Oil, erucic acid, and glucosinolate contents in winter hardy
rapeseed germplasms
Harbans L. Bhardwaj *, Anwar A. Hamama
Agricultural Research Station,Virginia State Uni6ersity,PO Box9061,Petersburg,VA23806,USA
Received 19 March 1999; accepted 17 November 1999
Abstract
The US industry uses :18 million kg of high erucic acid oil annually, mostly from imports. Therefore, a large overall market potential exists for development of annually renewable domestic sources of erucic acid. The present research was conducted to characterize the winter hardy rapeseed germplasm for oil, erucic acid, and glucosinolate contents for use in breeding programs to develop commercial production of rapeseed. Significant variation existed among the 455 accessions ofBrassica napus L. and the 44 accessions of Brassica rapa L. for oil, erucic acid, and glucosinolate contents.B.napushad significantly higher mean oil content in the seeds (37.4%) than theBrassica rapa
(36.6%). The glucosinolate content was higher in napusthan the rapa meal (49.2 vs. 43.8 mmol/g). The erucic acid
content was higher inrapa(32.6%) than thenapusaccessions (26.1%). Within species, the correlation between oil and glucosinolate contents was significantly negative among thenapusaccessions (−0.14), but was significantly positive amongrapa accessions (+0.39). The results indicate that plant material from eithernapusorrapa species could be used in breeding for increasing erucic acid content. Accessions with high, medium, and low contents of oil, erucic acid, and glucosinolate contents were identified. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Brassica napus;Brassica rapa; Erucic acid; Rapeseed; Glucosinolate
1. Introduction
The erucic acid from oil of industrial rapeseed
(Brassica napus L. and Brassica rapa L.) is a
valuable and renewable raw material for the man-ufacture of a wide array of industrial products. The US industry uses approximately 18 million kg of high erucic acid oil, mostly from imports (USDA, 1989). Thus, a large overall market po-tential exists for development of annually renew-able domestic sources of erucic acid (Van Dyne et al., 1990).
Rapeseed meal is unsuitable for livestock feed due to its high glucosinolate content and is a hindrance to domestic rapeseed production. Be-cause low glucosinolate meal can be used for
Contribution of Virginia State University, Agricultural Research Station. Journal Article Series No. 216. The use of trade names or vendors does not imply approval to the exclusion of other products or vendors that may also be suitable.
* Corresponding author. Tel.: +1-804-5246723; fax: + 1-804-5245950.
E-mail address:[email protected] (H.L. Bhardwaj)
Table 1
Characteristics of rapeseed accessions grown at Petersburg, VA during 1993/94 season
Characteristic B.napus(n=455) B.rapa(n=44)
Range
Mean Mean Range
29.6–49.2
Oil (%) 37.4 a* 36.6 b 29.5–40.8
Erucic acid (%) 26.1 b 0.3–56.2 32.6 a 12.9–50.3
Glucosinolates (mmol/g meal) 49.2 a 37.8–77.0 43.8 b 38.7–56.8
* Species means followed by different letters are significantly different according to Duncan’s multiple range test (5% level).
livestock feed, and high glucosinolate meal for potential pest control (Bhardwaj et al., 1996), either a reduction or an enhancement in glucosi-nolate content might be helpful in developing rapeseed as a renewable-domestic source of erucic acid.
The present research was conducted as a part of our efforts to develop rapeseed as a new alterna-tive winter cash crop in Virginia and adjoining areas and was necessitated by lack of winter hardy genotypes adapted to Virginia’s climatic conditions. During this period, commercial rape-seed was being produced in southern US states by using public and private rapeseed cultivars. A survey of historical weather data from 1961 – 1990 indicated that the average temperatures in Peters-burg, VA from September to May (the normal crop cycle for rapeseed production in Virginia) were 22, 16, 11, 5, 3, 4, 9, 14, and 19°C compared to the average temperatures for the same period being 24, 18, 13, 9, 7, 9, 14, 18, and 22°C in southern US states (Georgia and Alabama). These climatic differences indicated the need for devel-opment of winter hardy genotypes for production in Virginia.
In addition, information about the extent of variability in winter hardy rapeseed germplasm was not available. Previous studies (Auld et al., 1988; Mahler and Auld, 1989) reported the vari-ability for fatty acids and glucosinolate contents in rapeseed germplasm by evaluating the seeds collected from national and international sources. These seeds were analyzed without growing them in any single location. We were interested in char-acterizing the variability of all accessions grown under our environment, so that the climatic ef-fects during field production were eliminated. Our
specific objectives were to characterize variation among winter hardy rapeseed genotypes for oil, erucic acid, and glucosinolate contents; to deter-mine relationships between these characteristics; and to identify accessions with high, medium, or low contents of oil, erucic acid, and glucosinolates for use in future breeding programs.
2. Materials
2.1. Germplasm
A germplasm collection consisting of 938 acces-sions of Brassica napus L. and Brassica rapa L., provided by the North Central Regional Plant
Introduction Station of National Plant
Germplasm System of US Department of Agricul-ture, was evaluated at Randolph Farm, Virginia State University (37° 15% N and 77° 30.8% W) during the 1993 – 94 crop season. Thirty seeds of each accession were planted on 14 October 1993 in a completely randomized design with one repli-cation in rows 2-m long with 0.75-m spacing between the rows. The experiment was established
Table 2
Correlations between various characteristics of rapeseed acces-sions grown at Petersburg, VA during 1993/94 season
Overall
Variables B.napus B.rapa
−0.07
Oil and erucic acid −0.06 0.06 −0.14**
Oil and glucosinolates −0.11* 0.39** 0.03 0.06
Erucic acid and glucosi- 0.18 nolates
Table 3
Oil and erucic acid content of rapeseed accessions with high, medium, and low glucosinolate contents
B.napus
Glucosinolate content Overall B.rapa
Erucic acid (%) Oil (%)
* Means within columns, followed by similar letters are not different according to Duncan’s multiple range test (5% level).
on a uniform piece of land (Abel sandy loam: Fine Loamy mixed, thermic Aquatic Hapridult). All plots received 100 kg/ha of nitrogen, phospho-rus, and potassium. The experimental area re-ceived a pre-plant-incorporated treatment of Treflan herbicide (trifluralin). The number of emerged plants in each row were recorded two weeks after planting. All accessions that had a minimum of 12 plants per row were considered to have adequate plant stand. Among these acces-sions, those where at least 50% of the emerged plants had survived the winter and matured were classified as winter hardy. This material consisted of 499 accessions (455 fromB.napusand 44 from
B. rapa). These plots were harvested during first week of June, 1994 and the seeds of these acces-sions were evaluated for oil, erucic acid, and glucosinolate contents.
3. Methods
3.1. Oil extraction
The oil was extracted from 1 g of ground seed at room temperature by homogenization for 2 min in 10 ml hexane/isopropanol (3:2, v/v) with a Biospec Model 985-370 Tissue Homogenizer (Biospec Products, Inc. Racine, WI, USA) and centrifuged at 4000×g for 5 min (St. John and Bell, 1989). The oil extraction was repeated for each sample for three times to ensure full oil recovery. The hexane – lipid layer was washed and separated from the combined extract by shaking and centrifugation with 10 ml of 1% CaCl2 and
1% NaCl in 50% methanol. The washing proce-dure was repeated and the purified lipid layer was
removed by aspiration and dried over anhydrous Na2SO4. The oil percentage (g/100 g dry basis)
was determined gravimetrically after drying in an under vacuum oven at 40°C and stored under nitrogen at−10°C until analysis.
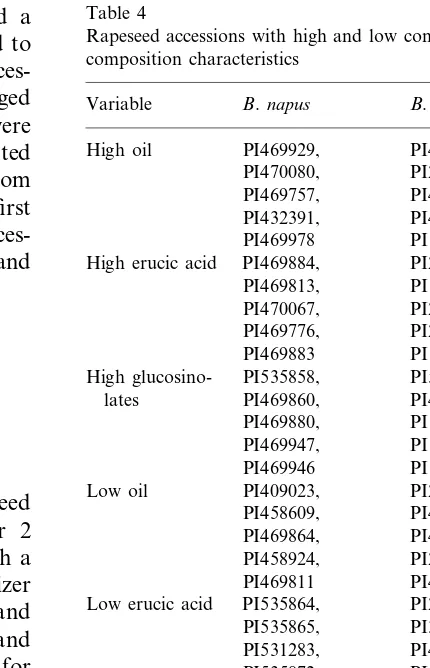
Table 4
Rapeseed accessions with high and low contents of three seed composition characteristics
B.napus
Variable B.rapa
High oil PI469929, PI458618, PI254543, High erucic acid PI469884, PI251326,
PI135821,
High glucosino- PI535858, PI537003, PI469860, Low erucic acid PI535864,
PI392024, Low glucosino- PI470077, PI470064,
PI470074, PI458614, lates
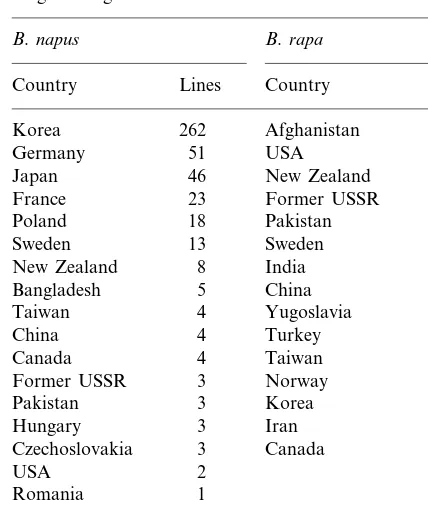
Table 5
Origins of rapeseed accessions that survived the winter under Virginia’s agro- climatic conditions
B.rapa B.napus
Country Lines Country Lines
Korea 262 Afghanistan 9
USA
51 6
Germany
46
Japan New Zealand 5
23
France Former USSR 4
Pakistan
18 4
Poland
Sweden 13 Sweden 3
India
Sugar Land, TX, USA). Helium was used as a carrier gas at a flow rate of 1 ml/min with a split ratio of 1:100. The column temperature was isothermal at 220°C. The injector and detector temperatures were 250 and 260°C, respectively. A Spectra Physics Model 4290 Integrator (San Jose, CA, USA) was used to determine relative concen-trations of the detected fatty acids.
Peaks were identified by reference to the reten-tion of FAME standards. The percentage of each peak was calculated as the percentage of the total area of all the peaks excluding the solvent.
3.4. Glucosinolate analysis
Total glucosinolates (mmol/g meal) were
deter-mined from 200 mg air-dried seed by homogeniza-tion with 3 ml 50 mM glycine – NaOH buffer (pH 9.0) for 15 s. After incubation at room tempera-ture for 10 min, 1 ml chloroform, 50 ml 10%
chlorhexidine di-acetate, 1.0 ml 100 mM citrate buffer (pH 5.0), and about 250 mg activated charcoal were added. A diastix strip (Fisher Scien-tific, Norcross, GA, USA) was immersed in the clear supernatant for 5 s, then allowed to stand for 2 min. The reflectance of the developed color was measured in ‘TRUBLUGLU’ meter (Systrix PTY, Wollongong, Australia), described by Tr-uscott et al. (1991).
3.5. Statistical analysis
The data were analyzed using the procedures of SAS Institute, Inc. (1989). The two Brassica spe-cies were compared for the 455 accessions of B.
napusas a group with the 44 accessions ofB.rapa
as a group using the completely randomized de-sign. The mean separation was based on Duncan’s Multiple Range Test (5% level). From the glucosi-nolate contents, the 499 accessions were classified as low, medium, or high using mean9standard deviation (Sokal and Rohlf, 1981). The mean glucosinolate content in accessions of both napus
and rapa was 48.8 mmol/g meal with a standard
deviation of 7.6. The accessions with glucosinolate content between mean91 standard deviation (41.2 – 56.4) were classified as medium, those with content less than 41.2 as low and those with content greater than 56.4 as high.
3.2. Fatty acid methyl esters (FAME) preparation
The oil samples (5 mg) were vortexed with 2 ml sulfuric acid/methanol (1:99, v/v) in 10 ml glass vials containing a Teflon boiling chip. The open vials were placed in a heating block at 90°C until the sample volume was reduced to 0.5 ml (Dah-mer et al., 1989). After cooling to room tempera-ture, 1 ml of hexane was added. The mixture was vortexed and dried over anhydrous Na2SO4. The
hexane phase containing FAME was transferred to a suitable vial and kept under N2at 0°C for gas
chromatographic analysis.
3.3. FAME analysis
One microlitre aliquot of FAME in hexane was injected into a SupelcoWax 10 capillary column (25 m×0.25 mm i.d. and 0.25 mm film thickness,
4. Results and discussion
The oil contents of the 499 accessions varied from 29.5 to 49.2% (Table 1). The 455 accessions
ofnapus, as a group, had significantly (5%
proba-bility level) higher oil content in the seeds (37.4%) than the 44 accessions of rapa, as a group (36.6%). The erucic acid content was higher in the
rapa (32.6%) than the napus accessions (26.1%). The glucosinolate content was higher in napus
compared to rapa seeds (49.2 vs. 43.8 mmol/g
seed). A significantly negative overall correlation (−0.11) existed between glucosinolate and oil content (Table 2). However, the correlations tween oil and erucic acid content and that be-tween erucic acid and glucosinolate content were not significant. Within species, the correlation be-tween glucosinolate and oil content (Table 2) was significantly negative amongnapusaccessions (−
0.14), but was significantly positive among rapa
accessions (+0.39).
Due to our interest in using high glucosinolate rapeseed meal as a biological pesticide (Bhardwaj et al., 1996), we compared the oil and erucic acid contents of rapeseed lines with high, medium, or low contents of glucosinolates. Significant differ-ences existed among the high, medium, and low glucosinolate groups for oil content in B. napus, but not in B. rapa. The low glucosinolate acces-sions had significant high oil content compared to the high glucosinolate napus accessions. This servation agrees with the negative correlation ob-served between glucosinolate and oil content in this species. The differences among the high, medium, and low glucosinolate groups for erucic acid content were not significant (Table 3).
Accessions with high and low contents of oil, erucic acid, and glucosinolate contents were iden-tified (Table 4). Among B. napus, PI469929 had the highest oil content (49.2%), PI469884 had the highest erucic acid content (56.2%), and PI535858 had the highest glucosinolate content (77.0mmol/g
meal). Among B.rapa, PI458618 had the highest oil content (40.8%), PI251326 had the highest erucic acid content (50.3%), and PI537003 had the highest glucosinolate content (56.8 mmol/g meal).
Among B. napus, PI409023 had the lowest oil content (29.6%), PI535864 had the lowest erucic
acid content (0.3%), and PI470077 had the lowest glucosinolate content (37.8 mmol/g meal). Among
Brassica rapa, PI254360 had the lowest oil content
(29.5%), PI263055 had the lowest erucic acid con-tent (12.9%), and PI470064 had the lowest glu-cosinolate content (38.7mmol/g meal). These lines
could form the basis of a breeding program to increase or decrease the amount of erucic acid, glucosinolates, and oil.
During the 1993 – 94 crop season, the average monthly minimum/maximum temperatures at Pe-tersburg, VA from September to May (the normal crop cycle for rapeseed production in Virginia) were: 16.7/28.3, 8.2/21.3, 4.2/16.7,−1.1/8.8,−
3.9/5.5,−1.6/10.7, 3.0/15.8, 9.8/24.8, and 11.0/
24.1°C, respectively. Based on average monthly temperatures from 1961 to 1990, the average tem-peratures in Petersburg, VA from September to May have been 22, 16, 11, 5, 3, 4, 9, 14, and 19°C. The historical weather data also indicates that mean annual winter temperature in Petersburg, VA is colder (15°C) than the southern areas (18°C). Currently, rapeseed is commercially pro-duced in the southern states consisting of Georgia and Alabama and attempts are being made to develop rapeseed production in Virginia. These observations indicate the need for developing win-ter hardy rapeseed genotypes because the geno-types developed for southern US may not be suitable for production in Virginia and other ar-eas with similar climatic conditions. An analysis of origin of plant material that survived under Virginia’s agro-climatic conditions indicated that for the napus species, 58% of the cold-tolerant lines originated in Korea (Table 5) and 11% orig-inated in Germany. For the rapa species, 20% of the cold tolerant lines originated in Afghanistan and 14% originated in US. This observation could be helpful for future plant collection and evalua-tion efforts. To enhance the cold tolerance in rapeseed, plant materials from Korea and other countries of similar climate should be collected and evaluated.
Our results indicate that breeding efforts to decrease glucosinolates in industrial rapeseed might be successful with either napus or rapa
the two species (Table 1). However, the napus
might be a more desirable source than rapa for increasing oil, erucic acid, and glucosinolate be-cause of its high contents (Table 1). For erucic acid, the napus entries with lower content than
rapa may be more amenable for development of low erucic acid lines.
Acknowledgements
This work was supported by the Cooperative State Research, Education, and Extension Service, US Department of Agriculture, under Coopera-tive Agreement No. 93-COOP-1-9523. Any opin-ions, findings, conclusopin-ions, or recommendations expressed in this publication are those of the authors and do not necessarily reflects the view of the US Department of Agriculture. The authors gratefully acknowledge the assistance of Dr Rick Luhman, USDA-ARS, North Central Regional Plant Introduction Station (NC6), for providing the seeds of the 938 Brassica accessions.
References
Auld, D.L., Mahler, K.A., Voorhis, A.A., 1988. Evaluation of the USDA Collection of Brassica for Fatty Acid Composi-tion and Glucosinolate content. Miscellaneous Series
Bul-letin No. 114. Agricultural Experiment Station, University of Idaho, Moscow, ID 83843, 36 p.
Bhardwaj, H.L., Hamama, A.A., Porter, D.M., Reese Jr, P.F., 1996. Rapeseed meal as a natural pesticide. In: Janick, J. (Ed.), Progress in New Crops. ASHS, Alexandria, VA, pp. 646 – 649.
Dahmer, M.L., Fleming, P.D., Collins, G.B., Hildebrand, D.F., 1989. A rapid screening technique for determining the lipid composition of soybean seeds. J. Am. Oil Chem. Soc. 66, 543 – 549.
Mahler, K.A., Auld, D.L., 1989. Fatty Acid Composition of 2100 Accessions of Brassica. Miscellaneous Series Bulletin No. 125. Agricultural Experiment Station, University of Idaho, Moscow, ID 83843, 172 p.
SAS Institute Inc., 1989. SAS/STAT User’s Guide, Version 6, fourth ed., vol. 2, Cary, NC, 846 p.
St. John, L.C., Bell, F.P., 1989. Extraction and fractionation of lipids from biological tissues, cell, organelles, and fluids. Biotechniques 7, 476 – 481.
Sokal, R.R., Rohlf, F.J., 1981. Biometry. W.H. Freeman and Company, New York, p. 859.
Truscott, R.J.W., Tholen, J., Buzza, G., McGregor, D.I., 1991. Glucosinolate measurement in rapeseed using reflec-tance. The TRUBLUGLU meter. In: McGregor, D.I. (Ed.), Proceedings of the Eighth International Rapeseed Congress Groupe Consultatif International de Recherche sur le Colza (GCIRC) and Canola Council of Canada, vol. 5 of 6, pp. 1425 – 1429.
USDA, 1989. Vegetable oils high in erucic acid — crambe and industrial rapeseed, Growing Industrial Materials. Office of Agricultural Industrial Materials. US Department of Agriculture, Washington, DC.
Van Dyne, D.L., Blase, M.G., Carlson, K.D., 1990. Industrial Feedstocks and Products from High Erucic Acid Oil: Crambe and Industrial Rapeseed. University of Missouri, Columbia, MO, 29 p.