www.elsevier.com / locate / livprodsci
Review article
Reticulo-rumen biohydrogenation and the enrichment of
ruminant edible products with linoleic acid conjugated isomers
a,b ,
*
a a aR.J.B. Bessa
, J. Santos-Silva , J.M.R. Ribeiro , A.V. Portugal
a˜ ´ ´ ´
Estac¸ao Zootecnica Nacional, Fonte Boa, Vale de Santarem, 2000 Santarem, Portugal
b
ˆ ´
Instituto de Ciencias Biomedicas Abel Salazar, UP, Porto, Portugal
Received 7 December 1998; received in revised form 13 April 1999; accepted 6 May 1999
Abstract
Conjugated linoleic acid (CLA) is presently under extensive research because of its potent anticarcinogenic effects and also because of its effects on the immune system and on lipid metabolism. The biological effects of these acids are briefly reviewed. Ruminant edible products are the richest natural sources of CLA because they are supposed to be mainly derived from rumen biohydrogenation of linoleic acid. The end product of the main and more effective linoleic acid biohydrogena-tion pathway is the trans-11 C18:1. The contents of CLA and trans C18:1 acids are positively correlated in rumen contents, fat depots and milk, although their ratio (trans C18:1 / CLA) varies. Nutritional strategies for the enrichment of ruminant products with CLA will currently be achieved by increasing the supply of linoleic acid in reticulo-rumen metabolism, although this will also result in an increase in trans-11 C18:1. The inclusion in the diet of soybean oil up to 12% does not greatly affect the trans C18:1 / CLA ratio. The potential deleterious effects of trans octadecenoic acids on human health have been considered even if no consensus is reached. Therefore it will be important to manipulate rumen biohydrogenation in order to increase CLA output with a low trans C18:1 / CLA ratio. Although further investigation is needed, scarce data already suggest this could be achieved by increasing the supply of linoleic rich fat and modifying the basal diet or include ionophores. 2000 Elsevier Science B.V. All rights reserved.
Keywords: CLA; Biohydrogenation; Trans fatty acids; Lipid supplementation; Lamb
1. Introduction trend, even if not fully supported by experimental
evidence as noted by Blaxter and Webster (1991). In Ruminant edible products are characterised by this context of ruminant fat devaluation, the outcome their highly saturated fat which has decreased con- of experimental data revealing linoleic acid conju-sumer interest. Repeated dietary recommendations to gated isomers as potent anticarcinogenic agents reduce saturated fat intake as a prevention of car- appears as a strong hope for the revaluation of diovascular diseases (Kritchevsky, 1998) support this ruminant products, because ruminant fat is the
richest natural source of these acids.
The dissemination of information by mass media, *Corresponding author. Tel.: 1351-043-760-202; fax: 1
351-often resulting from the simplistic and partial inter-043-760-540.
E-mail address: [email protected] (R.J.B. Bessa) pretation of scientific data, has proven to either
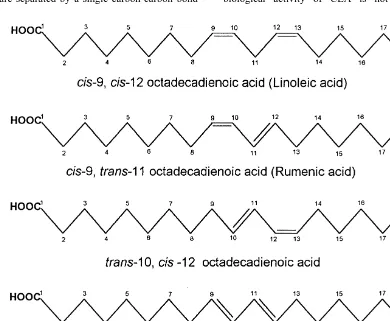
provoke alarmist reactions (as for trans fatty acids) (conjugated unsaturation) instead of the usual or promote consumer interest in specific foods (such methylene interrupted double bond system. CLA as eggs enriched in docosahexaenoic acid). We can, isomers include both cis –cis, cis –trans and trans – therefore expect increasing interest in foods ‘‘natu- trans geometry with double bonds at 9 and 11, 10 rally’’ enriched in these conjugated linoleic acid and 12 or 11 and 13 positions (Fig. 1). The pre-isomers. The objective of this work is to briefly dominant natural isomer in humans and animals is review some of the biological activities attributed to the cis-9, trans-11 octadecadienoic acid (Parodi, these fatty acids, their production in ruminants and 1977; Britton et al., 1992; Chin et al., 1992; Parodi, possible strategies to increase them in ruminant milk 1994). We will use the designation ‘‘rumenic acid’’,
and meat. recently proposed by Kramer et al. (1998a), as the
common name for this acid. Rumenic acid is consid-ered to be the most biologically active CLA isomer
2. Conjugated octadecadienoic acids because it is the predominant isomer incorporated in
membrane phospholipids (Ha et al., 1990). However, Conjugated octadecadienoic acids are a group of extensive incorporation of cis-11, trans-13 isomer in geometric and positional isomers of linoleic acid swine hepatic and myocardic phospholipids was (C18:2 n-6). They are collectively known as conju- observed when a commercial CLA mixture was fed gated linoleic acid (CLA) and have double bonds (Kramer et al., 1998b). Other results indicate that the which are separated by a single carbon-carbon bond biological activity of CLA is not restricted to
rumenic acid. Contrary to what is observed with hamsters (Nicolosi et al., 1997) fed atherogenic mixed conjugated octadienoic acids, Lee et al. diets.
(1998) demonstrated that rumenic acid alone does Ha et al. (1990) claimed that CLA was effective not inhibit the expression of stearoyl-CoA desaturase preventing peroxide formation from unsaturated fatty
mRNA. acids, although nothing in the structure of CLA
Like all conjugated dienes, CLA absorbs UV light suggest that it can possess such antioxidant activity. at 234nm. This allows it to be detected by UV These authors hypothesised that an oxidised deriva-spectrophotometry (Riel, 1963). This property has tive of CLA (formed by the introduction of a b -been extensively used to study the peroxidation of hydroxy acrolein moiety across the conjugated doub-unsaturated fatty acids in biological systems because le-bound system) is the active antioxidant species of the characteristic formation of conjugated diene rather than CLA itself. In later revaluations, van den fatty acid hydroperoxides (Logani and Davies, Berg et al. (1995) and Banni et al. (1998) concluded 1980). Conjugated unsaturation in both types of that CLA has only a minor effect preventing linoleic compounds causes difficulties in interpreting peroxi- acid oxidation, that was attributed to a competition dation data measured by this method (Banni et al., effect, due to the higher susceptibility of CLA to 1998), particularly when CLA content is high. oxidation. Some works even suggest that CLA can be a pro-oxidant agent (Schonberg and Krokan, 1995; Chen et al., 1997).
3. Biological activities of conjugated CLA participates in the modulation of the immune
octadecadienoic acids system, enhancing the mitogen-induced lymphocyte
blastogenesis, lymphocyte cytotoxic activity and The role of CLA in animal metabolism is currently macrophage killing ability (Michal et al., 1992; under strong investigation. The already known ef- Chew et al., 1997; Wong et al., 1997). CLA reduces fects can be very diverse, and are not fully integrated catabolic effects in skeletal muscles in birds, rats and into a comprehensive framework of knowledge. The mice after immune stimulation, without compromis-interest of the scientific community was triggered by ing the efficacy of the immune response (Cook et al., the accidental discovery of anticarcinogenic activity 1993; Miller et al., 1994). The proposed mechanism in fried beef extracts (Pariza and Hargraves, 1985) in to explain this action involves the reduction of which Ha et al. (1987) later isolated CLA isomers as prostaglandin E2 (PGE ) synthesis (Cook et al.,2 being responsible for the anticarcinogenic action. 1993). The inhibitory effects of CLA on PGE were2 Since then, the inhibitory action of CLA on several confirmed in several tissues and experimental con-chemical induced carcinogenesis models was demon- ditions (Liu and Belury, 1997; Sugano et al., 1997; strated, including epidermal (Ha et al., 1987; Belury Li and Watkins, 1998; Liu and Belury, 1998). The et al., 1996), mammary (Ip et al., 1991, 1994b; modulation of the eicosanoid synthesis by CLA Thompson et al., 1997) and gastrointestinal car- probably includes the elongation and desaturation cinomas (Ha et al., 1990; Liew et al., 1995). of CLA resulting in the synthesis of conjugated Physiological concentrations of CLA inhibits prolif- eicosatrienoic and eicosatetraenoic metabolites eration of several human cancer cell lines in vitro (Banni et al., 1996). These metabolites were found in (Shultz et al., 1992a,b; Schonberg and Krokan, phospholipids of the ovine hepatocyte (Banni et al., 1995). Anticarcinogenic effects of CLA appear to be 1996) and in the liver (Sebedio et al., 1997) and dose dependent, from 0.1 to 1% in the diet (Ip et al., mammary gland (Thompson et al., 1997) of rats fed 1991, 1994b). When extrapolated to humans, this CLA supplemented diets.
lipogenesis and increased lipolysis in adipose tissue developed for the large scale synthesis of CLA (Park et al., 1997). Furthermore, West et al. (1998) which may allow the production of CLA rich meat concluded that in mice fed high-fat and low-fat CLA and eggs through feeding regimes containing these supplemented diets, CLA reduces body fat by de- synthesised CLA’s. In monogastrics, the inclusion of creasing feed intake, by increasing metabolic rate CLA in diets is particularly promising because its and by decreasing the night-time respiratory quot- products have low CLA content and hydrogenation is ient. This lipid catabolic response is in agreement minimal in the gastrointestinal tract. Kramer et al. with observations by Belury et al. (1997), suggesting (1998b) introduced 1% of a CLA commercial mix that CLA acts as a typical hepatic peroxisome into pig diets which resulted in the enrichment of proliferator. Besides the effects on lipid metabolism, meat lipids with the isomers present in the mix. the proposed reduction of PGE synthesis in skeletal2
muscles induced by CLA (Cook et al., 1993) may
result in reduced proteolysis (Rodemann and Gold- 5. Rumen biohydrogenation and CLA synthesis
berg, 1982).
Rumenic acid was initially identified by Kepler et al. (1966) as being an intermediate agent in the
4. The dietary sources of CLA biohydrogenation of linoleic acid by the Butyrivibrio
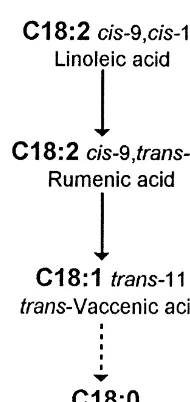
fibrisolvens rumen bacteria. The cis-12, trans-11 CLA is present in a great variety of feeds, octadecenoate isomerase which catalyses the trans-although usually in residual quantities (Forgerty et formation of linoleic acid into rumenic acid needs al., 1988; Chin et al., 1992; Lin et al., 1995). CLA the free COOH radical (Kepler et al., 1970). This concentration is higher in ruminant products, par- implies a prior lipolysis of galactolipids, phos-ticularly rumenic acid (Parodi, 1977 and 1994). The pholipids and triglycerides of the diet before isomeri-presence of the conjugated double bond system in sation takes place. The initial isomerisation is fol-milk was first described by Booth et al. (1935). Riel lowed by the saturation of cis-9 double bond through (1963) studied and reviewed the presence of total the reductase characterised by Hughes et al. (1982) conjugated dienes in milk fat and observed that they resulting in trans-vaccenic acid (trans-11 C18: 1), can vary between 2 and 28 mg / g, showing a marked the major trans isomer of ruminant tissues.
that the accumulation of trans octadecenoic acids in the reticulo-rumen ecosystem may also be useful as a response to environmental stress such as low pH, presence of ionophores and high concentrations of fatty acids.
In monogastrics, gut microorganisms also produce CLA (Chin et al., 1994b), although to a lesser extent because of the reduced size and the anatomical position of fermentative compartments.
As shown by Pollard et al. (1980) and Holman and Mahfouz (1981) in mice, rumenic acid may also be synthesised by the desaturation of trans-vaccenic acid (trans-11 C18:1) resulting from the action of hepatic microssomal D9 desaturases. This mecha-nism of CLA formation may also be observed in ruminants, where trans-11 C18:1 availability is greater than in monogastric animals. The abomasal infusion of trans-11 C18:1 and trans-12 C18:1 in Fig. 2. Pathway of linoleic acid biohydrogenation in the rumen
dairy cows induced an increase in rumenic acid (Harfoot and Hazlewood, 1988).
concentration in the milk as well as that of cis-9, Jenkins, 1998); rumen pH (Kalscheur et al., 1997); trans-12 C18:2 which was previously non existent and ionophores (Fellner et al., 1997; Sauer et al., (Corl et al., 1998). Bauman’s team at Cornell, Ithaca, 1998). Therefore, the reticulo-rumen micropopula- is presently studying this hypothesis (Bauman et al., tion may be extremely sensitive to these factors. 1998). It is not likely that trans octadecenoic acid In other biological systems, it was proven that availability limits this pathway in ruminants, because certain bacterial strains have direct octadecenoate the concentration of trans octadecenoic acids in the cis –trans isomerisation mechanisms in the cellular milk and fat depots is always much higher (3 to 12 membrane, thus reducing membrane fluidity as a times) than CLA. The possibility of humans syn-defence mechanism against lipophylic and toxic thesizing CLA from trans octadecenoic acids in the stress stimuli (Keweloh and Heipieper, 1996). If it is same way as ruminants (Parodi, 1994; Bauman et al., accepted that trans fatty acids may play a protective 1998) may bring new meaning to the role of trans role for bacteria which are subject to different octadecenoic acids, especially that of trans-11 sources of stress as proposed by Guckert et al. C18:1, which is present mainly in ruminant fat. (1986) and Sikkema et al. (1995), it is conceivable Importance was given to trans octadecaenoic
Table 1
a
Proportion of cis and trans octadecenoic isomers in cow milk fat (butter) (adapted from Wolff et al., 1995)
trans isomers % of total trans isomers cis isomers % of total cis isomers
trans-8 1.5 cis-8 0.5
trans-9 13.6 cis-9 94.0
trans-10 6.4 cis-10 0.2
trans-11 64.4 cis-11 4.2
trans-12 2.4 cis-12 0.1
trans-13 2.3 cis-13 0.5
trans-14 3.6 cis-14 0.1
trans-15 2.3
trans-16 3.0
a
isomers in this paper because they are found in large tadecaenoic isomers with precision and observed that quantities in ruminant tissues and because positive the milk fat content is negatively correlated with correlations between CLA and trans C18:1 isomer only one minor isomer (trans-10 C18:1). These concentrations were observed in milk (Jiang et al., results were confirmed by Newbold et al. (1998) 1996; Newbold et al., 1998), lamb muscle and who showed that trans-10 C18:1 concentration is not adipose tissue (Fig. 3) (Bessa et al., 1998). There- correlated with that of CLA or trans-11 C18:1. fore, the formation of CLA rich products implies a Bauman et al. (1998) go even further in suggesting parallel enrichment in trans C18:1 isomers. that the negative effects of CLA in milk (and body) The trans C18:1 / CLA ratio may vary widely. In fat synthesis could be due to isomers which have a reticulo-ruminal contents and bacteria, Bessa (un- trans-10 bond.
published findings) found values that ranged from 25 Trans acids may have negative effects not only on to 32. In growing lambs, we found values of 8.7 in animal productivity but also on human health (Wahle perirenal fat, 7.8 in subcutaneous fat and 3.4 in and James, 1993). There is considerable concern and Longissimus dorsi muscle lipids (Bessa et al., 1998). controversy regarding the latter, particularly in plas-In cow milk, Jiang et al. (1996) determined a value ma lipoprotein concentrations and composition of 4.0 for trans-11 C18:1 / CLA ratio. These differ- which are related to an increase in cardiovascular ences may be partially explained by the possible diseases (Judd et al., 1994; Precht and Molkentin, preferential incorporation of CLA into phos- 1995; Ackman, 1997; ASCN /AIN Task Force on pholipids, preferential secretion of CLA in milk or trans Fatty Acids, 1996). Further studies need to be perhaps by differences in D9 desaturase activity in carried out regarding the specific biological effects of
several tissues. each isomer and the confirmation that trans-11
The association between CLA and trans-oc- C18:1 may be a quantitatively relevant precursor for tadecenoic acids may limit the interest of increasing rumenic acid.
their concentration in ruminant products. Trans-oc-tadecenoic isomers have been blamed for the
de-crease of fat in cow milk (Gaynor et al., 1994; 6. Manipulation of rumen biohydrogenation as
Wonsil et al., 1994; Gaynor et al., 1995) which may a way to enrich products in CLA
compromise the interest in increasing CLA
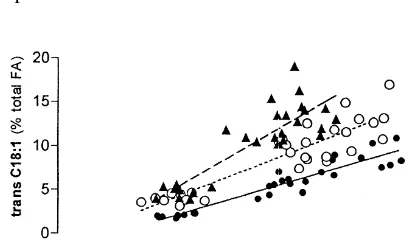
con-centration in milk. However, Griinari et al. (1998a) The level of CLA in milk reflects the quantity separated and identified cow milk trans-oc- which is available for intestinal absorption (Loor and Herbein, 1997; Chouinard et al., 1998b). Therefore, feed manipulation which allows high CLA output in the reticulo-rumen is likely to increase CLA availa-bility for deposition in tissues and secretion in milk. The increase of linoleic acid intake (C18:2 n-6) is one of the feeding strategies for CLA enrichment in ruminant fat since linoleic acid is the main precursor of CLA. The main available sources of linoleic acid in animal feeds are cereal and oilseed grains or oils obtained from these. Kelly et al. (1998a) sup-plemented dairy cows with peanut oil (rich in cis-9 C18:1), sunflower (rich in C18:2 n-6) and linseed (rich in C18:3 n-3), thus obtaining the following Fig. 3. The relationship between CLA and trans-C18:1 in fat CLA concentrations in milk fat: 13.3 mg / g, 24.4
2
from the m. Longissimus dorsi (? —, Y5 21.015.8 x; r 5 mg / g and 16.7 mg / g, respectively. We also obtained 2
0.90), adjacent subcutaneous fat (+ --- Y5 20.0317.9 x; r 5
2 a significant increase for CLA concentration in lamb
0.78) and perirenal fat (m– – –.Y5 21.4112.0 x; r50.83) of
fat, after having included soybean oil up to 7% of lambs fed dehydrated lucerne supplemented or not with soybean
al., 1997; Choi et al., 1997). In order to explain the great production of trans octadecenoic acid ob-served, it is necessary to admit to the possible oxidative shortening of carbon chains from these very long acids on a scale never demonstrated in the reticulo-rumen ecosystem or the direct cis –trans isomerization of oleic acid.
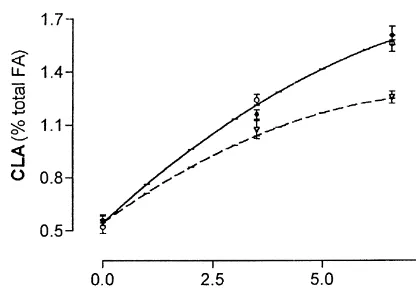
CLA levels in the milk of grazing animals are higher than those of animals in confinement (Kelly et al., 1998b; Griinari et al., 1998b). Pasture availability may explain the seasonal variation in CLA levels in milk observed by Riel (1963) in Canada and the high levels in sheep tissues in Australia (Forgerty et al., Fig. 4. Effect of soybean oil supplementation on CLA con- 1988). Green pasture may contain up to 3% fatty centration in m. Longissimus dorsi (? — Y50.5410.23 x 2 acids on a Dry Matter basis, of which about 90%
2 2
0.06 x ), subcutaneous fat (+--- Y50.5410.23 x 20.06 x ) and
2 will be unsaturated C18 acids (Murphy et al., 1995).
perirenal fat (,– – – Y50.5610.18 2 0.06 x ) of lambs fed
The effects of presentation and technological dehydrated lucerne (data from Bessa et al., 1998).
processing of lipid supplements on CLA production in the reticulo-rumen still need to be studied. For However, the concentration of CLA in milk fat example, milk fat from dairy cows fed extruded increased clearly when dairy cows were fed with low soybean contains 11 to 12% trans-11 C18:1 versus linoleic acid diets, such as linseed oil calcium soaps only 2.5% in cows fed natural soybean (Chouinard et (Chouinard et al., 1998a). The studied linolenic acid al., 1997). Although these authors did not determine biohydrogenation pathways do not consider CLA as the CLA level, we may suppose that its concen-an intermediary agent but they produce trconcen-ans-11 tration had the same variation because of the high C18:1 as one of the final products (Harfoot and positive correlation between trans-11 C18:1 and Hazlewood, 1988). Due to the extreme microbial CLA. In another study, Chouinard et al. (1998a) diversity in the reticulo-rumen, alternative pathways observed increases in CLA concentration in the milk may exist to linolenic acid biohydrogenation, involv- of cows fed diets containing extruded, toasted and
ing CLA production. micronised soybeans when compared to raw
soy-CLA concentration in milk fat also seems to have beans.
increased when dairy cows are fed fish oil sup- Therefore, work on biohydrogenation seems im-plemented diets (Chouinard et al., 1998a). It is not portant in order to increase CLA production and at clear if this results of an increase in CLA rumen the same time reduce the trans C18:1 / CLA ratio. production or of the action of D9 desaturase on This may be obtained by controlling the enzymatic trans-11 C18:1. In fact, the supplementation of process which regulates CLA conversion into trans-ruminant diets with fish oils or fish meals leads to 11 C18:1. Hughes et al. (1982) isolated and char-accumulation of trans octadecenoic acids in the acterised Butirivibrio fibrisolvens cis-9 trans-11 oc-rumen (Borsting et al., 1992; Scollan et al., 1997) tadecadienoate reductase and concluded it was a which are reflected in milk fat composition (Wonsil cytoplasmatic enzyme requiring iron and had a et al., 1994; Mansbridge and Blake, 1997). Fish oils maximum activity for pH values between 7.2 and are usually poor in linoleic and linolenic acids and 8.2.
Fig. 5. The trans C18:1 / CLA ratio (A) and CLA concentration in % of total fatty acids (B) in mixed rumen bacteria continuous culture contents. Effect of ionophores with or without linoleic acid infusion (LA) – (Adapted from Fellner et al., 1997).
Table 2
Relation between trans fatty acids and CLA in milk – Interaction between the basal diet and lipid supplement for dairy cows (according to data published by Griinari et al., 1996)
Type of diet Lipid supplement trans FA CLA trans FA / CLA
(mg / g) (mg / g) ratio
High forage Saturated 1.4 0.2 7
a
High forage Unsaturated 44.4 9.1 4.9
Low forage Saturated 6.0 0.2 30
a
Low forage Unsaturated 52.0 4.9 10
a
Corn oil – rich in C18:2 n-6.
trans C18:1 / CLA and in an increase in CLA propor- Acknowledgements
tion (Fig. 5).
The trans C18:1 / CLA ratio may also be decreased Work carried out in the scope of PRAXIS Project ´ by studying interactions between the basal diet and CA / 3 / 3.2 / 2000 / 95. We would like to thank Antonio the type of lipid supplement. Although the use of Horta for the critical reading of the manuscript and
´
increasing levels of lipids as supplements of the Ann Barreiro and Lucia Rato for the translation. same diet did not change that ratio (Bessa et al.,
1998), Griinari et al. (1996) showed that results depend on the type of basal diet being fed (Table 2).
References
Therefore, besides the traditional objective (i.e. total inhibition of biohydrogenation), new objectives
Ackman, R.G., 1997. Has evolution and long-term coexistence are defined for the manipulation of biohydrogenation adapted us to cope with trans fatty acids? J. Food Lipids 4, in the rumen. Attention is now centred on CLA, 295–318.
although little information is available on the effects ASCN /AIN Task Force on Trans Fatty Acids, 1996. Position paper on trans fatty acids. Am. J. Clin. Nutr. (63), 663–670. of a series of linoleic acid, non-conjugated isomers
Ashes, J.R., Siebert, B.D., Gulati, S.K., Cuthbertson, A.Z., Scott, on metabolism and human and animal health. These
T.W., 1992. Incorporation of n-3 fatty acids of fish oil tissue isomers positively correlate with CLA in the rumen and serum lipids of ruminants. Lipids 29, 629–631. content (Fellner et al., 1997), in milk fat (Sauer et Banni, S., Carta, G., Contini, M.S., Angioni, E., Deiana, M.,
´
of conjugated diene fatty acids in milk, dairy products, and Chin, S.F., Storkson, J.M., Liu, W., Albright, K.J., Pariza, M.W., 1994b. Conjugated linoleic acid (9,11- and 10,12-oc-lamb tissues. J. Nutr. Biochem. 7, 150–155.
tadecadienoic acid) is produced in conventional but not germ-Banni, S., Angioni, E., Contini, M.S., Carta, G., Casu, V., Lengo,
free rats fed linoleic acid. J. Nutr. 124, 694–701. ´
G.A., Melis, M.P., Deiana, M., Dessı, M.A., Corongiu, P.,
Choi, N.J., Kim, E.J., Maeng, W.J., Neville, M.A., Enser, M., 1998. Conjugated linoleic acid and oxidative stress. J. Am. Oil
Wood, J.D., Scollan, N.D., 1997. Rumen biohydrogenation of Chem. Soc. 75, 261–267.
fatty acids from different sources of fat. Proc. Br. Soc. Anim. Bateman, H.G., Jenkins, T.C., 1998. Influence of soybean oil in
Sci. 20, 19. high fiber diets fed to nonlactating cows on ruminal unsaturated
Chouinard, P.Y., Levesque, J., Girard, V., Brisson, G.J., 1997. fatty acids and nutrient digestibility. J. Dairy Sci. 81, 2451–
Dietary soybeans extruded at different temperatures: milk 2458.
composition and in situ fatty acid reactions. J. Dairy Sci. 80, Bauman, D.E., Corl, B.A., Baumgard, L.H., Griinari, J.M., 1998.
2913–2924. Trans fatty acids, conjugated linoleic acid and milk fat
Chouinard, P.Y., Corneau, L., Bauman, D.E., Butler, W.R., Chil-synthesis. Proc. Cornell Nutr. Conf., 95–103. liard, Y., 1998a. Conjugated linoleic acid content of milk fed Belury, M.A., Nickel, K.P., Bird, C.E., Wu, Y., 1996. Dietary different sources of dietary fat. J. Dairy Sci. 81 (Suppl. 1), 233. conjugated linoleic acid modulation of phorbol ester skin tumor Chouinard, P.Y., Corneau, L., Bauman, D.E., Metzger, L.E., promotion. Nutr. Cancer 26, 149–157. Barbano, D.M., 1998b. Milk yield and composition during Belury, M.A., Moya-Camarena, S.Y., Liu, K.L., Heuvel, J.P.V., abomasal infusion of conjugated linoleic acid in dairy cows. J.
1997. Dietary conjugated linoleic acid induces peroxisome Dairy Sci. 81 (Suppl. 1), 353.
specific enzyme accumulation and ornitine decarboxylase Cook, M.E., Miller, C.C., Park, Y., Pariza, M.W., 1993. Immune activity in mouse liver. J. Nutr. Biochem. 8, 579–584. modulation by altered nutrient metabolism: Nutritional control Bessa, R.J.B., Santos-Silva, J., Ribeiro, J.M.R., Portugal, A.V., of immune-induced growth depression. Poultry Sci. 72, 1301–
˜ ´
1998. Utilizac¸ao de oleo de soja como suplemento de luzerna 1305. ˜
desidratada na alimentac¸ao de borregos em crescimento. 2. Corl, B.A., Chouinard, P.Y., Dwyer, D.A., Bauman, D.E., Griinari,
˜ ´ ´
Efeitos na composic¸ao em acidos gordos dos depositos lip- J.M., Nurmela, K.V.V., 1998. Conjugated linoleic acid in milk ´ıdicos corporias. VIII Resumos do Congresso de Zootecnia, fat of dairy cows originates in part by endogenous synthesis
144. from trans-11 octadecenoic acid. J. Dairy Sci. 81 (Suppl. 1),
Blaxter, K.L., Webster, A.J.F., 1991. Animal production and food: 233.
real problems and paranoia. Anim. Prod. 53, 261–269. Dugan, M.E.R., Aalhus, J.L., Schaefer, A.L., Kramer, J.K.G., Booth, R.G., Kon, S.K., Dann, W.J., Moore, T., 1935. A study of 1997. The effect of conjugated linoleic acid on fat to lean seasonal variation in butter fats. II – A seasonal spectroscopic repartitioning and feed conversion in pigs. Can. J. Anim. Sci. variation in the fatty acid fraction. Biochem. J. 29, 133–137. 77, 723–725.
Borsting, C.F., Hvelplund, T., Weisbjerg, M.R., 1992. Fatty acid Fellner, V., Sauer, F.D., Kramer, J.K., 1997. Effect of nigericin, digestibility in lactating cows fed increasing amounts of monensin, and tetronasin on biohydrogenation in continuous protected vegetable oil, fish oil or saturated fat. Acta Agric. flow-through ruminal fermenters. J. Dairy Sci. 80, 921–928. Scand., Sect. A, Anim. Sci. 42, 148–156. Forgerty, A.C., Ford, G.L., Svoronos, D., 1988. Octadeca-9,11-Britton, M., Fong, C., Wickens, D., Yudkin, J., 1992. Diet as a dienoic acid in foodstuffs and in lipids of human blood and
source of phospholipid esterified 9,11-octadecadienoic acid in breast milk. Nutr. Rep. Int. 38, 937–944.
humans. Clin. Sci. (Colch) 83, 97–101. Fritsche, J., Steinhart, H., 1998. Amounts of conjugated linoleic Carro, M.D., Miller, E.L., Fabb, O.C., 1997. Rumen hydro- acid (CLA) in German foods and evaluation of daily intake. genation of long chain fatty acids from fish meal. Proc. Br. Zeitschrift fur Lebensmittel – Untersuchung und Forschung.
Soc. Anim. Sci. 20, 129. 206, 77–82.
Chen, Z.Y., Chan, P.T., Kwan, K.Y., Zhang, A., 1997. Reassement Gaynor, P.J., Erdman, R.A., Teter, B.B., Sampugna, J., Capuco, of the antioxidant activity of conjugated linoleic acids. J. Am. A.V., Waldo, D.R., Hamosh, M., 1994. Milk fat yield and Oil Chem. Soc. 74, 749–753. composition during abomasal infusion of cis or trans oc-Chew, B.P., Wong, T.S., Shultz, T.D., Magnuson, N.S., 1997. tadecenoates in Holstein cows. J. Dairy Sci. 77, 157–165.
Effects of conjugated dienoic derivatives of linoleic acid and Gaynor, P.J., Waldo, D.R., Capuco, A.V., Erdman, R.A., Douglass, beta-carotene in modulating lymphocyte and macrophage func- L.W., Teter, B.B., 1995. Milk fat depression, the glucogenic tion. Anticancer Res. 17, 1099–1106. theory, and trans-C18:1 fatty acids. J. Dairy Sci. 78, 2008– Chin, S.F., Liu, W., Storkson, J.M., Ha, Y.L., Pariza, M.W., 1992. 2015.
Dietary sources of conjugated dienoic isomers of linoleic acid, Griinari, J.M., Dwyer, D.A., McGuire, M.A., Bauman, D.E., 1996. a newly recognised class of anticarcinogens. J. Food Comp. Partially hydrogenated fatty acids and milk fat depression. J.
Anal. 5, 185–197. Dairy Sci. 79 (Suppl. 1), 177.
Griinari, J.M., Nurmela, K.V., Sairanen, A., Nousiainen, J.I., Muller, L.D., 1998b. Effect of intake of pasture on con-Khalili, H., 1998b. Effect of dietary sunflower oil and pasture centrations of conjugated linoleic acid in milk of lactating forage maturity on conjugated linoleic acid (CLA) content in cows. J. Dairy Sci. 81, 1630–1636.
milk fat from lactating dairy cows. J. Dairy Sci. 81 (Suppl. 1), Kepler, C.R., Hirons, K.P., McNeill, J.J., Tove, S.B., 1966. 300. Intermediates and products of the biohydrogenation of linoleic Guckert, J.B., Hood, M.A., White, D.C., 1986. Phospolipid ester acid by Butyrivibrio fibrisolvens. J. Biol. Chem. 241, 1350–
linked fatty acid profile changes during nutrient deprivation of 1354.
Vibrio cholerae: increases in the cis /trans ratio and proportion Kepler, C.R., Tucker, W.P., Tove, S.B., 1970. Biohydrogenation of of cyclopropyl fatty acids. Appl. Environ. Microbiol. 52, 794– unsaturated fatty acids. IV. Substracte specificity and inhibition 801. of linoleate delta12-cis,delta11-trans isomerase from Butyrivib-Ha, Y.L., Grimm, N.K., Pariza, M.W., 1987. Anticarcinogens from rio fibrisolvens. J. Biol. Chem. 245, 3612–3620.
fried ground beef: heat-altered derivatives of linoleic acid. Keweloh, H., Heipieper, H.J., 1996. Trans unsaturated fatty acids Carcinogenesis 8, 1881–1887. in bacteria. Lipids 31, 129–137.
Ha, Y.L., Grimm, N.K., Pariza, M.W., 1989. Newly recognised Kramer, J.K., Parodi, P.W., Jensen, R.G., Mossoba, M.M., anticarcinogenic fatty acids: Identification and quantification in Yurawecz, M.P., Adlof, R.O., 1998a. Rumenic acid: a proposed natural and processed cheeses. J. Agric. Food Chem. 37, common name for the major conjugated linoleic acid isomer
75–81. found in natural products. Lipids 33, 835.
Ha, Y.L., Storkson, J., Pariza, M.W., 1990. Inhibition of ben- Kramer, J.K., Sehat, N., Dugan, M.E., Mossoba, M.M., Yurawecz, zo(a)pyrene-induced mouse forestomach neoplasia by conju- M.P., Roach, J.A., Eulitz, K., Aalhus, J.L., Schaefer, A.L., Ku, gated dienoic derivatives of linoleic acid. Cancer Res. 50, Y., 1998b. Distributions of conjugated linoleic acid (CLA) 1097–1101. isomers in tissue lipid classes of pigs fed a commercial CLA Harfoot, C.G., Hazlewood, G.P., 1988. Lipid metabolism in the mixture determined by gas chromatography and silver ion-rumen. In: Hobson, P.N. (Ed.), The Rumen Microbial Eco- high-performance liquid chromatography. Lipids 33, 549–558. system, Elsevier, New York, pp. 285–322. Kritchevsky, D., 1998. History of recommendations to the public Holman, R.T., Mahfouz, M.M., 1981. Cis and trans-octadecenoic about dietary fat. J. Nutr. 128, 449S–452S.
acids as precursors of polyunsaturated acids. Prog. Lipid Res. Lee, K.N., Kritchevsky, D., Pariza, M.W., 1994. Conjugated 20, 151–156. linoleic acid and atherosclerosis in rabbits. Atherosclerosis 108, Hughes, P.E., Hunter, W.J., Tove, S.B., 1982. Biohydrogenation of 19–25.
unsaturated fatty acids. Purification and properties of cis-9, Lee, K.N., Pariza, M.W., Ntambi, J.M., 1998. Conjugated linoleic trans-11-octadecadienoate reductase. J. Biol. Chem. 257, acid decreases hepatic stearoyl-CoA desaturase mRNA
expres-3643–3649. sion. Biochem. Biophys. Res. Commun. 248, 817–821.
Ip, C., Chin, S.F., Scimeca, J.A., Pariza, M.W., 1991. Mammary Li, Y., Watkins, B.A., 1998. Conjugated linoleic acids alter bone cancer prevention by conjugated dienoic derivative of linoleic fatty acid composition and reduce ex vivo prostaglandin E2 acid. Cancer Res. 51, 6118–6124. biosynthesis in rats fed n-6 or n-3 fatty acids. Lipids 33, Ip, C., Scimeca, J.A., Thompson, H.J., 1994a. Conjugated linoleic 417–425.
acid. A powerful anticarcinogen from animal fat sources. Liew, C., Schut, H.A., Chin, S.F., Pariza, M.W., Dashwood, R.H., Cancer 74, 1050–1054. 1995. Protection of conjugated linoleic acids against 2-amino-Ip, C., Singh, M., Thompson, H.J., Scimeca, J.A., 1994b. Conju- 3-methylimidazo[4,5-f]quinoline-induced colon carcinogenesis gated linoleic acid suppresses mammary carcinogenesis and in the F344 rat: a study of inhibitory mechanisms. Carcino-proliferative activity of the mammary gland in the rat. Cancer genesis 16, 3037–3043.
Res. 54, 1212–1215. Lin, H., Boylston, T.D., Chang, M.J., Luedecke, L.O., Shultz, Jiang, J., Bjoerck, L., Fonden, R., Emanuelson, M., 1996. Occur- T.D., 1995. Survey of the conjugated linoleic acid contents of
rence of conjugated cis-9,trans-11-octadecadienoic acid in dairy products. J. Dairy Sci. 78, 2358–2365.
bovine milk: effects of feed and dietary regimen. J. Dairy Sci. Liu, K.L., Belury, M.A., 1997. Conjugated linoleic acid modula-79, 438–445. tion of phorbol ester-induced events in murine keratinocytes. Judd, J.T., Clevidence, B.A., Muesing, R.A., Wittes, J., Sunkin, Lipids 32, 725–730.
M.E., Podczasy, I.J., 1994. Dietary trans fatty acids: effects on Liu, K.L., Belury, M.A., 1998. Conjugated linoleic acid reduces plasma lipids and lipoproteins of healthy men and women. Am. arachidonic acid content and PGE2 synthesis in murine kera-J. Clin. Nutr. 59, 861–868. tinocytes. Cancer Lett. 127, 15–22.
Kalscheur, K.F., Teter, B.B., Piperova, L.S., Erdman, R.A., 1997. Logani, M.K., Davies, R.E., 1980. Lipid oxidation: Biological Effect of dietary forage concentration and buffer addition on effects and antioxidants - A review. Lipids 15, 485–495. duodenal flow of trans-C18:1 fatty acids and milk fat pro- Loor, J.J., Herbein, J.H., 1997. Secretion of cis-9,trans-11-18:2 in duction in dairy cows. J. Dairy Sci. 80, 2104–2114. milk fat of Holstein cows in response to infusion of conjugated Kelly, M.L., Berry, J.R., Dwyer, D.A., Griinari, J.M., Chouinard, linoleic acid into the abomasum. J. Dairy Sci. 80 (Suppl. 1),
P.Y., VanAmburgh, M.E., Bauman, D.E., 1998a. Dietary fatty 164.
Michal, J.J., Chew, B.P., Schultz, T.D., Wong, T.S., 1992. Inter- Sebedio, J.L., Juaneda, P., Dobson, G., Ramilison, I., Martin, J.C., action of conjugated dienoic derivatives of linoleic acid with Chardigny, J.M., Christie, W.W., 1997. Metabolites of
conju-b-carotene on cellular host defense. FASEB J. 6, A1102. gated isomers of linoleic acid (CLA) in the rat. Biochem. Miller, C.C., Park, Y., Pariza, M.W., Cook, M.E., 1994. Feeding Biophys. Acta 1345, 5–10.
conjugated linoleic acid to animals partially overcomes Shantha, N.C., Decker, E.A., Ustunol, Z., 1992. Conjugated catabolic responses due to endotoxin injection. Biochem. linoleic acid concentration in processed cheese. J. Am. Oil Biophys. Res. Commun. 198, 1107–1112. Chem. Soc. 69, 425–428.
Murphy, J.J., Connolly, J.F., McNeill, G.P., 1995. Effects on cow Shultz, T.D., Chew, B.P., Seaman, W.R., 1992a. Differential performance and milk fat composition of feeding full fat stimulatory and inhibitory responses of human MCF-7 breast soyabeans and rapeseeds to dairy cows at pasture. Livestock cancer cells to linoleic acid and conjugated linoleic acid in Prod. Sci. 44, 13–25. culture. Anticancer Res. 12, 2143–2145.
Newbold, J.R., Robertshaw, K.L., Morris, H.W., 1998. Associa- Shultz, T.D., Chew, B.P., Seaman, W.R., Luedecke, L.O., 1992b. tions between concentrations of fat and intermediates of Inhibitory effect of conjugated dienoic derivatives of linoleic ruminal biohydrogenation in milk of dairy cows. Proc. Br. Soc. acid and beta-carotene on the in vitro growth of human cancer Anim. Sci. 21, 224. cells. Cancer Lett. 63, 125–133.
Nicolosi, R.J., Rogers, E.J., Kritchevsky, D., Scimeca, J.A., Huth, Sikkema, J., deBont, J.A.M., Poolman, B., 1995. Mechanisms of P.J., 1997. Dietary conjugated linoleic acid reduces plasma membrane toxicity of hydrocarbons. Microbiol. Rev. 59, 201– lipoproteins and early aortic atherosclerosis in
hypercholes-222. terolemic hamsters. Artery 22, 266–277.
Sugano, M., Tsujita, A., Yamasaki, M., Yamada, K., Ikeda, I., Pariza, M.W., Hargraves, W.A., 1985. A beef-derived mutagenesis
Kritchevsky, D., 1997. Lymphatic recovery, tissue distribution, modulator inhibits initiation of mouse epidermal tumors by
and metabolic effects of conjugated linoleic acid in rats. J. 7,12-dimethylbenz[a]anthracene. Carcinogenesis 6, 591–593.
Nutr. Biochem. 8, 38–43. Park, Y., Albright, K.J., Liu, W., Storkson, J.M., Cook, M.E.,
Thompson, H., Zhu, Z., Banni, S., Darcy, K., Loftus, T., Ip, C., Pariza, M.W., 1997. Effect of conjugated linoleic acid on body
1997. Morphological and biochemical status of the mammary composition in mice. Lipids 32, 853–858.
gland as influenced by conjugated linoleic acid: implication for Parodi, P.W., 1977. Conjugated octadecadienoic acids of milk fat.
a reduction in mammary cancer risk. Cancer Res. 57, 5067– J. Dairy Sci. 60, 1550–1553.
5072. Parodi, P.W., 1994. Conjugated linoleic acids – An
anticar-van den Berg, J.J., Cook, N.E., Tribble, D.L., 1995. Reinvestiga-cinogenic fatty acid present in milk fat. Aust. J. Dairy Technol.
tion of the antioxidant properties of conjugated linoleic acid. 49, 93–97.
Lipids 30, 599–605. Pollard, M.R., Gunstone, F.D., James, A.T., Morris, L.J., 1980.
Wahle, K.W., James, W.P., 1993. Isomeric fatty acids and human Desaturation of positional and geometric isomers of monoenoic
health. Eur. J. Clin. Nutr. 47, 828–839. fatty acids by microsomal preparations from rat liver. Lipids
Werner, S.A., Luedecke, N.K., Shultz, T.D., 1992. Determination 15, 306–314.
of conjugated linoleic acid content and isomer distribution in Precht, D., Molkentin, J., 1995. Trans fatty acids: implications for
three cheddar-type cheeses: Effects of cheese cultures, process-health, analytical methods, incidence in edible fats and intake
ing and aging. J. Agric. Food Chem. 40, 1817–1821. (a review). Nahrung 39, 343–374.
West, D.B., Delany, J.P., Camet, P.M., Blohm, F., Truett, A.A., Riel, R.R., 1963. Physico-chemical characteristics of Canadian
Scimeca, J., 1998. Effects of conjugated linoleic acid on body milk fat. Unsaturated fatty acids. J. Dairy Sci. 46, 102–106.
fat and energy metabolism in the mouse. Am. J. Physiol. 275, Rodemann, H.P., Goldberg, A.L., 1982. Araquidonic acid,
prosta-R667–R672. glandin E2 and F2a influence rates of protein turnover in
skeletal and cardiac muscle. J. Biol. Chem. 257, 1632–1638. Wolff, R.L., Bayard, C.C., Fabien, R.J., 1995. Evaluation of Sauer, F.D., Fellner, V., Kinsman, R., Kramer, J.K., Jackson, H.A., sequential methods for the determination of butterfat fatty acid Lee, A.J., Chen, S., 1998. Methane output and lactation composition with emphasis on trans-18:1 acids. Application to response in Holstein cattle with monensin or unsaturated fat the study of seasonal variations in French butters. J. Am. Oil added to the diet. J. Anim. Sci. 76, 906–914. Chem. Soc. 72, 1471–1483.
Schonberg, S., Krokan, H.E., 1995. The inhibitory effect of Wong, M.W., Chew, B.P., Wong, T.S., Hosick, H.L., Boylston, conjugated dienoic derivatives (CLA) of linoleic acid on the T.D., Shultz, T.D., 1997. Effects of dietary conjugated linoleic growth of human tumor cell lines is in part due to increased acid on lymphocyte function and growth of mammary tumors lipid peroxidation. Anticancer Res. 15, 1241–1246. in mice. Anticancer Res. 17, 987–993.