www.elsevier.com / locate / livprodsci
Risks of transmission of spongiform encephalopathies by
reproductive technologies in domesticated ruminants
A.E. Wrathall
Veterinary Laboratories Agency( Weybridge), New Haw, Addlestone, Surrey KT15 3NB, UK
Abstract
This paper considers whether transmissible spongiform encephalopathies (TSEs or prion diseases) could be spread by artificial insemination, embryo transfer and other more advanced reproductive technologies which are used for genetic improvement and also for purposes such as production of recombinant drugs for medical use. Although the technologies are most used in cattle, they are increasingly used in sheep, goats and deer as well, all of which can be naturally affected by TSEs. In general, provided appropriate precautions are taken, the risks of TSE carriage specifically by the gametes (spermatozoa and oocytes) or by in-vivo-derived embryos per se appear to be negligible, but further research, some of which is already in progress, will be helpful to give assurance on this point. Greater concerns relate to the many biological products that are used in the technologies, e.g. pituitary hormones used for the superovulation of donors, and various tissues and blood products used in semen and embryo culture / transport media, some of which have the potential to carry TSE infectivity if derived from infected animals. The myriad instruments and items of technical equipment that are used also give cause for concern because if they become contaminated with TSEs they may, due to their construction, be impossible to sterilise properly. Crown Copyright 2000 Published by Elsevier Science B.V. All rights reserved.
Keywords: Transmissible spongiform encephalopathy; Reproductive technology; Embryo transfer; Disease control; Ruminants
1. Introduction research on the TSEs, including studies to elucidate
their routes of transmission.
Awareness of the transmissible spongiform en- Unconnected with TSEs, but nevertheless
cephalopathies (TSEs — also known as prion dis- momentous, was the revelation early in 1997 that a eases), particularly scrapie in sheep and bovine cloned sheep (‘Dolly’) had been produced in Edin-spongiform encephalopathy (BSE), has risen burgh, Scotland, by transfer of the nucleus from an dramatically in the past decade. The possibility of a adult cell (Wilmut et al., 1997). This has also zoonotic link between BSE and new variant Creut- stimulated new research and a vigorous debate on the zfeldt–Jakob disease (nvCJD) in man, first reported implications of cloning and other reproductive tech-in March 1996 (Will et al., 1996), added ‘fuel to the nologies in farm animals, including possible risks of fire’ and precipitated the so-called ‘beef crisis’ in the diseases such as TSEs being spread by their use UK, Europe and in other parts of the world. In the (Evans, 1999). These risks are small but they cannot wake of this there has been a huge increase in be dismissed altogether. The possibility, for example,
of a cloned, transgenic animal transmitting TSE to enable more oocytes from preferred females to be infectivity via recombinant products destined for utilised. Then in the third category is a range of medical use is an alarming but not implausible techniques, some relatively new, such as sexing, scenario if sanitary precautions are overlooked. This cloning and genetic modification, which select or paper explains how TSE transmission risks might modify gametes and embryos to obtain offspring arise in reproductive technologies and how they with specific characteristics. Taken together, these
should be avoided. Topics covered include the technologies not only supplement what can be
following. achieved by conventional breeding but also enable
the production of new foods, industrial products, and
• Brief summaries of the reproductive technologies drugs for use in human medicine. in domesticated ruminants.
• Conventional disease transmission risks via re- 2.2. Selective breeding productive technologies.
• Differences between conventional infectious dis- Although not strictly a reproductive technology it
eases and the TSEs. is apposite to mention selective breeding because
• Natural TSE transmission, especially during re- susceptibility to some TSEs is strongly affected by
production in ruminants. genetic factors. Selective breeding enables promising
• Risks of TSE transmission via reproductive tech- individuals to be identified and genetic lines to be
nologies, and their control. selected and improved in respect of commercially
important characteristics. One of these characteristics is disease resistance, and selection for resistance to
2. The reproductive technologies conventional diseases, such as mastitis and lameness,
has aroused much interest. Discovery of strong
2.1. Background correlations between certain genotypes and scrapie
susceptibility in sheep and goats (though apparently Selective breeding and the science of genetics not in cattle) was followed by development of blood have been used to good effect over the last 100 years tests to identify those genotypes (Dawson et al.,
to improve farm livestock, but the reproductive 1998; Goldmann et al., 1998). Combined with
technologies are now enabling even faster progress. selective breeding, these tests are now being used to Some, such as artificial insemination (AI) and em- increase the proportions of scrapie-resistant animals, bryo transfer in cattle, sheep and goats, are already particularly in sheep populations, and reproductive well established, and others such as cloning and technologies such as AI and embryo transfer are genetic modification are on the threshold of commer- enabling even faster progress. Conversely if selection cial development. Use of AI and embryo transfer is is made for other desirable factors without due also starting in other domesticated ruminants such as regard to TSE genetics, the result may be
popula-deer and water buffalo. tions of animals that are more susceptible to TSE
Much has been written elsewhere about the re- infections. This could also apply to production of productive technologies, so the information given cloned and transgenic animals which, for example in here is simply to help understand how TSE transmis- the case of individuals designed to produce recombi-sion risks might arise. Attention is mainly confined nant proteins for medical use, should ideally carry to use of the technologies in cattle, sheep, goats, and TSE-resistant genotypes.
deer, i.e. the ruminants reported to be susceptible to
TSE infection. Essentially the technologies fall into 2.3. Semen collection and artificial insemination three main categories. Firstly there is AI which (AI)
enables effective use of the male in breeding
reproduc-tive technology. It is used extensively in cattle, ing new technology involves production of sexed especially dairy breeds, but has also been developed semen by flow cytometry (see below) and for this for many other species. Partly due to the physical and other situations where available sperm numbers difficulty of insemination, seasonal breeding and are very low a modified AI technique has been their tendency to extensive types of husbandry, AI is proposed in which the semen is deposited deep not widely used in sheep, goats and deer. Disease within the ipsilateral uterine horn (ovulation side) to agents can often be found in the semen of infected increase the chance of a functional sperm reservoir in males, and others may contaminate during collection the active oviduct (Hunter and Greve, 1998). Epidur-and processing. Nevertheless it is well known that if al anaesthesia may be required in this case.
semen donors are properly selected and managed,
and if the semen is properly processed, AI is seldom 2.3.2. Semen collection and AI in sheep and goats a cause of disease transmission (Hare, 1985; Phil- Rams and bucks, like bulls, can be trained to pott, 1993; Eaglesome and Garcia, 1997). donate semen into an artificial vagina (small version of the bovine one), and their semen can be frozen.
2.3.1. Semen collection and AI in cattle Sometimes, however, semen is obtained by
electroe-Semen is routinely collected by stimulating the jaculation, which entails placing the instrument into bull to ejaculate into an artificial vagina, a device the rectum, and sedation or even general anaesthesia consisting of a strong rubber or plastic cylinder with is usually required. Insemination presents more a softer latex liner, and filled with warm water problems in sheep and goats than in cattle, partly (Parkinson, 1996a). The inner surface of the liner in because of seasonal breeding, and consequently contact with the penis is lubricated with petroleum oestrus synchronisation is often used in these jelly or liquid paraffin, and a rubber extension cone species. Further problems arise due to their smaller leads to a receptacle tube for the semen. Most bulls size and anatomical differences of the cervix. In-donate semen voluntarily but occasionally electroe- travaginal insemination with fresh semen can be jaculation is used, especially with untrained farm successful but pregnancy rates with frozen–thawed bulls. Electroejaculators (the electrodes) are inserted semen deposited into the vagina are low. The by the operator with gloved hand into the bull’s transcervical route is technically difficult and can rectum. Whatever the collection method, the semen is cause injury, so AI often involves surgery under diluted with a liquid extender and stored in plastic general anaesthetic or sedation and a local anaes-straws, usually in a frozen state. Extenders contain thetic (McKelvey, 1999). With the animal inverted in animal origin substances such as skimmed milk and / a restraining cradle small amounts of frozen–thawed or egg yolk in buffered saline with antibiotics, and semen are injected directly into the uterus (or cryoprotectants such as glycerol are also used if the oviduct) via laporotomy, or by laporoscopy using a semen is to be frozen. It should be noted that grasping probe and inseminating pipette.
although the semen is diluted with extender, the
spermatozoa are not separated from the seminal 2.3.3. Semen collection and AI in other
fluids. domesticated ruminants
Except perhaps in beef cattle where farmers may AI in domesticated deer, especially the red deer wish to breed within a narrow time period, it is not (Cervus elaphus scotticus), the larger North Ameri-normal practice to synchronise oestrus for AI. For can sub-species known as wapiti or Rocky Mountain insemination the straw is warmed if necessary to elk (Cervus e. nelsoni), the fallow deer (Dama thaw the semen, then fitted into a metal catheter-like dama), and various hybrids, is being developed along
routine, especially with frozen semen. Semen collec- FSH, and LH), and uterine prostaglandins (long tion and AI have been practised for many years in chain fatty acid compounds such as PGF2a). Apart
domestic buffalo (Bubalus bubalis) (Jainudeen, from the gonadotrophins these are all available as 1996), but are still in the development stages in synthetic analogues, so use of tissue extracts is bison (Bison bison) (Dorn, 1995). The techniques unnecessary, thus avoiding any associated disease
resemble those used for cattle. risks. The gonadotrophins, however, are a different
matter because they cannot be synthesised, and FSH 2.4. Oestrus synchronisation and superovulation has a key role, especially for superovulation. Several FSH preparations are commercially available (Gor-Oestrus synchronisation facilitates the supervision don, 1994; Christie, 1996) but the most effective and of breeding programmes and efficient use of sires by widely used are extracts from the pituitary glands of natural mating or AI. It is especially important in pigs or sheep. Equine chorionic gonadotrophin species such as sheep and buffalo which show little (eCG), extracted from pregnant horse blood, is sign of oestrus in the absence of a male. It is also a sometimes used, as also is the human menopausal vital component of embryo transfer programmes. gonadotrophin (hMG) which is extracted from urine, Synchronisation is commonly achieved by the use of but whilst their origins suggest they should be safer intravaginal devices (coils, sponges or other soft from a disease perspective than the pituitary-derived polyurethane / plastic appliances) impregnated with FSH products, both have disadvantages. Some years slow release synthetic progestagens, with or without ago it was reported (Looney and Bondioli, 1988) that oestrogen, which inhibit ovarian activity. Sometimes an effective bovine FSH had been produced by progestagen is given as a subcutaneous implant recombinant DNA technology but this has not been instead of by intravaginal device. Alternatively pros- marketed commercially. Due to their disease risks, taglandin injections spaced 10 to 12 days apart are cadaver-derived pituitary hormones have now been used to re-schedule ovarian activity. To overcome replaced by recombinant products in human medi-limitations caused by seasonal breeding in sheep, cine.
goats and deer, photoperiodic conditioning or
courses of melatonin treatment may be used. 2.5. Embryo collection and transfer
Withdrawal of progestagen, or injection of
prosta-glandin, is normally followed by synchronous Embryo transfer began in cattle and sheep in the
growth of follicle(s) and ‘rebound’ into oestrus 1950s and initially involved full-scale surgical inter-within 2 to 4 days. If, however, instead of natural vention. It was not until non-surgical techniques rebound, a series of injections of follicular stimulat- were developed for cattle in the 1970s that the ing hormone (FSH) is given to coincide with the technology began to be widely used commercially. decline of progestagen, this stimulates growth of Data from the International Embryo Transfer Society extra follicles and ‘superovulation’. Small FSH doses (IETS) (Thibier, 1998) show that almost 400,000 may lead to twins or triplets whereas high doses can bovine embryo transfers took place world-wide in potentially give many more embryos for collection 1997. Most were in-vivo-derived embryos (i.e. and transfer. Some programmes incorporate gonado- flushed from the uterus about a week after con-trophin releasing hormone (GnRH) injections to ception) but |30,000 were produced in vitro.
Annu-ensure timely release of endogenous FSH and / or al transfers in sheep and goats number at least 6000 luteinising hormone (LH) from the animal’s own and 10,000, respectively (unpublished data), with
pituitary gland. smaller numbers in buffalo and deer.
In view of their potential for carriage of TSE
and developed to the morula or blastocyst stage, but ethylene glycol as cryoprotectant, have been de-are as yet unhatched from the zona pellucida, they veloped which give good results and have the are collected from the uterine cavity by the non- advantage that they enable direct transfer of frozen– surgical flushing technique (Christie, 1996). This has thawed embryos without cryoprotectant removal similarities to AI, but requires more complex equip- (Voelkel and Hu, 1992). Fresh (i.e. unfrozen) em-ment and greater skill. Various types of ‘Foley’ bryos can be loaded directly into straws in the catheter of silicone rubber, plastic, etc. are used, original collection medium and transferred into some of which are stiffened with an inner metal recipients within a few hours of their collection. stylet during manipulation through the cervix into the Recipients are usually oestrus synchronised to uterus, whilst for others a metal tube (introducer) ensure synchrony between the maternal reproductive with inner rod (trochar) is passed through first, then cycle and the developmental stage of the embryo. the trochar is removed and the catheter is passed The straw containing the fresh or frozen–thawed through the introducer. Once in position a balloon- embryo is loaded into a transfer ‘gun’ of similar cuff on the catheter is inflated to prevent leakage; the construction, although longer, than an AI gun; the collection fluid medium is then injected through into embryo is then transferred non-surgically in a man-the uterine lumen and man-the embryos are flushed back ner similar to AI. The need to manipulate the cervix, via plastic tubing into a collection flask. As with AI, ovaries and uterus per rectum, and to deposit the the operator keeps a hand within the cow’s rectum to embryo well down the uterine horn on the side of the manipulate the reproductive tract, catheter, etc. by active corpus luteum, means that an epidural anaes-palpation through the rectal wall. At least one thetic is usually required prior to transfer.
assistant is also needed to handle the equipment and
to instill the flushing fluid. Donors are almost 2.5.2. Embryo collection and transfer in sheep and invariably given an epidural anaesthetic during em- goats
bryo collection. As with cattle, oestrus synchronisation and
Embryos are collected and processed in a fluid superovulation precede embryo collection from medium which essentially consists of buffered saline sheep and goats, and the hormonal regimes are with low levels of blood protein (e.g. fetal calf serum broadly similar. Insemination, especially if frozen
or bovine serum albumen) to maintain embryo semen is used, is by laporoscopy, as described
viability and prevent them sticking together. Anti- above. Embryos are usually collected 5 or 6 days biotics are also added to control bacterial contamina- post-insemination when at the unhatched morula or tion. Using a microscope, the embryos are picked out blastocyst stage, and this entails full surgical from the uterine flushings and examined to establish laporotomy with exteriorization of ovaries and their developmental stage and viability. For purposes uterus, laporoscopy using similar instruments to of disease control embryos are usually washed 10 those used for AI (McKelvey et al., 1986; McK-times, as specified in the Manual of the International elvey, 1999), or a combined approach. Small Foley Embryo Transfer Society (IETS), and are sometimes catheters, similar in design to the bovine type, are also treated with trypsin (a proteolytic enzyme from used, and flushing media tend to be of similar porcine or bovine pancreas) to ensure certain viruses composition to those for bovine embryos. Sanitary will be removed, if present (Stringfellow, 1998). processing and freezing of in-vivo-derived sheep and Embryos for freezing are passed through solutions of goat embryos are basically the same too. Embryos a cryoprotectant (e.g. glycerol), aspirated into plastic are transferred via laparotomy under general anaes-straws (0.25 ml), cooled in a freezing apparatus, then thesia, or by the laparoscopic method which resem-stored in a liquid nitrogen refrigeration tank. Eventu- bles that used for AI in sheep and goats.
ally, when thawed, the embryo is passed through
dilutions of glycerol or sucrose in buffered saline to 2.5.3. Embryo collection and transfer in other remove the cryoprotectant; it is then loaded into species
same way as for other ruminants (Asher and Dixon, blastocyst, without having to recover them surgic-1994; Fennessy et al., surgic-1994; Dorn, 1995). Embryos ally. Reasons why IVP embryos have proved less are collected surgically in smaller species of deer, as popular for commercial transfer include their lower in sheep and goats, but in larger species (e.g. wapiti / survival after cryopreservation, higher disease trans-elk), and in buffalo, non-surgical embryo collection mission potential, and a tendency for the resulting and transfer can be done under epidural anaesthesia, offspring to develop a foetal oversize problem with as in cattle (Misra et al., 1998). Deer and buffalo high mortality (Walker et al., 1996; Kruip and den embryos are amenable to freezing in the same way as Daas, 1997).
cattle embryos.
2.7.2. Oocyte collection and IVF /IVP methods in
2.6. Ultrasound scanning cattle
Essentially oocytes are aspirated from immature Ultrasound scanning, especially with the versatile ovarian follicles, then matured, fertilised and cul-(and expensive) B-mode apparatus, is a key element tured in vitro to the morula or blastocyst stage. They in some reproductive technologies (Taverne and are aspirated either from excised ovaries or whilst Willemse, 1989). In large ruminants a probe (trans- still in situ. In the former case the ovaries may have ducer) is held and directed by the operator from been removed surgically although more often they within the rectum whilst in small species it is applied are taken from batches of abattoir-slaughtered cattle to the external abdominal wall. The transducer is to a laboratory where follicular aspiration is done connected by a rubber ensheathed electrical cable to with hypodermic needle and syringe. The other the monitor where tissues of interest are imaged on method involves aspiration from the ovaries of live the screen. Apart from its obvious use for pregnancy cows by trans-vaginal oocyte recovery (TVOR) diagnosis, ultrasound can provide detailed images of using a purpose-built ultrasound transducer which ovarian follicles and corpora lutea. Also, as de- houses and guides the aspiration needle (Kruip et al., scribed later, a specifically designed transducer can 1991; Looney et al., 1994). TVOR is a skilled be positioned in the vagina of a cow to guide needles technique, and epidural anaesthesia plus a sedative for the aspiration of oocytes from ovarian follicles. are required. With the transducer inserted into the Sanitary aspects of scanning should not be over- vagina and the cow’s pelvic contents visualised on looked, particularly when transducers are placed the monitor screen, a long (e.g. 60 cm) single or
within the rectum or vagina. double lumen needle (with echo-reflective tip) is
guided through the anterior vaginal wall and across 2.7. In vitro fertilisation (IVF) and in vitro the peritoneal cavity into the ovary, the latter being
production (IVP) of embryos held adjacent to the transducer by the operator’s hand within the cow’s rectum. Ovarian follicles are
2.7.1. Background to IVP technologies penetrated by the needle and fluids plus oocytes are
Collection of oocytes, IVF, then culture of the aspirated via plastic tubing into a receptacle. The resulting zygotes, is principally used to produce transducer unit may be covered with a sanitary latex embryos in cattle (Gordon, 1994), but the technology cover when in use but has to be taken apart for is also being developed for sheep and goats (Alvarez cleaning afterwards, and needles must be sterilised if et al., 1999; Graff et al., 1999), deer (Pollard et al., re-used. TVOR can be used to collect oocytes 1995) and buffalo (Chauhan et al., 1996; Galli et al., repeatedly from the same animals, including those 1998). IVP embryos are less used in routine com- with reproductive problems and in early pregnancy, mercial embryo transfer than the in-vivo-derived thus enabling an almost unlimited supply of known type, but are widely used in research. The technology lineage (Garcia and Salaheddine, 1998). Oocytes is also the foundation for other more advanced from batches of abattoir ovaries, on the other hand, technologies such as cloning and transgenics since it are difficult to trace back to the donors, so disease makes available a range of developmental stages, risks are higher.
their subsequent in vitro maturation, IVF and culture straightforward, but aspiration during life by TVOR require controlled laboratory conditions, a variety of from smaller species such as sheep and goats is equipment, sophisticated media, and strict sanitary usually impractical, so surgery (laporotomy or standards to prevent contamination. Media tend to be laporoscopy) is the method of choice (Smith, 1994; similar to those for in-vivo-derived embryos, but Alvarez et al., 1999; Graff et al., 1999). Production with higher levels of serum and antibiotics, and of good quality embryos by IVF / IVP appears to be gonadotrophins, steroids, heparin and transferrin may less efficient than in cattle.
be added at different stages of the culture.
A straw of frozen semen is usually used for the 2.8. Semen and embryo sexing IVF. Prior to its addition to the oocytes the semen is
washed to remove cryoprotectant and seminal plas- Production of sexed offspring is of value in many
ma, then motile spermatozoa are selected by a situations and can be achieved either by semen
method such as filtration through glass wool, or sexing or by embryo sexing. In the case of bovine separation on discontinuous gradients of bovine semen, separation of male and female spermatozoa is serum albumen (BSA) or ‘Percoll’ (silica particles possible because their DNA content differs; those bound with polyvinylpyrrolidone) (Gordon, 1994). In with an X chromosome being about 4% heavier contrast to AI of live cattle, only the sperm fraction (Cran et al., 1993; Johnson et al., 1994). The is used for IVF, and, as described below, it is now technology involves addition of a fluorescent dye to possible to use sexed sperm to produce IVF embryos the diluted semen which is then passed through a of known gender. It is also possible to microsurgical- sophisticated flow cytometer. A laser beam activates ly inject a single sperm into the oocyte to initiate the dye-stained DNA which enables male and female apparently normal development, a technique known sperm to be identified and sorted within an electrical as intracytoplasmic sperm injection (ICSI). ICSI is field into separate collection tubes. Although |95%
often used in human IVF to enable infertile men to accurate, the sorting rate (about 1000 live sperm / s) is conceive (Kurinczuk and Bower, 1997) but has had too slow to provide enough sexed sperm to use for limited success in ruminants (Catt et al., 1996; Chen conventional AI in cattle; in fact what many would and Seidel, 1997; Keskintepe et al., 1997; Keskin- consider a minimum dose of 2.5 million live sperm
tepe and Brackett, 1999). (Moller et al., 1972) would take almost an hour,
The embryos, once fertilised, are usually co-cul- whilst a typical dose for frozen semen (25 million — tured for 7 to 9 days with somatic cells such as Parkinson, 1996b) would take up to 10 h. Sorted granulosa cells (from ovarian follicles), oviductal sperm, however, can be used for IVF where the low epithelial cells, or continuous cell lines from other numbers are adequate (Cran et al., 1993). Encourag-species, e.g. buffalo rat liver (BRL) cells, or monkey ing results have also been reported when small doses
5
kidney (Vero) cells. In the early days of IVF / IVP, of unfrozen, sexed semen (3310 sperm), obtained
newly fertilised bovine embryos were often trans- in minutes by a new sorting process, were given by ferred surgically into ligated oviducts of live sheep deep intrauterine AI to oestrus-synchronised heifers and ‘cultured’ there for a week before retrieval and (Seidel et al., 1998). Semen sexing as used for cattle transfer into recipients of the intended species. is possible in other ruminants (e.g. Cran et al., 1997) Temporary culture in sheep oviducts is still used but has not been developed commercially. Unfor-occasionally (e.g. Campbell et al., 1996; Wilmut et tunately the complexity of the flow cytometry equip-al., 1997; Galli et equip-al., 1998) although because there ment means that its effective sterilisation is impracti-are some potential disease risks this is not a wise cal.
practice. Embryo sexing has been possible for several years
in cattle, but its cost and the fact that half the 2.7.3. Oocyte collection and IVF /IVP in other embryos are of the ‘wrong’ sex have restricted its
species commercial use. One method involves microsurgical
sex chromosome is present (Seidel and Seidel, Kato et al., 1998; Stice et al., 1998). The technique 1991). Unfortunately this is slow and rather inaccu- for removing the chromosomes from an oocyte (no rate, and the biopsied embryos tend not to survive distinct nucleus at this stage) and replacing with a well after freezing. Damage to the zona pellucida donor cell nucleus resembles embryo biopsy, and also breaches the protective barrier against patho- requires similar microsurgical equipment. Whatever gens, so embryos for export must undergo washing their type, the donor cells are usually co-cultured and other necessary treatments before microsurgery. initially on rodent cell feeder layers in a medium A second sexing method also involves a biopsy but containing a high level (e.g. 10%) of foetal bovine in this case the sex (Y) chromosome is detected by serum. They are then dissociated with trypsin and the polymerase chain reaction (PCR) (Herr and Reid, replication is arrested by further culture in a medium 1991). Although faster and more accurate than with little serum (e.g. 0.5%). A whole cell is inserted karyotyping, the PCR method is quite expensive. The beneath the zona pellucida of the recipient oocyte microsurgical equipment required to hold the em- and, to provoke integration of the donor nucleus into bryo, cut through the zona pellucida and remove a the surrogate cytoplasm, the oocyte is placed be-biopsy is complex, expensive, and difficult to steril- tween metal wire electrodes on a glass microscope ise. Fortunately, only the holding and cutting instru- slide and subjected to electrical pulses with ‘elec-ments have contact with the embryo and, being trofusion’ equipment.
cheaply fashioned from glass tubing or rod using a Subsequent to nuclear transfer the resultant microforge, these can be discarded after a single use. zygotes are cultured to morula or blastocyst stage There appear to be few reports of embryo sexing in before transferring into recipients. Sometimes they
ruminant species other than cattle. are cultured in ligated oviducts of sheep (e.g.
Camp-bell et al., 1996), although this is not ideal due to
2.9. Cloning infection risks. Culture with high levels of serum and
co-culture cells can be successful (e.g. Bourhis et al., Cloning, the production of genetically identical 1998; Kato et al., 1998) but, as with IVP embryos, animals, can be achieved in various ways, the fetal oversize, congenital abnormalities and neonatal simplest being embryo division or disaggregation to death have been linked to culture conditions and produce identical twins, triplets, etc. Triplet calves exposure to serum during these very early stages and quadruplet sheep have been produced by sepa- (Lees et al., 1998).
ration and transfer of individual blastomeres from Probably the best known clone, ‘Dolly’, born in four- and eight-cell stage embryos (Willadsen, 1981; July 1996, was the result of transplanting a nucleus
Willadsen and Polge, 1981), but a more practical from a culture of mammary gland cells from a
method for producing identical twins is to split 6-year-old sheep (Wilmut et al., 1997). Since then morulae or early blastocysts and to transfer each half cloned calves have been produced using nuclei from into one or two synchronised recipients (Willadsen cultured oviductal epithelium and ovarian follicle and Godke, 1984; Seidel and Seidel, 1991). Split cumulus cells from an adult cow (Kato et al., 1998). embryos, like biopsied ones, have the problem of These results are significant because previous at-lowered viability after freezing, but pregnancy rates tempts to clone mammals by nuclear transfer using over 50% can be obtained with singly transferred, any cells other than those from very early embryos, fresh demi-embryos, which means over 100% per or cell lines derived from them, had failed (Stice et
original embryo. al., 1998), so it had been assumed that nuclear
Identical animals can also be produced by nuclear totipotency is lost early in development.
post-natal life, possibly as a consequence of defec- only about 5% of injected zygotes survive to be tive nucleocytoplasmic interactions and genetic dis- transferred and, of these, only a tiny proportion ease (Morris, 1999; Renard et al., 1999). Adult cell incorporate the transgene in a balanced way to nuclear transfer can, however, be of value for become productive transgenic adults (Eyestone et al., preserving endangered breeds and species, as shown 1998). A higher rate of transgenic offspring has by Wells et al. (1999a) who produced a healthy calf recently been reported in cattle by using a re-from the last surviving Enderby Island cow by plication-defective retroviral vector to introduce transplanting granulosa cell nuclei into enucleated genes into metaphase II (arrest phase) oocytes (Chan oocytes from a conventional cow. Interest in the et al., 1998).
nuclear transfer technology is also reviving with Another way of producing transgenic animals
prospects of combining it with genetic modification utilises nuclear transfer technology plus a process technologies to create cloned transgenic animals. known as ‘transfection’. This has a major advantage over microinjection in that potential nuclear donor
2.10. Genetic modification (transgenesis) cells can be checked to ensure the desired gene is
technologies in cattle and sheep incorporated before they are used (Cibelli et al., 1998). Cell lines, usually of embryonic or foetal Genetic modification involves taking a gene from origin, are initially propagated in culture, as for one organism (the donor) and inserting it into the cloning by nuclear transfer. The desired gene con-genome of another. If successful, the modified struct is linked to a marker gene (e.g. one conferring (‘transgenic’) individual has a new gene which resistance to a specified cell toxin) and these genes functions to produce a protein characteristic of the are together inserted into the cultured cells by donor organism. Traditional techniques for gene transfection, a process usually achieved by concur-insertion such as pronuclear injection and transfec- rent exposure of the cells and the construct to a tion (see below) achieve rather random integration cationic lipid transfection reagent (Schnieke et al., into the genome, but more precise ‘gene targeting’ 1997). After further culture in a medium containing methods are being developed which will enable them levels of the toxin lethal to non-transfected cells, to be inserted (or deleted) at specific locations on colonies of the resistant cells (i.e. transgenic ones particular chromosomes (Wilmut, 1998). Gene dele- with marker gene) are selected for further propaga-tion, for example, can be used to delete the prion tion. These are then used for nuclear transfer in the protein (PrP) gene, and individuals modified in this sure knowledge that the resulting embryos, if they way should not then succumb to TSE. In ruminants, survive, will develop into transgenic offspring. however, precision gene targeting is a relatively Aliquots of the cells can be frozen down and used
futuristic technology. again and again as nuclear transfer donors.
The primary steps in traditional genetic modi- Since only cells expressing the transgene are used, fication are to identify and extract the DNA sequence fewer nuclear transfers and pregnancies are needed of the specified gene; this is then used to produce the to obtain transgenic individuals. Moreover, because desired gene ‘construct’. Next, many copies (usually multiple cloned transgenic individuals are obtained hundreds) of the prepared construct are inserted into in the first generation, testing a few individuals for the genome of an individual embryo. Typically the health and production is predictive for all present and constructs are injected microsurgically into one of future clones of that type. Donor cell populations can the pronuclei of a single cell embryo (zygote), also be karyotyped beforehand to ensure that trans-whereupon the latter is cultured for some days in genic animals will be of the desired sex. Thus, if vitro (or, for example, in a sheep’s ligated oviduct). transgenic females are needed to produce specific Embryos surviving this culture period are transferred milk proteins (e.g. for pharmaceutical use) this is into recipient females, hopefully to develop into possible in the first generation.
have been reported too, and it is evident that these and often require substantial use of biological materi-technologies do carry risks as well as benefits. For als. Disease risks associated with oestrus synchroni-example, transgenic goats (and pigs) intended for sation and superovulation arise mainly because production of valuable proteins in milk (for human donors and recipients may be treated with potentially therapy) were found eventually to develop peculiar contaminated hormonal products.
mammary lesions (Ebert and Schindler, 1993). The IVP embryos are the foundation for most
ad-foetal oversize problems, already mentioned, which vanced reproductive technologies, including cloning seem to be associated with in vitro culture are also and transgenics, but due to the properties of their impeding progress with genetic modification tech- zonae pellucidae, which seem to make them ‘sticky’, nologies. Another more hypothetical risk is that they are less amenable to pathogen removal by carriage of or susceptibility to infection, including washing than in vivo derived embryos (Stringfellow infection with TSE agents, might permeate a narrow- and Wrathall, 1995; Marquant-Le Guienne et al., ly based, genetically modified population and remain 1998; Trachte et al., 1998; Booth et al., 1999; in it undetected, only to manifest itself many years Langston et al., 1999) The potential for pathogen
later. exposure during oocyte collection, IVF and culture is
further increased by batch production methods, and by the many substances of animal origin, including
3. Overview of the risks of disease transmission cell cultures, which are routinely used (Bielanski,
by reproductive technologies 1998). Most laboratories collect oocytes weekly but
culture to the morula / blastocyst stage takes up to 9
3.1. General comments days, so inevitably there is some overlap, with
attendant risks of introducing new infections into It must be emphasised at the outset that the risks ongoing batches.
of transmitting infectious diseases by AI, embryo transfer and other reproductive technologies are
extremely small, especially if established sanitary 4. Important characteristics of the TSEs which
protocols are followed. Broadly it can be said that influence transmission risks
in-vivo-derived embryos seem to carry the lowest
disease risks, with the risks of semen and IVP The TSEs are unique diseases, having many
embryos somewhat higher, but the disease risks of characteristics which set them apart from those moving live animals are greater than any of these. caused by conventional infectious agents such as Surgical procedures create added disease risks if bacteria and viruses. For this reason they are often they are used in reproductive technologies. In small termed ‘unconventional infections’. Notable features ruminants, for example, it is normal for embryo of the TSEs include the following.
transfer and sometimes AI to be done surgically, or
at least by laporoscopy, so the risks are inevitably • The obscure nature of their causal agents which higher than for techniques without intentional pene- seem to contain no nucleic acid; thus, despite tration of the peritoneal cavity. Disease risks of major variations between agent strains, the ge-surgery, and also of ultrasound and manual interven- netic coding mechanism is a mystery.
tions per rectum or per vagina in the larger rumin- • The extreme resistance of the agents to inactiva-ants, are largely those of mechanical transfer of tion by standard physical and chemical treatments infection from one animal to another by contami- such as dry heat and radiation, and many chemi-nated instruments, or by the operator’s hands (Divers cal disinfectants.
et al., 1995), and are not necessarily due to carriage • The epidemiology (natural spread of TSEs be-of infection via the gametes or embryos per se. The tween animals) is poorly understood, and there is advanced technologies such as semen and embryo scant information about threshold infective doses. sexing, cloning and genetic modification, all tend to • Their incubation periods are very long, i.e. years
carry higher risks simply because they involve rather than days, weeks or months, but once
• The absence of quick and effective tests for ticular infectious disease is never transmitted by presence of infection in living, preclinical (and parents to their offspring during natural breeding, clinical) cases, and also for infectivity in tissues then prima facie, it is unlikely that it will ever be
and fluids. carried specifically by semen, oocytes or embryos.
• The fact that host genetic factors can strongly Nevertheless, transmission could occur in artificial influence TSE susceptibility and incubation breeding due to contamination by other tissues, or if periods, particularly in sheep and goats (and man contaminated equipment is used. Possible natural
and mouse). disease transmission routes are:
These and other features of TSEs are now consid- • horizontal (or lateral) transmission: the spread of
ered in more detail. infection between unrelated animals via direct or
indirect contact at any time, or to the offspring
4.1. Nature of TSE agents after parturition;
• vertical transmission: the spread of infection from In all TSEs a characteristic proteinase-resistant parent (male or female) to offspring via
germ-res Sc
insoluble protein, referred to as PrP or PrP , plasm (spermatozoa or oocytes) at fertilisation, or accumulates in the central nervous system (CNS). in utero during prenatal life;
Many now believe TSE infections are caused by • maternal transmission: the spread of infection
Sc
particles consisting solely of PrP , or ‘prions’, and from the dam to her offspring either vertically that infectivity arises when the natural, soluble cell (via female germplasm, or across the placenta), or
c
glycoprotein (PrP ) is transformed by abnormal horizontally in the immediate post-parturient
Sc
folding at the molecular level into insoluble PrP or period (via milk, saliva, faeces, etc.). prion protein (Prusiner, 1995; Weber and Aguzzi,
c
1997). However, in recent work, when PrP was Despite much study, natural routes of TSE
trans-Sc
transformed in vitro into protease resistant PrP , the mission are still poorly understood. Long incubation latter was found not to be infectious (Hill et al., periods make it difficult to link clinical cases to their
1999a). original sources of infection, and genetic
predisposi-TSE strains are subtypes of predisposi-TSE infectious agents tions passed from parents to their offspring, especial-capable of maintaining their distinctive phenotypic ly in sheep scrapie, make epidemiological interpreta-characteristics such as disease incubation periods, tions even more complicated. Most TSEs can be CNS lesion profiles, and possibly tropisms for spe- transmitted experimentally by injecting or feeding cific cell types, when passaged within a host species, infected material (e.g. brain from affected animals) to or even within other species. A minority view is that others of the same species, and sometimes to differ-nucleic acid might exist as small, hitherto uniden- ent species, but the relevance of this to natural
Sc,
tified virus-like particles associated with the PrP transmission routes is uncertain.
and that the agent strain variations are due to genetic Epidemiological studies have shown that most polymorphisms (Farquhar et al., 1998; Hunter, cases of BSE in the UK arose from dietary exposure 1999). However, proponents of the protein-only to infected meat and bonemeal (Wilesmith et al., hypothesis (e.g. Collinge et al., 1996; Safar et al., 1988; Kimberlin and Wilesmith, 1994) and this, 1998) argue that strain-specific properties of TSE though unintentional, was a man-made transmission agents are encoded by conformational patterns of the route. The mean incubation period for BSE is about
Sc
PrP protein. 5 years, with a probable range of 2 to 7 years, and it
is believed that cows giving birth in the latter stages 4.2. Natural transmission (epidemiology) of TSEs of incubation, or after clinical onset, are more likely
the dam, the risk thereafter was enhanced, rising to explanation (van Keulen et al., 1999). Some (e.g.
|10% in the last 6 months of the maternal incuba- Ridley and Baker, 1998) have proposed that the
tion period, and possibly more in those born after disease is entirely due to genetic causes; however, clinical onset. Some remain sceptical about these the fact that genetically susceptible sheep in scrapie-data, arguing that the apparent maternal transmission free countries do not succumb indicates otherwise. might in fact be due to genetic variation in suscep- As for maternal transmission, this probably occurs tibility to BSE. However, there is little evidence that transplacentally or soon after birth. Moderate levels
Sc
the known PrP gene polymorphisms in cattle affect of infectivity and PrP have been found in placentae susceptibility, as they do in sheep. Further, as (Pattison et al., 1974; Race et al., 1998) and this is pointed out by Donnelly et al. (1997), the fact that thought to contaminate and persist in pens and the maternal transmission risk is positively correlated pastures, leading also to horizontal transmission. It with incubation stage makes a genetic explanation has been found that scrapie tends to be common in unlikely. Maternal transmission in cattle is now sheep born to infected dams in infected environments generally accepted therefore, though how and when (Hourrigan and Klingsporn, 1996). Whether maternal the calves are exposed, i.e. transplacentally during transmission occurs only in late incubation in sheep, gestation, at parturition, or in the early post-natal as in cattle, is not clear, although Hoinville (1996) period via maternal secretions / excretions, is still mentions that the risk to lambs born the year before unknown. Experimental and epidemiological inves- onset of the dam’s clinical signs is similar to that in tigations have shown no clear evidence for horizontal lambs born in the year of disease onset.
transmission of BSE in cattle. Scrapie cases in goats are uncommon and most
Scrapie, which affects sheep and goats, is the can be traced to contacts with affected sheep. It is commonest of the natural TSEs, and at least in sheep also assumed from tissue infectivity studies that there is good evidence for both horizontal and natural transmission routes and pathogenic mecha-maternal transmission (Dickinson et al., 1974; Hoin- nisms resemble those in sheep (Andrews et al., 1992; ville, 1996; Wrathall, 1997; Woolhouse et al., 1998). Wood et al., 1992). Onset of natural scrapie usually However, because sheep carry a variety of different occurs between 2 to 4 years, but after experimental PrP gene polymorphisms which strongly influence inoculation the incubation can be as little as 20 susceptibility, the epidemiology of scrapie in differ- months. Maternal transmission in goats, although ent sheep breeds and populations is very complex. In probable, has not been documented. Until recently it infected flocks with a high proportion of genetically was believed that genetic factors had little effect on susceptible animals the disease tends to be common, scrapie incubation in goats, but new studies (Gol-with clinical signs typically appearing at 2 to 4 years dmann et al., 1996, 1998) have revealed PrP gene of age, whereas in those with many resistant animals polymorphisms which do have a marked influence. clinical scrapie is rare and mainly occurs in older Chronic wasting disease (CWD), a naturally oc-sheep. In some countries, such as Australia and New curring TSE in deer, was first reported in North Zealand, despite significant numbers of genetically America in 1967. Affected species include Rocky susceptible sheep (Hunter and Cairns, 1998; Bossers Mountain elk or wapiti, mule deer (Odocoileus et al., 1999), scrapie is not seen, which suggests an hemionus), white-tailed deer (Odocoileus
vir-absence of endemic infection (and a need to avoid its ginianus) and certain hybrids. Both captive and free
importation). In countries with endemic scrapie, on ranging animals have been affected (Williams and the other hand, selective breeding for resistance, Young, 1992; Spraker et al., 1997) and there are based on PrP genotyping blood tests, has good mounting concerns about spread into the expanding potential for control of the disease (Dawson et al., deer farming industry in North America (Zeman et
1998). al., 1998). Horizontal transmission of CWD is
Sc
to have been studied. The infective agent responsible the presence of PrP does not necessarily equate for CWD differs from those of scrapie and BSE in with TSE infectivity, so bioassays, usually in mice, that it does not readily transmit experimentally to are also required. These are very time consuming mice or hamsters; transmissions to ferrets, mink and and expensive to perform. In peripheral tissues, as
Sc
a goat have been reported however (Bartz et al., distinct from the CNS tissues, neither PrP nor TSE 1998). Little is known about susceptibility of other infectivity seem to be associated with pathological domesticated ruminants to TSEs, though one case of lesions.
BSE in a bison has occurred in a British zoo (MAFF, A guide to potential levels of TSE infectivity in
1997). various tissues is shown in Table 1 which is based
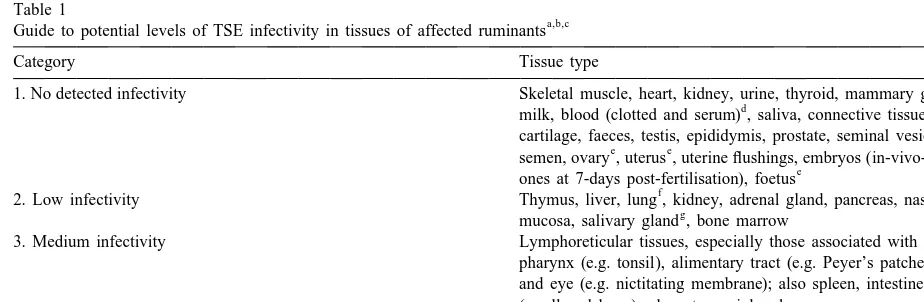
mainly on bioassay results from naturally and ex-4.3. TSE infectivity in different body tissues perimentally affected sheep, goats and cattle in many different studies, most of which are covered in detail Understanding the risks of TSE transmission by elsewhere (e.g. Hoinville, 1996; Wrathall, 1997; reproductive technologies requires a knowledge of MAFF, 1998). Despite all the studies, allocation of TSE infectivity in different types of tissue, and how tissues to infectivity categories is fraught with
diffi-Sc
it is detected. Presence of PrP , the characteristic culty, and it is unlikely that Table 1 is wholly proteinase-resistant protein of the TSEs, can be accurate. Doubts arise for various reasons, not least demonstrated in the CNS and some other tissues of because some of the published work lacks detail on affected animals by immunohistochemical and im- methodology. Results also vary according to stage of munoblotting tests which, if applied to biopsies of infection, PrP genotype, breed and species. For accessible tissues such as lymph node, tonsil or example, in cattle with BSE (as compared to sheep nictitating membrane, can also be useful for ante- and goats with scrapie) infectivity has been detected mortem diagnosis of TSEs (O’Rourke et al., 1998; by mouse bioassay in relatively few tissues, i.e. Race et al., 1998; Schreuder et al., 1998). However, CNS, trigeminal ganglion, retina, distal ileum and
Table 1
a,b,c
Guide to potential levels of TSE infectivity in tissues of affected ruminants
Category Tissue type
1. No detected infectivity Skeletal muscle, heart, kidney, urine, thyroid, mammary gland
d
milk, blood (clotted and serum) , saliva, connective tissue, skin, cartilage, faeces, testis, epididymis, prostate, seminal vesicle,
e e
semen, ovary , uterus , uterine flushings, embryos (in-vivo-derived
e
ones at 7-days post-fertilisation), foetus
f
2. Low infectivity Thymus, liver, lung , kidney, adrenal gland, pancreas, nasal
g
mucosa, salivary gland , bone marrow
3. Medium infectivity Lymphoreticular tissues, especially those associated with the pharynx (e.g. tonsil), alimentary tract (e.g. Peyer’s patches) and eye (e.g. nictitating membrane); also spleen, intestines (small and large), placenta, peripheral nerve
4. High infectivity Central nervous system (e.g. brain, spinal cord), eye, dorsal
h h h
root ganglia, pituitary gland , pineal gland , dura mater
a
Information condensed from many original reports (see text).
b
Infectivity critically dependent on incubation stage, assay sensitivity and many other factors (see text).
c
In cattle few non-CNS tissues have been shown to harbour infectivity; i.e. distal ileum, bone marrow.
d
Inconclusive evidence exists that certain white blood cells might carry infectivity (see text).
e
Detected by Hourrigan (1988, 1990) in sheep by mouse bioassay but not confirmed by other workers.
f
If killed by stunning / pithing, infected brain emboli may lodge in lung (and possibly other tissues).
g
Detected by Pattison and Millson (1962) in goat salivary gland by bioassay in goats, but not confirmed.
h
possibly bone marrow, and other tissues have tested appear to be proportionately much lower than in negative. Whereas bioassays, if positive, are valuable sheep and goats with scrapie.
for categorising the risk of particular tissues,
confi-dence in negative results obtained when heterologous 4.4. TSE spread within the body of an infected species (e.g. mice) are used for the tests may be animal
misplaced, particularly if only a few samples are
tested, or the tissue dilution factor is high. Testing At this juncture it is important to consider how for BSE infectivity in bovine tissues by inoculating TSE infection spreads to the CNS from its portal of mice could be as much as 1000-fold less sensitive entry into the body. The traditional view, based on than testing in cattle, the natural host species (Wells experimental work with scrapie in mice (Kimberlin et al., 1998). Thus, even if a particular ruminant and Walker, 1988; Fraser et al., 1992) is that after tissue tests negative in mice, analogies with other initial replication in the lymphoreticular tissues, species (including man), plus a basic knowledge of especially those of the alimentary tract and spleen, TSE pathogenesis, may indicate that it would be infection spreads along autonomic nerve fibres to the prudent to allocate it to a potentially non-negative spinal cord, and thereafter to the brain. Replication in risk category until proved otherwise. Possible exam- the CNS occurs first in those parts of the spinal cord ples include the spleen and placenta in cattle. (or brain) from which nerves connect to the sites of In Table 1, in contrast to similar tables in some primary infection, which implies spread of the agent other publications (e.g. WHO, 1997; Advisory Com- via the peripheral nervous system. An alternative (or mittee, 1998), potentially high infectivity tissues (e.g. additional) route about which there is currently much CNS) are placed in the high numerical category, i.e. speculation is that infectivity might be carried in the category 4, whilst those without detectable infectivi- blood by mobile cells from the lymphoreticular ty are mainly in category 1. This is to facilitate a system.
quantitative approach to risk.
Apart from CNS and some contiguous tissues 4.4.1. Possibility of haematogenous carriage of which can carry high levels of infectivity it is evident TSE infectivity
from Table 1 that several peripheral tissues can also Follicular dendritic cells (FDCs) are known to support at least some TSE replication. Of particular play a key role in TSE replication in the importance in this respect are lymphoreticular tis- lymphoreticular tissue (McBride et al., 1992; van sues, especially those (e.g. tonsil and Peyer’s pat- Keulen et al., 1996; Hill et al., 1997, 1999b) but, ches) associated with the alimentary tract. The spleen being non-mobile cells, they are unlikely to carry is another potential source of infectivity in sheep and infection into the blood stream themselves. Recent goats, although none has been found in spleen or work (Blattler et al., 1997; Klein et al., 1997, 1998; lymph nodes of cattle with BSE. Nevertheless, BSE Collinge and Hawke, 1998) suggests that B lympho-infectivity has been detected to a limited extent in cytes, which are not only mobile and circulate in the bone marrow of affected cattle (Wells et al., 1998, blood (part of the ‘buffy coat’ fraction), but also 1999b), and, surprisingly, it has also been found in interact closely with FDCs, might act as ‘carriers’ of the spleen of sheep challenged with BSE (Foster et infectivity. If correct this has important implications
al., 1996a). for the reproductive technologies.
Occurrence of scrapie and BSE infectivity in Direct evidence for TSE infectivity in blood has spleen of sheep, and possible BSE infectivity in the come mainly from studies with hamsters, mice and bone marrow (but not spleen) of cattle, might mean humans. For example, Casaccia et al. (1989)
demon-that TSE agents infect different types of strated low levels in concentrated blood from
presence of infectivity in samples of buffy coat, sions or use of blood products in any species, plasma, and Cohn plasma fractions I-plus-II-plus III, including humans. Thus the risks, if they exist, must but not in the red blood cell component or in Cohn be very small, and should be balanced against the plasma fractions IV or V (the albumen fraction) from established benefits of using blood products. clinically ill mice that had been inoculated with a
human CJD strain. Brown also claims to have 4.4.2. Iatrogenic transmissions
detected CJD infectivity in plasma, buffy coat and The term ‘iatrogenic transmission’ means inadver-whole blood of human patients (Brown, 1998). So tent and preventable induction of disease by medical / far as ruminants are concerned, apart from an early veterinary treatments or procedures (Webster’s report of transmission of a scrapie-like illness to Medical Dictionary, 1986) so it includes disease mice by inoculation of serum from an affected ram induced by reproductive technologies. Iatrogenic (Gibbs et al., 1965), infectivity does not appear to transmissions of CJD in the medical field have had have been detected in blood or serum from scrapie- much publicity, with at least 80 known cases arising affected sheep and goats, or in blood clots, serum or from transplants of dura mater from cadavers which the buffy coat from BSE-affected cattle that were were subsequently shown or suspected of having had inoculated into mice. It is important to reiterate, CJD (Brown, 1998). Smaller numbers have resulted however, that failure to detect infectivity does not from transplantation of eye tissues (cornea and necessarily mean it is absent. Samples of concen- sclera) (Duffy et al., 1974) and also from use of trated blood or of specific types of blood cells from contaminated neurosurgical instruments or in-preclinically and clinically affected ruminants have tracerebral electrodes. In a recent case-control study seldom been tested, and bioassays, particularly in of risk factors for sporadic CJD in humans in mice, may be incapable of reliably detecting very Australia a range of surgical procedures were found low and intermittent levels of haematogenous infec- to be significantly associated with development of
tivity (Bolton, 1998). the disease (Collins et al., 1999). The largest number
The possibility of haematogenous carriage of TSE (over 100) of known iatrogenic CJD cases, however, infectivity not only raises concerns about the use of has arisen from the use of pituitary hormones blood and blood products but, a priori, would imply (growth hormone and gonadotrophins) which were that most tissues and some secretions and excretions extracted from what were presumably infected from TSE-affected animals could also be potentially human cadavers (Brown, 1998). Gonadotrophins infected. Concerns about haematogenous infectivity were mainly used for treatment of infertility and in have been particularly acute in the medical field IVF programmes. CJD incubation periods after CNS where, because certain individuals who died of CJD surgery or ocular exposure were often short (1 or 2 had been blood donors during their pre-clinical years) whereas incubations following parenteral in-phase, the risks of transmission via blood or blood jection of pituitary hormones tended to be longer (5 components are being taken very seriously (Will and to 35 years).
Kimberlin, 1998). Among blood components per- Iatrogenic TSE transmissions have occurred in the ceived as a risk is serum albumen, which is used in veterinary field too, the best known example being human IVF, and, to the dismay of those practitioners an incident in the UK over 60 years ago in which and their patients who had already used them, some tissues (brain, spinal cord and spleen) from young batches of this and other blood products have had to sheep which must have been incubating scrapie were
be withdrawn. In that the haematogenous TSE treated with formalin and used to make a vaccine
been iatrogenic scrapie occurred recently in Italy in IETS (Stringfellow and Seidel, 1998). Quality con-sheep and goats vaccinated against the mycoplasmal trols should be based on these.
disease, contagious agalactia, with a vaccine consist- Procedures involving surgery or laparoscopy used ing of homogenised, filtered ovine brain, mammary for AI and embryo collection and transfer in small gland and lymph nodes (Capucchio et al., 1998). Of ruminants generally carry higher risks than the non-a totnon-al of over 1000 gonon-ats non-and 1000 sheep on three surgical procedures used to collect and transfer farms, 18.5% of the goats and 1.15% of the sheep embryos in cattle and other large ruminants. The
developed scrapie. same applies to TVOR from live donors of whatever
These incidents of iatrogenic TSE are salutary species, since this also involves invasion of the
warnings of the hazards of using contaminated abdominal cavity.
instruments or infected biological materials for
medi-cal and veterinary purposes, including the reproduc- 5.2. Risks of transmission by instruments and tive technologies. Vigilance is essential to minimise equipment
the risks.
Although the risks of TSE transmission by instru-ments and equipment in reproductive technologies
5. Special risks of TSE transmission by are small, they are important. Disinfection
proce-reproductive technologies dures for use in embryo laboratories are detailed in
the IETS Manual (Schiewe, 1998) which emphasises TSE transmission risks in reproductive tech- the need to avoid chemical residues toxic to the
nologies include: semen and embryos. TSE agents, however, are
extremely resistant to most standard disinfection
• those associated with people, protocols and pro- procedures and, to complicate matters, some
instru-cedures; ments used in the reproductive technologies are
• those associated with instruments and equipment; either too fragile to withstand effective physical or
• those associated with materials of animal origin; chemical treatments, or so complicated that
sub-• those associated with the gametes and / or em- sequent removal of toxic residues would be
im-bryos per se. possible.
Advice on preventing TSE transmission in
hospi-5.1. People, protocols and procedures tals has been given by the Advisory Committee
(1998) on Dangerous Pathogens, and its recom-Avoidance of disease transmission by reproductive mendations for surgical instruments are also relevant technologies depends heavily on the people who to reproductive technologies. Briefly, instruments are carry them out, especially those with a duty to ensure categorised according to whether they are used for effective sanitary precautions. Team leadership, in- human patients without known TSE exposure or tegrity and training are critical. As the technologies symptoms, for symptomless patients having had become increasingly complex, national and interna- exposure (e.g. iatrogenically), or for patients with tional regulatory bodies have to devote more and actual or suspected TSE symptoms. The Committee more effort to formulating protocols and regulations. assigned these patient groups to categories (iii), (ii) Good progress has been made, including the setting and (i), respectively, but to facilitate an additive up of AI centres and embryo transfer and production approach the order here is reversed, i.e.:
teams supervised by veterinarians which is the basis
known or suspected to be clinically affected with equipment used on animals with clinical BSE,
TSE. scrapie or CWD would be in category 3, and ought
never to be reused. Category 2 applies to most other The Advisory Committee (1998) recommends that situations, i.e. instruments used on clinically normal instruments for high risk patients should be of a animals in countries or regions where the relevant single use type and be destroyed by incineration after TSEs are considered endemic.
use, whereas for medium risk patients, provided the Except for salvage of genetic material, or for surgery does not involve the CNS or eye, the research, reproductive technologies are unlikely to be instruments can be reused if they undergo specified used in clinically affected animals. Nevertheless, if TSE decontamination procedures. Instruments for required, the best option in large ruminants would be CNS or eye surgery in medium risk patients should to collect embryos by non-surgical uterine flushing, be incinerated, as in the high risk category. No and with single-use, disposable equipment. In small special procedures are suggested for instruments in ruminants non-sterilisable laporoscopy equipment the low risk category apart from conventional clean- must be eschewed in favour of conventional surgery ing and disinfection / sterilisation. Extrapolation of with fully disposable instruments. In medium risk these recommendations to the instruments and equip- (category 2) situations in cattle there should be few ment used for reproductive technologies in ruminants limitations to AI, embryo collection and transfer (i.e.
seems appropriate. in-vivo-derived embryos) where disposable
instru-Specific decontamination procedures recom- ments and equipment can mainly be used, and the
mended for instruments used on medium risk pa- metallic items such as catheter introducers and tients (ruminants in our case) are either chemical insemination / embryo transfer ‘guns’ can be effec-disinfection with sodium hypochlorite (20,000 ppm tively sterilised to TSE standards. Manufacturers do for at least 1 h), or autoclaving in a porous load claim that certain ‘silicone’ items such as Foley steam steriliser at 134–1378C for a single cycle of at catheters and endotracheal tubes can be sterilised by
least 18 min (or six successive cycles of 3 min each) autoclaving, but it is doubtful whether this is pos-(Advisory Committee, 1998). Another recommenda- sible to TSE standards, so these should be used once, tion, from the World Health Organisation (WHO, then incinerated. In the relevant risk situations 1997), states ‘if instruments are to be re-used, they precautions must be taken not only with operating should be immersed in 1 N sodium hydroxide instruments per se, but also with operators’ clothing solution for 1 h, cleaned, and then autoclaved at (gowns, drapes, gloves etc.) and with anaesthetic 1348C for 1 h’. It must be emphasised, however, that equipment (laryngoscopes, endotracheal tubes, etc.).
many instruments and pieces of equipment used in The temptation to re-use disposable items to save reproductive technologies cannot withstand such money must be resisted. Surgical and laboratory rigorous procedures. Also listed by the Advisory premises should be kept clean and disinfected regu-Committee (1998) are chemical and physical dis- larly. A further point to emphasise regarding re-infection methods that are not effective against TSE productive technologies in larger ruminants (of any agents, including several that are routinely used in risk category) is that disposable arm-length gloves the reproductive technologies, e.g. alcohols, should always be used during rectal manipulations, iodophors, phenolics, ethylene oxide, autoclaving in and should be changed between animals.
dry heat or moist heat at 1218C for 15 min, and Some reproductive technologies, including TVOR
agents (bacteria and viruses) by fibreoptic endo- Whilst many materials of animal origin carry little scopes in hospitals has been reviewed by Hanson et or no TSE risk, the potential risks of others are high. al. (1991) who emphasise that these agents can lodge To maximise safety, therefore, they should be select-in channels and on damaged surfaces of such select-instru- ed with care according to their geographical origin, ments. They also showed that contamination by species origin and tissue type. Methods of collection blood and other body fluids will exacerbate matters. should also be considered, and steps should be taken To prevent transmission of human immunodeficiency where possible to remove or reduce any potential virus (HIV) thorough cleaning followed by disinfec- infectivity which might be present. These precau-tion with 2% glutaraldehyde was advocated, but tions will now be discussed in more detail.
these procedures would not be effective against
TSEs. Glass micropipettes, needles and other mi- 5.3.1. Geographical origin of the material
crosurgical instruments used for embryo biopsies, Movements of biological materials across national ICSI, cloning and related technologies might also borders for medical and veterinary use are strictly pose risks, so these should never be reused. Likewise controlled due to concerns that they might introduce materials such as ‘Percoll’, used to select viable exotic diseases. This applies not only to the range of sperm for IVF, must be disposed of after use to materials used in reproductive technologies but also avoid any residue of contamination. Microscopes, to vaccines, antisera and other biologicals, and to the micromanipulators, incubators, electroporators and raw materials used for their production, so choice of other ancillary items tend to have little direct contact disease-free sources is crucial (Owusu, 1995; WHO, with the gametes, embryos or tissues, nevertheless it 1998).
is important to ensure they do not become contami- The geographical origin is especially important
nated. with regard to the TSEs. Procurement decisions
should take account of veterinary infrastructure, 5.3. Risks associated with materials of animal disease surveillance systems, statistics on TSE
occur-origin rence, and whether control policies are being
effec-tively applied in the specific exporting countries or Guidelines for the international movement of regions. The reliability of veterinary certification is livestock embryos, published by the OIE in its also critical, and if the health or traceability of International Animal Health Code (1998), include materials and their donors is in any doubt, the risks the following statement: ‘any biological product of must be scored accordingly. The OIE in its Interna-animal origin, including co-culture cells and media tional Animal Health Code (OIE, 1998) has pro-constituents used in oocyte [and embryo] recovery, posed for BSE (chapter 3.2.13), and will probably maturation, fertilisation, culture, washing and storage also do so for scrapie (chapter 3.3.8), that the status should be free of living microorganisms. Media of different countries and zones be formally categor-should be sterilised by approved methods according ised on the basis of key risk criteria. These OIE to the IETS Manual (1998) and handled in such a Code proposals are complex and still under discus-manner as to ensure that sterility is maintained’. sion, but disease-free (category 1) countries or zones Adherence to these guidelines is not easy, particu- would in essence be those able to demonstrate an larly with regard to TSEs, so it is no surprise that absence of animal TSEs, whereas category 2 coun-another international expert group (WHO, 1997) tries would be those claiming absence but not yet concluded ‘ . . . the ideal situation would be to avoid having met all the specified criteria. Category 3 and the use of bovine materials in manufacture of 4 countries / zones would be those with known low or medicinal products, as well as the use of materials high incidences of the TSE(s), respectively. In the from other animal species in which TSEs naturally case of BSE, for example, high incidence countries occur’. Unfortunately animal materials are used to would probably have more than 100 cases per year. some extent in virtually all reproductive tech- Materials of animal origin for use in reproductive nologies, and often several such materials are used. technologies should preferably come from category 1 Almost invariably they are crucial for technological countries where, if their status is maintained, the