAH may protect
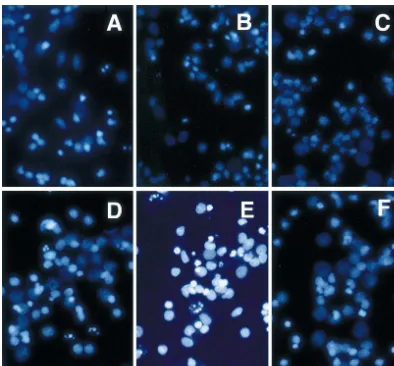
Fig. 5. Hoechst 33258 staining of cultured cortical neurons with and without exposure to 1 mM glutamate (Glu). Apoptotic neurons with fragmented nuclei were widespread in cultures infected with adenoviral vectors without an inserted gene (Ad1W/ Glu(1)) or with no infection (control / Glu(1)), while neurons infected with AdPAFAH(AdPAF / Glu(1)) did not show marked apoptotic features. A, control / Glu(2); B, Ad1W/ Glu(2); C, AdPAFAH / Glu(2); D, control / Glu(1); E, Ad1W/ Glu(1) and F; AdPAFAH / Glu(1).
Damage to neurons from 1 mM glutamate exposure was of mitochondrial function was relatively mild after 1 mM estimated by the MTT assay 24 h after exposure to glutamate exposure in the present study, in accordance glutamate for 60 min. Significantly more MTT product with our previous observation that neuronal death in was present 24 h after exposure to 1 mM glutamate for 60 culture was mainly apoptosis 24 h after exposure to 1 mM min in neurons infected with AdPAFAH than in those glutamate [10]. With Hoechst 33258 staining of neurons 24 infected with Ad1W or those without infection (Fig. 3). h after exposure of 1 mM glutamate for 60 min, apoptotic Tissue-type, 40 kDa monomer PAFAH (isoform II) acts as neurons with fragmented nuclei were widespread in cul-an cul-antioxidcul-ant phospholipase that protects cells from tures infected with Ad1W or with no infection (control), oxidative stress [15]. Plasma-type PAFAH has an amino while neurons infected with AdPAFAH did not show acid sequence similar to isoform II [8,9,24] and also has marked apoptotic features (Figs. 4 and 5). Thus, like the the ability to catabolize oxidized phospholipids [11,21,22]. tissue type, 40-kDa PAFAH monomer, plasma-type Overstimulation of glutamate receptors induces cell death PAFAH has an antioxidant action that protects cells from by reactive oxygen intermediates that result from impair- death due to oxidative stress.
ment of mitochondrial function [6,26]. The present results suggest that like tissue-type isoform II, plasma-type PAFAH can protect neurons from apoptosis by reducing
References
oxidative stress.
Mild glutamate excitotoxic insults lead to transient
[1] M. Ankarcrona, J.M. Dypbukt, E. Bonfoco, B. Zhivotovsky, S.
mitochondrial depolarization and apoptosis, while intense Orrenius, S.A. Lipton, P. Nicotera, Glutamate-induced neuronal insults produce irreversible mitochondrial depolarization death: a succession of necrosis or apoptosis depending on
[2] K. Asano, S. Okamoto, K. Fukunaga, T. Shiomi, T. Mori, M. Iwata, Saito, A. Takeshita, Percutaneous transluminal gene transfer into Y. Ikeda, K. Yamaguchi, Cellular source(s) of platelet-activating- canine myocardium in vivo by replication-defective adenovirus, factor acetylhydrolase activity in plasma, Biochem. Biophys. Res. Cardiovasc. Res. 30 (1995) 97–105.
Commun. 261 (1999) 511–514. [15] A. Matsuzawa, K. Hattori, J. Aoki, H. Arai, K. Inoue, Protection [3] R. Brett, M.G. Rumsby, Evidence of free radical damage in the against oxidative stress-induced cell death by intracellular platelet-central nervous system of guinea pigs at the prolonged acute and activating factor-acetylhydrolase II, J. Biol. Chem. 272 (1997) early relapse stages of chronic relapsing allergic encephalomyelitis, 32315–32320.
Neurochem. Int. 23 (1993) 35–44. [16] D.A. Musser, A.R. Oseroff, The use of tetrazolium salts to de-[4] M.R. Elstad, D.M. Stafforini, T.M. Mcintyre, S.M. Prescott, G.A. termine sites of damage to the mitochondrial electron transport chain Zimmerman, Platelet-activating factor acetylhydrolase increases in intact cells following in vitro photodynamic therapy with photo-during macrophage differentiation, J. Biol. Chem. 264 (1989) 8467– frin II, Photochem. Photobiol. 59 (1994) 621–626.
8470. [17] H. Niwa, K. Yamamura, J. Miyazaki, Efficient selection for high-[5] Z.S. Derewenda, Y.S. Ho, PAF-acetylhydrolases, Biochim. Biophys. expression transfectants with a novel eukaryotic vector, Gene 108
Acta 1441 (1999) 229–236. (1991) 193–200.
[6] L.L. Dugan, S.L. Sensi, L.M. Canzoniero, S.D. Handran, S.M. [18] K. Nogami, Y. Hirashima, S. Endo, A. Takaku, Involvement of Rothman, T.-S. Lin, M.P. Coldberg, D.W. Choi, Mitochondrial platelet-activating factor (PAF) in glutamate neurotoxicity in rat production of reactive oxygen species in cortical neurons following neuronal cultures, Brain Res. 754 (1997) 72–78.
exposure to N-methyl-D-aspartate, J. Neurosci. 15 (1995) 6377– [19] T. Ohmori, Y. Hirashima, M. Kurimoto, S. Endo, A. Takaku, In vitro
6388. hypoxia of cortical and hippocampal CA1 neurons: glutamate, nitric
[7] M. Hattori, H. Arai, K. Inoue, Purification and characterization of oxide, and platelet activating factor participate in the mechanism of bovine brain platelet-activating factor acetylhydrolase, J. Biol. selective neuronal death in CA1 neurons, Brain Res. 743 (1996)
Chem. 268 (1993) 18748–18753. 109–115.
[8] K. Hattori, M. Hattori, H. Adachi, M. Tsujimoto, H. Arai, K. Inoue, [20] D.M. Stafforini, S.M. Prescott, T.M. McIntyre, Human plasma Purification and characterization of platelet-activating factor platelet-activating factor acetylhydrolase: Purification and charac-acetylhydrolase II from bovine liver cytosol, J. Biol. Chem. 270 terization, J. Biol. Chem. 262 (1987) 4223–4230.
(1995) 22308–22313. [21] D.M. Stafforini, E.N. Rollins, S.M. Prescott, T.M. McIntyre, The [9] K. Hattori, H. Adachi, A. Matsuzawa, K. Yamamoto, M. Tsujimoto, platelet-activating factor acetylhydrolase from human erythrocytes
J. Aoki, M. Hattori, H. Arai, K. Inoue, cDNA cloning and Purification and properties, Biol. Chem. 268 (1993) 3857–3865. expression of intracellular platelet-activating factor (PAF) [22] K.E. Stremler, D.M. Stafforini, S.M. Prescott, T.M. McIntyre, acetylhydrolase II. Its homology with plasma PAF acetylhydrolase, Human plasma platelet-activating factor acetylhydrolase. oxidatively J. Biol. Chem. 271 (1996) 33032–33038. fragmented phospholipids as substrates, J. Biol. Chem. 266 (1991) [10] Y. Hirashima, M. Kurimoto, K. Nogami, S. Endo, M. Saitoh, O. 11095–11103.
Ohtani, T. Nagata, A. Muraguchi, A. Takaku, Correlation of [23] E.B. Tarbet, D.M. Stafforini, M.R. Elstad, G.A. Zimmerman, T.M. glutamate-induced apoptosis with caspase activities in cultured rat McIntyre, S.M. Prescott, Liver cells secrete the plasma form of cerebral cortical neurons, Brain Res. 849 (1999) 109–118. platelet-activating factor acetylhydrolase, J. Biol. Chem. 266 (1991) [11] K. Karasawa, M. Yato, M. Setaka, S. Nojima, Purification and 16667–16673.
characterization of platelet-activating factor acetylhydrolase from [24] L.W. Tjoelker, C. Wilder, C. Eberhardt, D.M. Stafforini, G. Dietsch, peritoneal fluid obtained from guinea pigs after endotoxin shock, J. B. Schimpf, S. Hooper, H.L. Trong, S. Cousens, G.A. Zimmerman, Biochem. (Tokyo) 116 (1994) 374–379. Y. Yamada, T.M. McIntyre, S.M. Prescott, P.W. Gray, Anti-in-[12] K. Karasawa, O. Kuge, K. Kawasaki, M. Nishijima, Y. Nakano, M. flammatory properties of a platelet-activating factor acetylhydrolase,
Tomita, K. Yokoyama, M. Setaka, S. Nojima, Cloning, expression Nature 374 (1995) 549–553.
and characterization of plasma platelet-activating factor- [25] H. Ueno, J.-J. Li, H. Tomita, H. Yamamoto, Y. Pan, Y. Kanegae, I. acetylhydrolase from guinea pig, J. Biochem. (Tokyo) 120 (1996) Saito, A. Takeshita, Quantitative analysis of repeat adenovirus-838–844. mediated gene transfer into injured canine femoral arteries, Arterios-[13] H.-S. Lee, A.S. Csallany, Measurement of free and bound malon- cler. Thromb. Vasc. Biol. 15 (1995) 2246–2253.