www.elsevier.com / locate / livprodsci
A secure health status associated with the production and trade
of in vitro derived cattle embryos
a ,
*
a b´
B. Guerin
, Brigitte Le Guienne , M. Thibier
a
´ ´
Union Nationale des Cooperatives d’Elevage et d’Insemination Artificielle, BP 65, 94703 Maisons-Alfort, France b
AFSSA, BP 19, 94701 Maisons-Alfort, France
Abstract
The number of in vitro produced embryos used worldwide is increasing. In some countries such as Canada and the Netherlands, genetics improvement programs use in vitro produced embryos extensively. Controlling the sanitary risks associated with the production of these embryos relies on a different set of guidelines compared with the use of in vivo produced embryos. There are particular risk factors: (1) the health status of the semen used for in vitro fertilization, (2) the health status of the oocytes of the donor cow; (3) the freedom from contamination of the media and reagents used, and (4) the environmental conditions associated with oocyte maturation, in vitro fertilization, cultivation and embryo transfer. Some of these risks have already been studied, especially the principal pathogens found in bull semen (Brucella sp., Haemophilus
somnus, Campylobacter fetus, Leptospira sp. etc.). The oocytes may also be contaminated, either intracellularly (Campylo-bacter fetus) or more frequently when virus (BHV-1, BVDV) or (Campylo-bacteria (Leptospira hardjo), Campylo(Campylo-bacter fetus) located
in the ovarian follicle, are adsorbed onto the zona pellucida. Granulosa or cumulus cells, or even oviductal cells, can be a source of infection when contaminated with virus (BHV-1, BVDV). For the production of in vitro embryos that present no health risk, one must rely on using closely controlled and monitored cell lines, media and reagents that are guaranteed free of products of animal origin. 2000 Elsevier Science B.V. All rights reserved.
Keywords: In vitro embryos; Sanitary risks; Oocyte maturation
1. Introduction then, these embryos have been used widely in Canada, the United States and also in Europe, mainly The first in vitro embryos were produced in the in the Netherlands, Ireland, Italy and France (Table early 50s (Dauzier et al., 1954), but the technique 1), some of the most advanced countries as far as was really developed during the mid-80s, especially genetics are concerned.
in bovines, once the in vitro culture phase had been The constant improvements of these production mastered. (Le Guienne and Thibier, 1988). Since protocols, which both enhance productivity and cut costs together with improvements in embryo freezing techniques, has led to the development of much international embryo exchange. International trade
*Corresponding author. Tel.: 133-14-353-5100; fax:1
33-14-and exchange of genetic material are accompanied
353-5101.
´
E-mail address: [email protected] (B. Guerin) by the risk of disease transmission, particularly when
´
272 B. Guerin et al. / Livestock Production Science 62 (2000) 271 –285
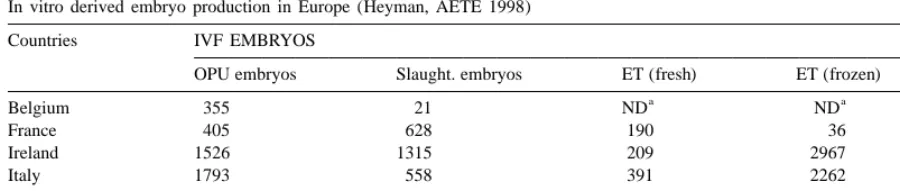
Table 1
In vitro derived embryo production in Europe (Heyman, AETE 1998)
Countries IVF EMBRYOS
OPU embryos Slaught. embryos ET (fresh) ET (frozen) Frozen
a a
Belgium 355 21 ND ND 5907
France 405 628 190 36 6287
Ireland 1526 1315 209 2967
Italy 1793 558 391 2262 6381
Netherlands 3632 1778 951 10 000
UK 1009 227 280 138 8016
a
ND no data.
animals themselves are moved from one country to vitro produced embryos involves examining the another. Some countries originally free of certain different steps of their production. Generally, those diseases have unwittingly imported such pathogens risks refer to:
with livestock. As far as embryos are concerned, the
international scientific community, and the regula- 1. the oocytes, associated cells (cumulus and granul-tory authorities of a large number of countries have osa cells) and follicular environmental conditions looked closely at these health risks associated with 2. the semen used for oocyte fertilization
the international exchange of genetic material. 3. the sanitary quality of the environment and re-Much research was originally undertaken on in agents used to cultivate those embryos and man-vivo derived embryos, and this has led to the ipulate them
publication of precise protocols, published in the 4. storage and shipment conditions latest issue of the ‘‘International Embryo Transfer
Society’’ Manual (Stringfellow and Seidel, 1998).
The current document has three different objec-Sanitary safety associated with embryo transfer was
tives: so obvious that this technique was qualified as ‘‘the
safest way to exchange genetic between countries or
1. to present the protocols used worldwide to gener-continents’’ (Thibier, 1990a).
ate in vitro produced embryos As far as in vitro-produced embryos are
2. to identify the different parameters, which might concerned, it soon became evident that such embryos
cause health risks could require a different set of rules from in
vivo-3. to propose rules that can guarantee the sanitary derived embryos. The biophysical characteristics of
quality of the embryos produced in these con-the zona pellucida as well as con-the different production
ditions. steps of these embryos are indeed peculiar, and
explain why the risks associated with their handling are different.
From a strictly sanitary point of view, in vitro- 2. Production of in vitro-derived embryos
derived embryos could however be more
advantage-ous than in vivo-produced embryos because their The in vitro produced embryos can be obtained sanitary quality can in theory be better controlled. It from oocytes collected from donor females, after is indeed possible to control all parameters at stake ovum pick-up (OPU) or from slaughterhouse in the making of such embryos with a certain degree ovaries. The follicles of 5 to 8 mm of diameter are of reliability and to define the guidelines to be manipulated with the help of a sterile needle. After followed at each step in order to avoid any risks. collection the oocytes are immediately put in a
Table 2
In vitro production of embryos from non-fertile cows (Guyader-Joly, unpublished)
Oocytes collected after Holstein Villard de Lans
OPU (n510, 24 sessions) (n52, 5 sessions)
Number Average / Number Average /
session session
Collected oocytes 315 13.1 157 31.4
Inseminated oocytes 269 11.2 114 22.8
Developed embryos 118 4.9 43 8.6
Transferred embryos 72 3.0 21 4.2
% of developed embryos 43.9% 37.7%
% gestation after 35 days 65.3% 66.7%
% gestation after 90 days 61.1% 57.1%
2.1. Production from oocytes donors collected 2.1.1. Superovulation treatment and pick-up period The pick-up of oocytes can be performed several after ovum pick up (OPU) session
times a week (n52–3) on females, without any ovarian stimulation. Periods between pick-up ses-The oocytes can be harvested by OPU from
pre-sions can be more important when females are pubertal females (Yang et al., 1997; Fry, 1999)
superovulated. The treatment consists of injecting heifers or cows (Donnay et al., 1997; Lacaze et al.,
eight decreasing doses of FSH, whose concentration 1997). An interesting development of this technique
depends on the age of the animal: generally 70% of a led to picking oocytes up from pregnant cows during
complete dose for cows, 50% for heifers and 25% or the first third of the gestation period (Guyader-Joly
less for pre-pubertal females. et al., 1997).
The stimulation treatment seems to be mandatory One of the most outstanding applications is its
for pre-pubertal animals in order to increase the utilization to produce embryos from non-fertile
number of oocytes picked up: 26.4 oocytes for females (Looney et al., 1994; Hasler et al., 1995;
sessions after FSH treatment vs. 3 oocytes picked up Guyader-Joly, unpublished, Table 2).
Table 3
In vitro derived embryo production on pregnant and non-pregnant cows (from Guyader-Joly et al., 1997)
a b
Oocytes collected Non pregnant cows Pregnant cows
by OPU Result / session Result / session
Number n526 donors, Number Montbeliarde Holstein
55 sessions (n55, 12 sessions) (n56, 10 sessions)
Collected oocytes 871 15.161.7 308 13.163.8 15.462.5
Inseminated 680 12.460.9 224 7.862.3 11.962.2
oocytes
Developed embryos 268 4.960.5 71 3.361.2 3.161.4
Transferred 166 3.160.4 46 2.360.9 1.961.0
embryos
% of developed 39.4% 38.1% 26.1%
embryos
% of gestation after 56% 37% 52.6%
35 days
a
Collection once a week, with superovulatory treatment. b
´
274
B
.
Guerin
et
al
.
/
Livestock
Production
Science
62
(2000
)
271
–
285
Table 4
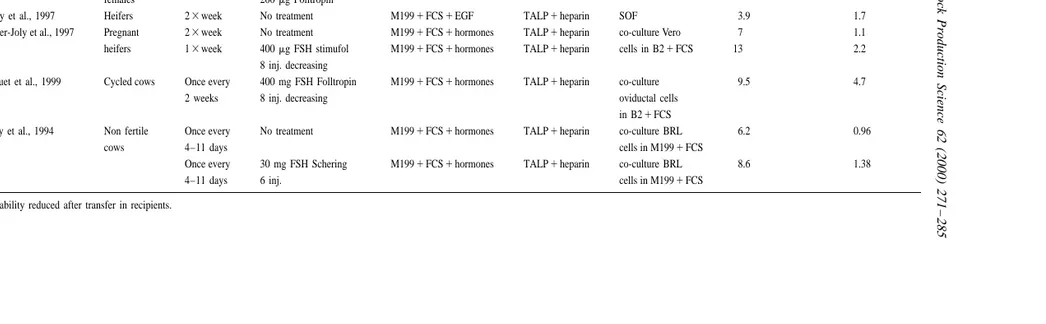
OPU-IVF embryos: Production compared in different systems
Animal Frequency Superovulatory treatment In vitro maturation Fertilization Culture No. oocytes / session No. transferable
of collect. embryos / session
a
Fry, 1999 Prepubertal 13month Sponge P454 inj. FSH M1991FCS1hormones TALP1heparin SOF 36.5 8.1
females 200mg Folltropin
Donnay et al., 1997 Heifers 23week No treatment M1991FCS1EGF TALP1heparin SOF 3.9 1.7
Guyader-Joly et al., 1997 Pregnant 23week No treatment M1991FCS1hormones TALP1heparin co-culture Vero 7 1.1
heifers 13week 400mg FSH stimufol M1991FCS1hormones TALP1heparin cells in B21FCS 13 2.2
8 inj. decreasing
Bousquet et al., 1999 Cycled cows Once every 400 mg FSH Folltropin M1991FCS1hormones TALP1heparin co-culture 9.5 4.7
2 weeks 8 inj. decreasing oviductal cells
in B21FCS
Looney et al., 1994 Non fertile Once every No treatment M1991FCS1hormones TALP1heparin co-culture BRL 6.2 0.96
cows 4–11 days cells in M1991FCS
Once every 30 mg FSH Schering M1991FCS1hormones TALP1heparin co-culture BRL 8.6 1.38
4–11 days 6 inj. cells in M1991FCS
without any treatment (Mermillod et al., 1997). On Day 7, the embryos are checked on mor-The use of FSH enables to stretch the period phological criteria and ranked in three categories: between sessions without reducing the number of morula, blastocystes or degenerated embryos. embryos produced during a given period. According
to Lacaze et al. (1997), the number of oocytes picked 2.1.3. Factors of variation
up and producing embryos are respectively 13.8 and The oocyte’s donor is one of the main factors of 1.8 for females picked up twice a week without variation, since the number of embryos produced for ovarian stimulation versus 15.1 and 2.1 for the same each session may vary depending on the female from animals picked up once a week after superovulation. 0.7 to 10.9 (P,0.0002). As far as males used for The same type of treatment applied to pregnant fertilization are concerned, the variation is less heifers enabled them to produce also the same important and varies from 1.3 to 9.2 embryos number of embryos per week (Guyader-Joly et al., produced per session (P,0.001) (Twagiramungu et 1997) without disturbing the process of gestation al., 1999).
(Table 3). According to Hagemann et al. (1999), the size of
The period in between two collections may also be the picked up follicles can also be another factor, two, three or four weeks (Bousquet et al., 1999). The given that the number of embryos produced from use of ovarian stimulation on nonfertile females also oocytes obtained from follicles from 3 to 5 mm in enables one to increase the number of oocytes picked diameter is significantly higher than the one from up (8.6 compared to 6.2) as well as the number of follicles from more than 6 mm.
embryos produced per session (1.38 vs. 0.96) Another influential factor may also be the system (Looney et al., 1994). of cultivation. Indeed, the number of embryos pro-One of the benefits of using the ovarian stimulat- duced for each session varies from 1.05 after ‘‘co-ing treatments is the reduction of the number of culture’’ with BRL cells to 1.28 after ‘‘culture’’ in sessions needed to produce a defined number of the SOF environment (P,0.001, more than 1000 embryos, helping thus to cut production costs by sessions analyzed, Merton and Mullaart, 1999). 42% (whenever females are used once a week after
stimulation, instead of twice a week without
treat-ment). 3. Production from slaughterhouse ovaries
2.1.2. Protocols of production of in vitro embryos The gamete cells can also be picked up in the The conditions of both in vitro maturation and slaughterhouse after evisceration, which enables one fertilization are almost similar between the different to access the ovaries easily. In this case, ovaries are teams (Table 4). The in vitro maturation environ- transported to the lab in phosphate buffer saline ment widely used is the M199 mixed with fetal calf (PBS) kept under control at 258C. The cumulus– serum, hormones and growth factors if necessary. oocytes complex is taken out from 2 to 6 mm Most of the production teams use the Tyrode’s diameter follicles, and treated as for oocytes obtained medium containing albumin, lactate and pyruvate after OPU sessions.
(TALP) to perform the in vitro fertilization with the This operation can lead to the recovery of several presence of heparin (Ball et al., 1983; Parrish et al., tens of gamete cells for each ovary.
1986). However, the techniques of in vitro culture vary from co-cultivation with the cells of the oviduct
or with cell lines (Vero cells or Buffalo Rat Liver – 4. Sanitary risks associated with donor females
BRL) to cultivation in a semi-defined environment
such as the synthetic oviduct fluid (SOF) or possibly 4.1. Sanitary risks linked to the follicular and with the Rosenkrans’ medium CR1 or the potassium peri-oocyte environment
synthetic oviduct medium (KSOM). These are
per-formed under mineral oil (Takahashi and First, The risk is mainly linked to the oocytes
´
276 B. Guerin et al. / Livestock Production Science 62 (2000) 271 –285
includes the cells of the cumulus and those of be taken so as to be able to avoid contamination granulosa as well as the follicular liquid. Parallel to from one herd to another or within the same herd this, the hygiene of collection is also a risk factor, from one animal to another.
which one has to master.
The pick-up of oocytes is always associated with
the presence of cells and biological fluids, which 5. Hygienic rules and sanitary precautions
potentially present a risk for contamination. At the
time of collection, follicular fluid and cells from 5.1. Hygienic rules ovarian tissue are always mixed with cumulus and
granulosa cells. Rules to be followed for the collection of oocytes The contamination of all these cells is possible and by OPU have been described elsewhere (Nibart et has been demonstrated especially in the case of al., 1998). They are linked to material and equipment infection by bovine viral diarrhea virus, BVDV, that must be perfectly adapted and cleaned and (Avery et al., 1993; Bielanski and Dubuc, 1994; permanently ready to use.
Booth et al., 1992,1995) and by the infectious bovine Briefly these rules address the following: ´
rhinotracheitis (IBR) virus (Guerin et al., 1989,1997;
Bielanski et al., 1993). • Intra-vaginal probe, support and needle guide must be sterile
4.2. Sanitary risks associated with the oocytes • Needle must be disinfected and rinsed with a sterile physiological solution before use
The major risk is associated with the intrafollicular • No equipment should be ever used for more than contamination of the oocyte that theoretically can be one donor without disinfecting
either cellular or superficial by absorbing pathogenic • Collection liquid (PBS) unit must be confined to agents onto the zona pellucida. The oocyte intracel- one individual
lular contamination has only been suggested for C. • Technicians must be dressed with clean clothes fetus (Bielanski, 1994) within the frame of ex- and boots
perimental contamination, which remains rather dif- • Protection gloves must be disposable
ferent from the conditions of natural infection. • Filter membranes used for oocytes must be sterile BVDV and bovine herpes virus 1 (BHV-1) are and used only for one donor
undoubtedly the pathogenic agents most widely • Oocytes must be kept in sterile flask containing found in the oocytes’ environment (Bielanski et al., sterile medium
´
1993; Guerin et al., 1997). These viruses easily • Transportation of the oocytes to the lab must be adhere to the zona pellucida of the oocytes or the done in adequate conditions necessary to avoid
´
embryos (Singh et al., 1982; Guerin et al., 1989; any contamination. Bielanski and Dubuc, 1993).
5.2. Sanitary precautions 4.3. Sanitary risk associated with ovum pick-up
The health status of the herd of origin of the donor OPU is a very important step that must be handled cow is important. It should be free of contagious using sophisticated hygienic procedures. A contami- diseases and should comply with the sanitary stan-nation at this step can have a very detrimental effect dards of the country. At least, the herd must be free and lead to an important reduction in terms of of tuberculosis and brucellosis. The donor female embryo production. should also be free of clinical signs of contagious
The practical organization for in vitro derived diseases (Nibart et al., 1998).
Table 5
samplings, 2–3 weeks apart, the second one being
Percentage of hatched embryos after cultivation of frozen embryos
done at the time of the oocyte collection. In addition
in ethylene glycol in two different media (Guyader-Joly,
un-an aliquot of the follicular fluid cun-an be tested for published) relevant pathogens and particularly for IBR and
BSA ‘A’ substitute
BVD viruses.
Number of cultivated embryos 37 49 Number of hatched embryos 22 31
Percentage 59.5% 63.3%
6. Sanitary risks associated with the production of in vitro-derived embryos
oocyte–spermatozoa–co-culture cells system. So, for These risks are linked to the different steps example, complex culture media are now exclusively associated with the production of in vitro embryos: made from amino acids from a plant origin, and it maturation, fertilization, culture, storage and also has no detrimental effect on either the results of with the conditions applied to handle them, which fertilization or the in vitro development rates concerns material and culture media. (Guyader-Joly, unpublished Table 5).
Later on, the operations of transfer will also have The value of adding antibiotics to those media lies to be performed in the best conditions as far as in the fact that it removes the pathogens (S. agalac-hygiene and asepsis are concerned, by following the tiae, A. pyogenes, E. coli) that could accidentally be recommended classical guidelines very strictly associated with the oocytes or embryos during ovum (Mapletoft and Stookey, 1998). pickup or culture phases (Otoi et al., 1992a,b).
One has to take great care in preparation of those 6.1. Environmental factors additives to the media because it has been demon-strated that these products could easily be contami-Environmental factors will be of capital impor- nated especially by BVD, parainfluenza, blue tongue tance during all the steps in the making of in vitro- and herpes viruses (Rossi et al., 1980; Van Soom et
´
produced embryos. One will have to make sure that al., 1994; Guerin et al., 1997; Brock, 1998). How-labs, working area, incubators, and all devices in ever, the contamination of serum batches by BVD contact with embryos are perfectly clean and handled virus can be associated with development rates that with hygienic care. do not significantly differ from those obtained from
´
In specialized labs, individuals units are set aside virus free serum (Guerin et al., 1997). This fact for particular tasks with restricted access. The most stresses the basic necessity of the best quality serum. advanced set-up also contains a laminar flow The risk of contamination from non-conventional chamber with close attention to cleaning and dis- agents such as prion has also been thought of. The infecting procedures. conclusions of very complete analysis seems to indicate however that it is rather low (Wrathall,
6.2. Culture media 1999).
Some current research is looking at the technique They must be able to provide the physiological of substituting macromolecules (sodium hyaluronate, needs to the embryo so they will vary in their polyvinyl alcohol, polyvinylpyrrolidone, VF 5) hav-composition depending on whether they are used for ing surfactant activity to reduce the health risk maturation, fertilization, culture, or preservation of (Guyader-Joly et al., 1992; Palasz et al., 1993; Seidel the embryos. Usually, they contain products from et al., 1990; Palasz et al., 1995).
animal origin, especially bovine oestrus serum, fetal
calf serum, bovine serum albumin or growth factors, 6.3. Contamination of co-culture cells such as numerous amino acids of animal origin.
´
278 B. Guerin et al. / Livestock Production Science 62 (2000) 271 –285
belong to the oocyte’s donor (homologous system) or tion, culture) and subsequently lead to the contami-´
come from cows from slaughter (heterologous sys- nation of the embryo finally produced (Guerin et al.,
tem). 1992; Bielanski et al., 1992a,b,c). However, a
rel-The contamination of these cellular systems was evant treatment with hyaluronic acid during the shown for bacteria (Bielanski and Stewart, 1996) and preparation phase can reduce or definitely eradicate also for the BVD and BHV-1 viruses, which are this contamination (Bielanski et al., 1992a,b,c). frequently found in these conditions in Canada, the The use of bull sperm at this stage has to be United States or France (Booth et al., 1992,1995; associated with very strict sanitary quality as far as
´
Bielanski et al., 1993; Guerin et al., 1997). The rate well-identified pathogens are concerned.
of isolation appears to relate to the importance of the However, the semen also needs to be prepared disease in the individual countries. under strict aseptic conditions despite the fact that The use of cell lines (BRL or Vero), where the bull sperm is never sterile from a bacteriological controlling the absence of any viral, bacterial (among point of view. This is mainly due to:
which are the mycoplasmas) or fungi contamination
is easy to perform, enables one to reach the required • the multiple microorganisms found in the ejacu-quality level, and to operate in totally secured lates
disease free conditions. One might also consider • or an abnormal location in the genital tract getting rid of the co-culture phase by using SOF (accessory glands, testicles)
(totally synthetic medium) during this step. These • or their presence as normal contaminant in the SOF could be controlled as required and would urethra, preputial cavity or penis
ensure totally secure higher sanitary conditions. • or because they may be present in the environ-ment during the pick-up (collection room). 6.4. Sanitary risks associated with semen
The use of egg yolk (fresh egg or preparation The production of in vitro embryos involves a made from industrial egg yolk) frequently leads to fertilization step of the oocytes in extremely well the presence of bacterial species of avian origin in controlled conditions. Some experimental work has the sperm. The latter are not killed by the antibiotics
´
shown (Barlow et al., 1986; Guerin et al., 1992; usually added to the extenders (gentamicin, penicil-Bielanski and Loewen, 1994) that bulls persistently lin, streptomycin, linco-spectinomycin).
infected by BVDV shed virus in semen. Similar The sperm microflora is made up of three kinds of observations have been published for the BHV-1 and microorganisms: (1) the permanent pathogenic for many other pathogenic agents. Some viruses agents: in this group are found the pathogenic might even penetrate with the spermatozoa and species such as Brucella sp., Mycobacterium tuber-therefore induce contamination of the embryo during culosis and paratuberculosis, Campylobacter fetus the fertilization phase (Nussbaum et al., 1993). etc.) and some viruses (FMDV, BVDV, BHV-1, Some bacterial species such as Stenotrophomonas EBLV, BTV etc.) (Afshar and Eaglesome, 1990); (2) maltophilia, Pseudomonas putida, P. aeruginosa, A. the opportunistic pathogenic agents: in this group are calcoaceticus or Flavobacterium have been iden- classified the bacteria species called the opportunistic tified in some infertility cases based on in vitro pathogenic agents such as Escherichia coli, Staphy-embryos production (Stringfellow et al., 1997a,b; lococcus aureus, Streptococcus faecalis, Pseudo-Lee et al., 1997). monas aeruginosa, Haemophilus somnus etc. (Bar-As detailed above, the spermatozoa used for the in tlett et al., 1976; Wierzsbowski, 1981; Humphrey et
´
contamina-tion of the preputial cavity: Micrococcus, Staphylo- used to produce semen, bulls will be regularly and coccus, Corynebacterium sp., Enterobacteriaceae, individually tested at least every year for several
¨
Bacteroıdes sp., Clostridium etc., (Bonadonna, diseases such as tuberculosis, brucellosis, leucosis,
1971). IBR-IPV, Campylobacteriosis, Trichomoniasis.
From a bacteriological point of view, it is almost
impossible to have a totally sterile semen even if 6.4.2. Hygienic measures and lab best practices: some antibiotics are used in the extenders. These mastering the microbiological quality of semen antibiotics that some protocols require to add to the In order to produce high bacteriological quality fresh semen before dilution rather than adding it to semen, some measures are of fundamental impor-the ready made extenders, never have a 100% tance:
bactericidal activity on the bacterial flora of the
semen whatever bacterial species are considered. As • the hygiene of the bull and the bull pen floor a matter of fact, semen sterilization by addition of • the hygiene of the collection (cow, collection substances with antibacterial activity is an illusion. room, collector)
With regard to viruses, the antibiotics have no • the treatment of the semen (sterile glassware, high impact on them and it is therefore impossible to bacteriological quality extenders)
think about eradicating them or inactivating them • the clean state and hygiene of the lab and the
through this process. devices used to handle the semen
Because of this, mastering the health risk associ- • the staff, which has to be competent and trained ated with the semen has to be dealt with by good in hygiene and disinfecting techniques
management. Most of this is incorporated into the • working spaces (cleaned from a bacteriological International Sanitary Guidelines and especially in point of view)
the European document (EEC Directive, 1988; • the containers, which have to be cleaned and
Thibier, 1990b). regularly disinfected.
In order to improve the quality of the semen, some
centers have committed themselves to implementing The bacteriological quality of the liquid nitrogen a quality audit. One of its main objectives is to used is also one of the important parameters. To this reduce the contamination of the ecosystem associated extent, recycling nitrogen from one container to with the preparation of semen. Some measures, another must not be done.
which are among the lab’s best practices, are listed and are the foundation of the specification document
for semen production. 7. Interactions between pathogenic agents and in vitro derived embryos
6.4.1. Regulation measures: mastering the health
risks associated with transmissible infectious The fist significant successes regarding in vitro diseases derived embryo production date back from the late These measures are in accordance with the fun- 80s (Le Guienne and Thibier, 1988). This time gap damental principal of the careful selection of the compared with in vivo produced embryos explains source of young sires for AI. The young male has to why there are so few researches about the interac-be selected either from a herd free of disease or from tions between pathogenic agents and in vitro derived a dam free of disease. It also has to be confirmed as embryos nowadays.
free of disease in its original herd before being The main principles previously set for in vivo-accepted as a donor sire. A batch of health tests will produced embryos may also be applied to in vitro be done before entering the collection center, over a derived embryos. One should not directly extrapolate period of isolation that may vary from 30 to 60 days from an agent to another or also from a species to depending on the regulations. This will help to another as rightly stated by Hare (1985).
´
280 B. Guerin et al. / Livestock Production Science 62 (2000) 271 –285
Table 6
´
In vitro fertilization, cleavage and development rates of embryos after use of BVDV infected semen (from Guerin et al., 1992)
Groups Non-infected semen Infected semen
a b c
Fertilization (%) 61 / 64 (95.3%) 61 / 81 (79%)
b c
Cleavage (%) 56 / 61 (91.8%) 47 / 64 (73.4%)
b c
Blastocyste / cleavage (%) 11 / 56 (19.6%) 1 / 47 (2.1%)
a
Estimated after observation of cleaved embryos or number of pronuclei after staining. b
Author, please supply footnote. c
Author, please supply footnote.
pathogenic agents than in vivo-derived embryos, After experimental infection of in vitro derived ´
mainly because of the differences in the structure of embryos by BHV-1 or BVDV (Guerin et al., 1990; the zona pellucida that allows the adsorption of Bielanski et al., 1993) or by Leptospira hardjo pathogenic agents. This peculiar characteristic is (Bielanski and Surujballi, 1996) embryos are con-very important when assessing the risks associated taminated by these agents. Moreover a very signifi-with these embryos (Shi and Wrathall, 1989). Avail- cant reduction in the number of transferable embryos able researches so far were done either on slaughter- occurred in these conditions with BVDV (Table 6) house ovaries from all coming cows, some of them (Hare, 1986; Bielanski and Hare, 1988; Allietta et being naturally infected with BVDV, or after an al., 1995; Bielanski and Dubuc, 1995; Stringfellow et experimental infection of donor cows. al., 1997a,b).
After experimental or natural infection by BHV-1 Nevertheless, in special conditions, it is very and a subsequent dexamethasone treatment, in vitro- interesting to note that embryos seem to have some derived embryos and oviductal cells are contami- noticeable antiviral activity especially directed
´
nated (Guerin et al., 1989, 1990; Bielanski and against BVDV (Bielanski and Loewen, 1994; Dubuc, 1994). Experimental contamination of the Zurovac et al., 1994; Bielanski and Dubuc, 1995; oocytes during the maturation phase also leads to a Palma et al., 1996) and for BHV-1 (Van Roose et al., contamination of the embryos (Bielanski et al., 1997). Similar activity is also observed after
ex-1987). perimental infection of donor cows with BHV-1
´
These surveys have clearly shown that the sanitary (Guerin et al., 1997, unpublished).
control of the different phases of embryo production This particular effect and the low number of viral could be wisely done on maturation and culture particles surrounding embryos could explain why the fluids. It has also proven that such a control has a BVD is not transmitted after embryo transfer of in major interest as much for the virus as for bacteria vitro derived embryos collected from viremic donors (Stringfellow et al., 1983; Brock et al., 1991; Bielan- (Van Soom et al., 1994).
ski et al., 1994) since there seems to be no efficient treatment (equivalent to the one using trypsin for in
vivo produced embryos) on in vitro derived embryos 8. Recommendations associated with sanitary
(Avery et al., 1993; Tsuboi and Imada, 1996; Bielan- guarantees linked to the production of in vitro
ski et al., 1997). derived embryos
Sanitary washes recommended for in vivo
technical recommendations aimed to define interna- laboratory as it has been done in France for 10 ´
tional rules related to handling and production of in years in cattle (Thibier and Guerin, 1993). vitro-produced embryos. Two chapters are especially
linked with transmission of diseases through in vitro- 8.2. Sanitary status of donor animals produced embryos (Bielanski, 1998) and sanitary
rules (Nibart et al., 1998). In addition, one specific Two distinct cases must be here considered, appendix to the OIE International Animal Health according to the origin of the oocytes: slaughter-Code (4.2.2.5.) has been approved giving guidelines house ovaries vs. OPU. In the first case, the sanitary to the Chief Veterinary Officers (OIE, 1992). status of the herd of origin must be known at the Application of these rules is targeted to facilitate latest before transfer so that embryos could be international trade of these embryos in association destroyed if all necessary guarantees are not pro-with a very high level of sanitary guarantees. vided. Since such productions are often conducted in
several batches of embryos obviously not coming from a single cow, a precise identification of the 8.1. The concept of officially approved embryo
group of donor cows for a given batch of embryos is production teams
required so that tracability can be perfectly estab-lished and used if required. If oocytes are obtained One of the main principles is to authorize embryo
from well-known donor animals, it is easy to get production only through a team of well-trained
guarantees on the sanitary status of the herd of origin technicians and veterinarians that must be officially
so that animals can be submitted to complementary approved by a veterinary authority (Thibier, 1993).
sanitary controls. This system has been first set up for bovine in
vivo-derived embryo transfer and is completely
8.3. Washing procedures adaptable to in vitro embryo production. Given the
peculiar characteristics of in vitro-produced embryos,
During cultivation that must be conducted under distinct authorizations would be required if a single
sterile conditions, saprophytic bacteria that can at-team wishes to have both activities approved
simul-tach to the zona pellucida can accidentally contami-taneously. The system has been accepted worldwide
nate media. The 10 sanitary washes done in PBS and by IETS, OIE and by the European Union as well.
the 100-fold dilution in each bath will reduce these Four conditions are needed for a team to be officially
bacterial problems (IETS Manual, 1998). Trypsin approved by the veterinary authorities:
can be added although not mandatory because of the lack of data showing its efficiency in the in vitro • the team should be supervised by a well-trained
systems. Choice and composition of media especially veterinarian upon sanitary and technical
proce-in term of products of animal origproce-in should also be dures to produce such embryos
particularly supervised to avoid unexpected contami-• the team should be able to work in satisfactory
nation. conditions both in terms of housing and
equip-ment, especially those required for the laboratory
8.4. Sanitary control of media and co-culture cells where oocytes are treated and embryos produced.
• the team should commit itself to strictly follow
The sanitary controls that must be applied to the procedures mentioned above (IETS Manual,
media and cells are fully described in the IETS 1998)
Manual. Briefly: • the team should be regularly submitted to the
inspection of the veterinarian authorities and be
• when used for in vitro embryo production, all also submitted to sanitary controls of the
degener-biological products must be strictly controlled and ated or non-fertilized embryos, maturation and
guaranteed free from microorganisms (virus, bac-flushed fluids stored for this purpose. This
´
282 B. Guerin et al. / Livestock Production Science 62 (2000) 271 –285
useful for cell cultures used during the co-cultiva- 9. Conclusion
tion phase (Fray et al., 1995).
• sera must be free from antibodies and be checked In vitro embryo production is a recent technique before use (IETS Manual, 1998). They must be that offers a lot of opportunities in terms of increas-heated at 568C during at least 30 min and be kept ing the value of breeding production or selection in under freezing conditions (T# 2188C). cattle. The rate of embryos produced from slaughter-• permanent control of the inactivation procedures house oocytes or from OPU oocytes are now equiva-used to inactivate pathogens throughout fabrica- lent. It allows this biotechnology to be more widely tion: heating at 658C for at least 3 h, gamma used especially for pregnant cows with high genetic irradiation at 2.5 mR, pH 5 treatment for 2 h, value, for infertile cows or for endangered breeds. membrane filtration (0.22 mm). One of the major interests is also to use different • a control of the batches at the end of their bulls for fertilization of the oocytes collected from a
production (especially for their bacterial sterility) single cow.
• addition of antibiotics is required even though an Success in in vitro embryo production depends on entire bactericidal action is impossible to achieve the ability to perform series of technical steps, with (gentamicin 25–50 mg / ml or kanamycin 50 mg / unrelenting attention to detail, while minimizing ml) (Bielanski and Surujballi, 1996; Bielanski factors reported to have a negative effect on the and Stewart, 1996) outcome. Sound sanitary and hygienic measures at all stages are vital to ensure success. The rules Other associated measures lead to set up precise associated with in vitro-derived embryo production rules in terms of importation for blood products of are connected with technical competencies, official cattle origin (EEC directive 96 / 405) or to authorize agreement and veterinary supervision of the team, a importation only from free countries (New Zealand, perfect risk assessment depending on a lot of sci-Canada) and under the condition that these products entific studies targeted to improve the sanitary have been conveniently treated so that all guarantees guarantees associated with this technique.
related to the absence of pathogens of cattle origin A tight compliance with the rules published in the can be provided. IETS Manual allows all users worldwide to be really Only a strict compliance with the rules recom- efficient and to work with a high level of guarantee, mended worldwide (IETS Manual, 1998; OIE, 1998) which has no equivalent in the world of breeding and would allow users to have all sanitary guarantees animal reproduction because all steps are rigorously associated with the production and use of in vitro controlled and performed by highly technical and derived embryos. well-trained technicians working in well-equipped
laboratories and clean laboratory environment. 8.5. Official recommendations The guarantees associated with in vitro-produced
embryos are higher than for in vivo-derived em-In vitro embryo production procedures have been bryos. Such a comparison allows considering that the ruled by the European Union in 1994. Rules and risk of transmission of infectious diseases through technical procedures that must be complied with are this biotechnology is close to zero. The fundamental clearly defined and rely on the rules published by concept of ‘‘the safest way to exchange genetic from OIE and IETS. European countries have to organize a sanitary point of view’’ can be then extended to in agreement of the officially authorized teams under vitro-produced embryos. In the future, an increase in strictly controlled conditions above mentioned. the use of in vitro-produced embryos can be pre-Hundreds of embryos are produced and traded dicted and will likely be associated with deep throughout the world in strong sanitary security and freezing which is up to date, the limiting factor of total reliability in terms of control of epidemiological the development of international trade of such
Bielanski, A., Dubuc, C., Hare, W.C.D., 1992b. Failure to remove
References
bovine diarrhea virus (BVDV) from bull semen by swim-up and other separatory sperm techniques associated with in vitro Afshar, A., Eaglesome, M.D., 1990. Viruses associated with fertilization. Reprod. Dom. Anim. 27, 303–306.
bovine semen. Vet. Bull. 60, 93–109. Bielanski, A., Dubuc, C., 1994. In vitro fertilization and culture of ´
Allietta, M., Guerin, B., Marquant-Le Guienne, B., Thibier, M., ova from heifers infected with bovine herpesvirus-1 (BHV1). 1995. The effect of neutralization of BVD/ MD virus present in Theriogenology 41, 1211–1217.
bovine semen of IVF and development of bovine embryos. Bielanski, A., Dubuc, C., Hare, W.C.D., Myers, D.J., Eaglesome, Theriogenology 43, 156, Abstract. M.D., 1992. Inactivation of bovine herpesvirus- I and bovine Avery, B., Greve, T., Ronsholt, L., Botner, A., 1993. Virus diarrhea virus in association with preimplantation bovine screening of a bovine in vitro embryo production system. Vet. embryos using photosensitive agents. Theriogenology 38, 663–
Rec. 132, 660. 664.
Ball, G.D., Leibfried, M.L., Lenz, E.W., Ax, R.L., Bavister, B.D., Bielanski, A., Eaglesome, M.D., Ruhnke, L H., Hare, W.C.D., First, N.L., 1983. Factors affecting successful in vitro fertiliza- 1989. Isolation of Mycoplasma bovis from intact and microin-tion of bovine follicular oocytes. Biol. Reprod. 28, 717–725. jected preimplantation bovine embryos washed or treated with Barlow, R.M., Nettleton, P.F., Gardiner, A.C., Greig, A., Camp- trypsin or antibiotics. J. in vitro Fert. Emb. Trans. 6, 236–241. bell, J.R., Bonn, J.M., 1986. Persistent bovine virus diarrhea Bielanski, A., Jordan, L., 1996. Washing or washing and trypsin virus infection in a bull. Vet. Rec. 118, 321–324. treatment is ineffective for removal of non-cytopathic bovine Bartlett, D.E., Larson, L.L., Parker, W.G., Howard, T.H., 1976. viral diarrhea virus from bovine oocytes or embryos after Specific pathogen free (SPF) frozen bovine semen: A goal. In: experimental viral contamination of in vitro fertilization sys-Proc. 6th NAAB Technical Conference on AI and Reproduc- tem. Theriogenology 46, 1467–1476.
tion, pp. 11–22. Bielanski, A., Singh, E.L., Hare, W.C.D., 1987. The in vitro Bielanski, A., Dubuc, C., Hare, W.C.D., 1992a. Failure to remove exposure to bovine rhinotracheitis virus of zona pellucida – bovine diarrhea virus BVDV from bull semen by swim up and micromanipulated bovine embryos with the zona pellucida other separatory sperm techniques associated with in vitro damaged or removed. Theriogenology 28, 495–501. fertilization. Reprod. Dom. Anim. 27, 303–306. Bielanski, A., Surujballi, O., 1996. Association of Leptospira Bielanski, A., 1994. Effect of Campylobacter fetus on in vitro borgpetersenii serovar hardjo type hardjo bovis with embryos fertilization and early in vitro development of bovine embryos. produced by in vitro fertilization. Theriogenology 46, 45–55. Theriogenology 41, 163, Abstract. Bonadonna, T., 1971. Semen pollution and its sanitary and Bielanski, A., 1998. Potential for disease control or transmission technological significance. IId Jug. Symp. A. I. and Reprod.
by embryos produced in vitro: a review of current literature. In: Dom. Anim. 24–36.
Stringfellow, D.A., Seidel, S.M. (Eds.), Manual of the Interna- Booth, P.J., Stevens, D.A., Collins, M.E., Brownlie, J., 1992. tional Embryo Transfer Society, pp. 45–53, Chapter 3. Detection of bovine viral diarrhea virus in ovarian and oviduc-Bielanski, A., Hare, W.C.D., 1988. Effect in vitro of bovine viral tal tissue. J. Reprod. Fert. Abstract Series No. 9, July 1992, p.
diarrhea virus on bovine embryos with the zona pellucida 28, Abstract no. 42.
intact, damaged and removed. Vet. Res. Comm. 12, 19–24. Booth, P.J., Stevens, D.A., Collins, M.E., Brownlie, J., 1995. Bielanski, A., Stewart, B., 1996. Ubiquitous microbes isolated Detection of bovine viral diarrhea virus antigen and RNA in from in vitro fertilization IVF system. Theriogenology 45, 269, oviduct and granulosa cells of persistently infected cattle. J.
Abstract. Reprod. Fertility 105, 17–24.
Bielanski, A., Lutze-Wallace, T., Sapp, T., Jordan, L., 1997. The Bousquet, D., Twagiramungu, H., Morin, N., Carbonneau, G., efficacy of trypsin disinfection of in vitro fertilized bovine Durocher, J., 1999. In vitro embryo production in the cow: an embryos exposed to bovine herpesvirus 1. Anim. Reprod. Sc. effective alternative to the conventional embryo production
47, 1–8. approach. Theriogenology 51, 59–70.
Bielanski, A., Loewen, K., 1994. In vitro fertilization of bovine Brock, K.V., 1998. Quality control for materials of animal origin oocytes with semen from bulls persistently infected with used in embryo production and transfer. In: Manual of the bovine viral diarrhea virus. Anim. Reprod. Sci. 35, 183–189. International Embryo transfer society. Stringfellow, D.A., Bielanski, A., Loewen, K.S., Del Campo, M.R., Sirard, M.A., Seidel, S.M., (Eds.), 135-140.
Willadsen, S., 1993. Isolation of bovine herpesvirus-1 (BHV1) Brock, K.V., Redman, D.R., Vickers, M.L., Irvine, N.E., 1991. and bovine viral diarrhea virus BVDV in association with the Quantitation of bovine virus diarrhea virus in embryo transfer in vitro production of bovine embryos. Theriogenology 40, flush fluids collected from a persistently infected heifer. J. Vet.
531–538. Diagn. Invest. 3, 99–100.
´ Bielanski, A., Dubuc, C., 1993. In vitro fertilization of bovine Dauzier, L., Thibault, C., Winterberger, S., 1954. La fecondation
oocytes exposed to bovine herpesvirus-1 BHV-1. Reprod. Dom. in vitro de l’oeuf de lapine. C.R. Acad. Sci., Paris 238,
Anim. 28, 285–288. 844–855.
´
284 B. Guerin et al. / Livestock Production Science 62 (2000) 271 –285
EEC Directive, 1988. EEC Council directive 88 / 407 laying down Choi, Sh., Yang, B.C., Kim, K.N., Jung, S.C., 1997. Identifica-the animal health requirements applicable to intra-Community tion of bacteria derived from frozen bovine semen that resulted trade in and imports of semen of domestic animals of the in contamination during in vitro fertilization. Theriogenology bovine species. OJEC L94 / 10-L94 / 21. 46, 375, Abstract.
Fray, M.D., Jenner, L.J., Prentice, H., Ross, J., Brownlie, J., 1995. Looney, C., Lindsey, B.R., Gonseth, C.L., Johnson, D.L., 1994. Cryopreserved granulosa cells support the development of in Commercial aspects of oocyte retrieval and in vitro fertilization vitro produced bovine (IVP) embryos. J. Reprod. Fert. Ab- IVF for embryo production in problem cows. Theriogenology
stract. Ser. 15, 210. 41, 67–72.
Fry, C., 1999. Personal communication Stringfellow, D.A., Seidel, S.M. (Eds.), 1998. Manual of the ´
Guerin, B., Le Guienne, B., Chaffaux, St., Harlay, T., Allietta, M., International Embryo Transfer Society, 3rd Edition, p. 170. Thibier, M., 1989. Contamination des ovocytes et des em- Mapletoft, R.J., Stookey, J.M., 1998. General sanitary procedures
´ ´ ` ´
bryons fecondes in vitro apres infection experimentale de and welfare considerations associated with in-vivo production vaches donneuses par le virus Herpes Bovin de Type 1 (BHV of embryos. In: Stringfellow, D.A., Seidel, S.M. (Eds.), Manual
´ ´
1). Rec. Med. Vet. 165, 827–833. of the International Embryo Transfer Society, pp. 55–66, ´
Guerin, B., Marquant-Le Guienne, B., Chaffaux, S., Allietta, M., Chapter 4.
Harlay, Th., Thibier, M., 1990. Effets de la contamination par Mermillod, P., Peynot, N., Lonergan, P., Khatir, H., Driancourt, ´
le BHV-1 sur la maturation et la fecondation in vitro des M.A., Renard, J.P., Heyman, Y., 1997. Developmental potential ´ ´
ovocytes de bovins. Rec Med. Vet. 166, 911–917. of oocytes collected from 8 to 15 day old unstimulated or FSH ´
Guerin, B., Chaffaux, S., Marquant-Le Guienne, B., Allietta, M., treated calves. Theriogenology 47, 294, Abstract.
Thibier, M., 1992. IVF and IV culture of bovine embryos using Merton, J.S., Mullaart, E., 1999. Comparison of the effect of two semen from a bull persistently infected with BVD. bovine in vitro culture systems, TCM 199 / FCS / BRL co-Theriogenology 37, 217, Abstract. culture and SOFaaBSA, on embryo production and pregnancy ´
Guerin, B., Nibart, M., Marquant-Le Guienne, B., Humblot, P., rate. Theriogenology 51, 248, Abstract.
1997. Sanitary risks related to embryo transfer in domestic Nibart, M., Marquant-Le Guienne, B., Humblot, P., 1998. General species. Theriogenology 47, 33–42. sanitary procedures associated with in vitro production of Guyader-Joly, C., Charbonnier, G., Durand, M., Marquant-Le embryos. In: Stringfellow, D.A., Seidel, S.M. (Eds.), Manual of
´
Guienne, B., Thibier, M., 1992. Taux de gestation apres the International Embryo Transfer Society, pp. 67–77, Chapter ´
transfert dembryons bovins produits in vitro sur des genisses 5.
asynchrones. El. et Ins. 250, 1–4. Nussbaum, O., Laster, J., Loyter, A., 1993. Fusion of enveloped Guyader-Joly, C., Ponchon, S., Thuard, J.M., Durand, M., Nibart, viruses with sperm cells: Interaction of Sendai, Influenza and M., Marquant-Le Guienne, B., Humblot, P., 1997. Effects of Semliki Forest viruses with bull spermatozoa. Exp. Cell. Res. superovulation on repeated ultrasound guided oocyte collection 206, 11–15.
and in vitro production in pregnant heifers. Theriogenology 47, OIE, 1998. International Animal Health Code; OIE Publisher,
157, Abstract. Paris, France, 452 pp.
Hagemann, L.J., McMillan, W.H., Tervit, H.R., 1999. Analysis of Otoi, T., Tachikawa, S., Kondo, S., Suzuki, T., 1992a. Effect of potential production of transferable quality IVP bovine em- antibiotic treatment of in vitro fertilized bovine embryos to bryos: an OPU perspective. Theriogenology 51, 434, Abstract. remove adhering bacteria. J. Vet. Med. Sci. 54, 763–765. Hare, W.C.D., 1985. Diseases transmissible by semen and embryo Otoi, T., Tachikawa, S., Kondo, S., Suzuki, T., 1992b. Effect of
transfer techniques. OIE serie technique no. 4, Paris; 119 pp. exposure of bovine immature oocytes to bacteria on in vitro Hare, W.C.D., 1986. BVD virus infection and embryo transfer. Vet. development capacity. J. Reprod. Develop. 38, 61–66.
Rec. 10 May: 544. Palasz, A.T., Alkemade, S., Mapletoft, R.J., 1993. Sodium hy-Hasler, J.F., Henderson, W.G., Hurtgen, P.J., Jin, Z.Q., McCauley, aluronate as a substitute for biological proteins in bovine and
A.D., Mower, S.A., Neely, B., Shuey, L.S., Stokes, J.E., mouse embryo freezing. Cryobiology 30, 172–178.
Trimmer, S.A., 1995. Production, freezing and transfer of Palasz, A.T., Tornesi, M.B., Archer, J., Mapletoft, R.J., 1995. bovine IVF embryos and subsequent calving results. Media alternatives for the collection, culture and freezing of Theriogenology 43, 141–152. mouse and cattle embryos. Theriogenology 44, 705–714. Humphrey, J.D., Little, P.B., Barnum, D.A., Doig, P.A., Stephens, Palma, G.A., Modl, J., Wolf, G., Beer, M., Brem, G., 1996. Effect
L.R., Thorsen, J., 1982. Occurrence of Haemophilus somnus in of non-cytopathic bovine viral diarrhea virus on the develop-bovine semen and in the prepuce of bulls and steers. Can. J. ment of in vitro produced bovine embryos. In: Proceed. 13th Comp. Med. 46, 215–217. ICAR meeting, Sydney, Australia, Vol. 2, pp. 13–G.
´
Lacaze, S., Marquant-Le Guienne, B., Delalleau, N., Richet, S., Parez, M., Guerin, B., 1983. Sanitary and hygienic procedures for Maunas, S., Nibart, M., Humblot, P., 1997. Centralized in vitro controlling ubiquitous microflora. In: Proc. Int. Symp. Microb. embryo production after ultrasound guided bovine oocyte Tests for the International Exchange of Animal Genetic collection: effects of parity and superovulation treatment. Material, Stalheim Edition, pp. 43–49.
Theriogenology 47, 161, Abstract. Parrish, J.J., Susko-Parrish, J.L., Leibfried-Rutledge, M.L., Le Guienne, B., Thibier, M., 1988. Premiers blastocystes bovins Critser, E.S., Eyestone, W.H., First, N.L., 1986. Bovine in vitro
´
Rossi, C.R., Brigman, C.R., Kiesel, G.K., 1980. Viral contamina- Thibier, M., 1993. Health surveillance of the transfer of bovine tion of bovine lung cultures and bovine fetal serum. Am. J. Vet. embryos fertilized in vitro. Rev. Sci. Techn. Off. Int. Epiz. 12,
Res. 41, 1680–1681. 773–787.
Seidel, Jr. G.E., Elsden, R.P., Brink, Z., 1990. Cryopreservation of Thibier, M., Guerin, B., 1993. La securite sanitaire dans le´ ´ ´ bovine embryos in media with chemically defined macro- transfert d’embryons bovins collectes in vivo, fecondes in vitro´ ´ ´ molecules. Theriogenology 33, 322, Abstract. ou clones. Bull. Acad. Vet. de France. 70, 183–194.´ ´ Shi, S., Wrathall, A.E., 1989. The importance of the zona Trachte, E.A., Stringfellow, D.A., Riddell, K.P., Gallik, P.K.,
pellucida for disease control in livestock by embryo transfer. Riddell, M.G., Wright, J.C., 1997. Washing and trypsin treat-Br. Vet. J. 145, 129–140. ment of IVF embryos exposed to bovine viral diarrhea virus. Singh, E.L., Thomas, F.C., Papp-Vid, G., Eaglesome, M.D., Hare, Theriogenology 47, 383.
W.C.D., 1982. Embryo transfer as a mean of controlling the Tsuboi, T., Imada, T., 1996. Non cytopathogenic and cyto-transmission of viral infections II: The in vitro exposure of pathogenic bovine viral diarrhea – mucosal disease viruses do pre-implantation bovine embryos to infectious bovine rhinot- not affect in vitro development into the blastocyst stage. Vet. racheites virus. Theriogenology 18, 133–140. Microb. 49, 127–134.
Stringfellow, D.A., Riddell, K.P., Brock, K.V., Riddell, M.G., Twagiramungu, H., Morin, N., Brisson, C., Carbonneau, G., Galik, P.K., Wright, J.C., Hasler, J.F., 1997a. In vitro fertiliza- Durocher, J., Bousquet, D., 1999. Animal factors that influence tion and in vitro culture of bovine embryos in the presence of the in vitro production of bovine embryos. Theriogenology 51, non-cytopathic bovine diarrhea virus. Theriogenology 48, 171– 334, Abstract.
183. Van Roose, G., Nauwynck, H., Van Soom, A., Vanopdenbosch, E.,
Stringfellow, D.A., Scanlan, C.M., Hannon, S.J., Panagala, V.S., de Kruif, A., 1997. Susceptibility of zona-intact and zona-free Gray, B.W., Galik, P.A., 1983. Culture of uterine flushings, in vitro-produced bovine embryos at different stages of de-cervical mucus, and udder secretions collected post-abortion velopment to infection with bovine herpesvirus-1. Theriogenol-from heifers artificially exposed to Brucella abortus. ogy 47, 1389–1402.
Theriogenology 20, 77–83. Van Soom, A., Van Opdendosch, E., Mahmoudzadeh de Kruif, A., Stringfellow, J.S., Hathcock, T.L., Riddell, K.P., Stringfellow, 1994. In vitro production of Belgium blue cattle embryos: D.A., Galik, P.K., Riddell, M.G., Carsoon, R.L., 1997b. Intro- Some data on the risk of viral infections. Vlaams Diergeneeskd duction of Stenomonas maltophilia through semen used for in Tijdschr. 63, 139–145.
vitro production of bovine embryos. Theriogenology 46, 382, Wierzsbowski, S., 1981. Bull semen opportunistic pathogen and
Abstract. ubiquitary microflora. Diseases Control in Semen and
Em-Takahashi, Y., First, N.L., 1992. In vitro development of bovine bryos. 23rd FAO Meeting, 21–28.
embryos: influence of glucose, lactate, pyruvate, amino acids Wrathall, A.E., 1999. Risks of transmission of Spongiform and vitamins. Theriogenology 37, 963–978. Encephalopathies by reproductive technologies in domesticated Thibier, M., 1990a. Le transfert embryonnaire: le moyen le plus ruminants. EAAP Zurich 1999, Abstract book, p 120.
e
ˆ ´
sur, au plan sanitaire, d’echanges de genes. In: Actes 6 Yang, X., Presicce, G.A., Du, F., Jiang, S., 1997. Pregnancies and
´ ´
reunion de l’Association europeenne de transfert embryonnaire calves derived from prepuberal calf oocytes. Theriogenology ´
(AETE), Fondation Marcel-Merieux, Lyon, France, pp. 67–81. 47, 163, (Abstract).