Bundit Tengjaroenkul, Bonnie J. Smith
), Thomas Caceci,
Stephen A. Smith
Department of Biomedical Sciences and Pathobiology, Virginia – Maryland Regional, College of Veterinary Medicine, Virginia Polytechnic Institute and State UniÕersity, Blacksburg, VA, 24061, USA
Accepted 19 July 1999
Abstract
Regional distribution of intestinal enzymes in Nile tilapia was investigated using enzyme histochemistry. Samples from adult fish were obtained from the five major intestinal segments. Activities of maltase, leucine aminopeptidase, dipeptidyl aminopeptidase IV, lipase, non-specific esterases, and alkaline phosphatase were examined in each segment. All enzymes were present at specific sites along the first four intestinal segments. Strong reaction for maltase was present in the third intestinal segment, while aminopeptidases and alkaline phosphatase were detected in the first three parts. The most intense activity for lipase was present in the first two parts, while non-specific esterases were observed in the first four portions. Activities of all these enzymes were demonstrated in the brush border. Non-specific esterases were also present in the cytoplasm of the enterocytes. In addition to its brush border localization in the cranial segments, dipeptidyl aminopeptidase IV was also observed in the basal lamina of all segments, including the terminal segment. These results suggest that the first four regions play the most important role in both digestion and absorption of nutrients. In addition, the great length of the portions of the intestinal tract participating in digestion provides abundant surface area for nutrient absorption, and is likely one factor involved in the rapid growth rate characteristic of tilapian fish.q2000 Elsevier Science
B.V. All rights reserved.
Keywords: Oreochromis niloticus; Tilapia; Fish; Digestion; Intestine; Enzyme; Nutrition
)Corresponding author. Tel.:q1-540-231-9024; fax:q1-540-231-7367; E-mail: [email protected]
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
1. Introduction
As in other vertebrates, the ability of fish to utilize ingested nutrients depends on the presence of appropriate enzymes in appropriate locations in the wall and along the lumen of the intestinal tract. Generally, distribution and intensity of intestinal enzyme
Ž
activity along the gut varies with feeding habits and intestinal morphology Cockson and Bourne, 1972; Hofer and Schiemer, 1981; Kuz’mina, 1984; Kuz’mina and Smirnova,
.
1992; Sabapathy and Teo, 1993 . Though tilapian fish have been categorized as herbivorous, many are well-known for their ability to utilize a wide variety of foods
Ž including aquatic larvae and insects as well as algae, weeds and macrophytes
Lowe-.
McConnell, 1975; Bowen, 1982; Trewavas, 1983 . Various intestinal enzymes involved in digestive and absorptive processes have been reported in tilapian fish, such as
Ž
amylase, pepsin, trypsin, esterases and alkaline phosphatase Nagase, 1964; Cockson .
and Bourne, 1972; Moriarty, 1973; Klaren et al., 1993; Li and Fan, 1997 . As with other herbivorous fish, tilapia demonstrate greater activity of carbohydrase than protease and a
Ž
lesser lipase activity compared to carnivorous and omnivorous fish Fish, 1960; Agrawal .
et al., 1975; Das and Tripathi, 1991; Opuszynski and Shireman, 1995 .
Tilapia enjoy worldwide prominence in foodfish aquaculture, and are also growing in importance as a laboratory animal. Recently, their complex gross intestinal morphology has been described as being composed of five major segments, a notable difference from
Ž .
other cultured fish Smith et al., in press . To date, neither the distribution along the intestinal tract nor the cellular location of their intestinal enzymes has been completely characterized. The present study began this characterization by using enzyme histochem-istry to investigate the occurrence, distribution and cellular localization of selected intestinal enzymes in the Nile tilapia.
2. Materials and methods
2.1. Preparation of tissue sections
Six mature Nile tilapia, Oreochromis niloticus, age 10–12 months, were obtained from the same brood at the Aquatic Medicine Laboratory of the Virginia–Maryland Regional College of Veterinary Medicine at Virginia Polytechnic Institute and State
Ž .
University. The fish were 15.7–18.1 cm mean 16.95"S.D. 0.81 cm in total length
Ž .
and weighed 72–97 g mean 83.33"S.D. 9.05 g . Tilapia were fed two times a day
Ž .
with a commercial fish feed Koi pond nuggets, PMI Feeds, St. Louis, MO . At the time Ž
of tissue collection, fish were anesthetized with tricaine methanesulfonate MS-222, .
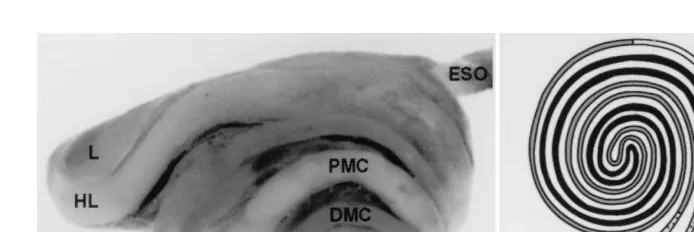
Sigma, St. Louis, MO and then killed by cervical separation. Intestinal samples were obtained from the five specific segments: the hepatic loop, proximal major coil, gastric loop, distal major coil, and the mid-portion of terminal part well proximal to the rectum ŽFig. 1. ŽSmith et al., in press . All tissue specimens were embedded in optimal critical.
Ž .
Ž . Ž . Ž . Ž .
Fig. 1. Photomicrograph right view A and schematic drawing ventral view B of the five intestinal segments. HL, hepatic loop; PMC, proximal major coil; GL, gastric loop; DMC, distal major coil; TP, terminal portion of the intestine.
2.2. Chemicals
Substrates, diazonium salts and conditions of incubation media for the enzyme studies are summarized in Table 1. All chemicals in this study were obtained from Sigma. The buffers for each enzyme were specifically prepared as follows: 0.1 M citric acid-phosphate buffer for maltase; 0.1 M phosphate buffer for leucine aminopeptidase, dipeptidyl aminopeptidase IV, lipase, and non-specific esterases; and 0.1 M Tris buffer for intestinal alkaline phosphatase.
2.3. Fixation, staining and enzyme examination
Before incubation in media, fresh cryostat sections intended for study of maltase ŽMalt , lipase Lip , non-specific esterases NSE and intestinal alkaline phosphatase. Ž . Ž . ŽIAP were fixed for 5 min in 10% neutral buffered formalin. For detection of leucine.
Ž . Ž .
aminopeptidase LAP and dipeptidyl aminopeptidase IV DAP IV , tissue sections were fixed for 1 min in equal parts of cold absolute acetone and chloroform. Preparation of incubation media and procedures for the enzyme examinations followed established
Ž
techniques using substituted naphthol methods Lojda et al., 1979; Bancroft and Hand,
Table 1
Summary of incubation techniques for enzyme histochemistry M-2-NU
: Methoxy-2-naphthylamide; Malt, maltase; LAP, leucine aminopeptidase; DAP IV, dipeptidyl aminopeptidase IV; Lip, lipase; NSE, non-specific esterases; IAP, intestinal alkaline phosphatase.
Ž .
Enzyme Substrate Diazonium salt pH Time min
Malt b-naphthyl-a-glucoside Pararosaniline 6.9 60 U
LAP L-Leucyl-4-M-2-N Fast Blue B 7.4 30 U
DAP IV Glycyl-prolyl-4-M-2-N Fast Blue BB 7.4 30
Lip a-naphthyl palmitate Pararosaniline 7.4 60
NSE Naphthol AS-D acetate Pararosaniline 7.4 10
.
1987; Knospe and Plendl, 1997 . After incubation, the tissue sections were coverslipped with mounting medium and examined under a light microscope. Enzyme activities were evaluated as strong, weak or absent depending on the staining intensity of the azo dye in
Ž .
the tissue Hirji and Courtney, 1982; Gawlicka et al., 1995 . Intestinal sections incubated without substrate were used as controls.
3. Results
Intestinal enzymes demonstrated a marked difference in regional distribution and
Ž .
localization along the intestinal length Table 2 . Activities of various enzymes were detected at characteristic sites along the first four intestinal segments. Most of these activities were localized along the brush border, but non-specific esterases were also present in the cytoplasm of the enterocytes, and DAP IV was also detected in the basal lamina of all segments.
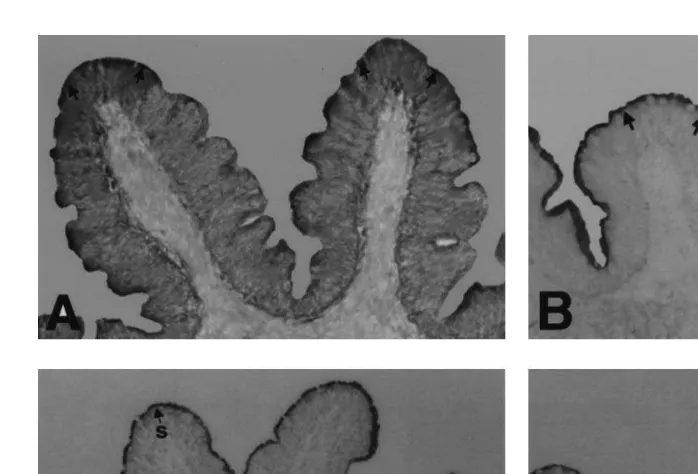
Ž Maltase activity was found in the brush border of the columnar epithelial cells Fig. .
2A in the first four intestinal segments, with the most intense staining observed in the third segment. Leucine aminopeptidase and DAP IV were detected in the microvilli of
Ž .
the enterocytes Fig. 2B and C, respectively in the first four intestinal segments. Both peptidases demonstrated stronger activities in the first three intestinal segments than in
Ž . the fourth. Weak DAP IV staining was also observed in the basal lamina Fig. 2C of all
Ž .
intestinal segments. Lipase activity was detected in the brush border Fig. 2D of the first three parts of the intestine, with the most intense enzyme reaction observed in the first two regions. Uniform non-specific esterase activity was found in the first four intestinal regions. The esterase reactions were present in the microvilli, and also in
Ž .
cytoplasm of the enterocytes Fig. 3A . Intestinal alkaline phosphatase activity was detected in the first four intestinal regions. The strongest reaction was observed in the first three intestinal segments, with a rapid decrease in intensity taking place at the
Table 2
Distribution and localization of the enzymes along the intestinal tract of the Nile tilapia
Ž . Ž . Ž .
Level of the staining intensity:qq strong ,q weak ,y absent . Intestinal segment
Ž . Ž .
Fig. 2. Intestinal sections showing enzyme staining arrows . A Maltase in the microvilli of the first major
Ž . Ž . Ž . Ž .
coil 66=. B Leucine aminopeptidase in the brush border of the second major coil 50=. C Dipeptidyl
Ž . Ž .
aminopeptidase IV with strong reaction s in the microvilli in comparison to the weaker intensity w in the
Ž . Ž .
basal lamina. Counterstained with hematoxylin 40=. D Lipase in the brush border of the first major coil
Ž40=..
transition from the third to the fourth segment. The enzyme reaction was localized in the
Ž .
brush border of the columnar epithelial cells Fig. 3B,C . Higher magnification also Ž demonstrated this enzyme activity in the supranuclear cytoplasm of the enterocytes Fig.
. 3C .
Ž . Ž .
Fig. 3. Intestinal sections showing enzyme staining arrows . A Non-specific esterases in the brush border
Ž . Ž .
and cytoplasm of the columnar epithelial cells of the gastric loop 100=. B Alkaline phosphatase in the
Ž . Ž . Ž .
microvilli of the gastric loop 40=. C Alkaline phosphatase in the brush border in white and supranuclear
Ž . Ž .
4. Discussion
Digestion and absorption of food particles and molecules generally takes place along the brush border of the columnar epithelial cells, where numerous digestive and absorptive enzymes are localized. Examples of such enzymes include maltase,
dipepti-Ž
dases, lipase and alkaline phosphatase Hirji and Courtney, 1982; Kuz’mina and .
Gelman, 1997 . In the Nile tilapia as in other teleost fish, these enzymes are variously distributed along the length of the intestine.
4.1. Maltase
Teleost fish generally absorb carbohydrates in the form of monosaccharides, with Ž
herbivorous fish typically relying more on this pathway than carnivorous fish Budding-.
ton et al., 1987 . Because maltase hydrolyses the disaccharide maltose to produce the monosaccharide glucose, the result of this study showing the greatest maltase activity occurring in the gastric loop suggests that the middle intestinal region is the most active region in formation of glucose. The resulting glucose may be absorbed there andror more distally along the intestinal tract. Interestingly, the distribution of maltase activity observed in the present work corresponds well with the earlier work on amylase, the
Ž .
enzyme that hydrolyzes maltose to glucose Stevens and Hume, 1995; Horn, 1998 . Ž .
Nagase 1964 reported the greatest amylase activity from the middle portion of the intestine of Tilapia mossambica, similar to that observed here in the Nile tilapia for maltase. A similar topographic distribution pattern of these two enzymes has plain functional significance: amylase would produce the substrate for maltase activity.
Variation in distribution of maltase activity along the intestinal length have been
Ž .
reported in numerous fish species. Bream Abramis brama demonstrated uniform
Ž .
enzyme reaction along the length of the intestinal tract Kuz’mina, 1985 , whereas Ž
rainbow trout showed greater activity in the cranial than in the caudal region Costanzo
. Ž .
et al., 1983 . Peak maltase activity in pike Esox lucius was present in the middle gut
Ž . Ž .
segment Kuz’mina, 1985 , while in the ayu or sweet-fish Plecoglossus altiÕelis
Ž .
maximal activity was observed in the caudal intestinal region Kawai and Ikeda, 1971 . Optimal pH for maltase activity has also been reported to vary among fish. For the Nile
Ž
tilapia, the optimum pH for this enzyme reaction has been reported as 6.0 Yamada et
. Ž
al., 1996 ; for bream and roach, 7.0 to 8.0, and for pike, 8.0 Kuz’mina and Nevalenny, .
1983 . Intestinal pH in the immediate post-gastric segment of tilapia has been reported
Ž .
as relatively low, about 5.5–6.0 Moriarty, 1973 , differing widely from the reported optimal pH of maltase activity at 6.9. Thus, weak maltase activity in the most cranial
Ž .
segment the hepatic loop of the tilapian intestine may relate at least in part to the pH of this segment.
4.2. Peptidases
Protein digestion in tilapia begins with the hydrolysis of proteins and polypeptides by Ž
the action of pepsin, trypsin and chymotrypsin Fish, 1960; Nagase, 1964; Cockson and .
Ž
the absorption of small peptides across the intestinal lumen and into the blood Kim and .
Erickson, 1985 . Previous studies have also demonstrated that di- and tripeptides are Ž
transported more efficiently than free amino acids Ash, 1980; Boge et al., 1981; .
Stevens and Hume, 1995 . Our results suggest that the hydrolysis of polypeptides into peptides and amino acids, as well as the absorption of short peptides in Nile tilapia, takes place mainly in the first three gut regions. These findings are in agreement with
Ž .
Bowen 1981 , who stated that proteins were completely digested and absorbed in the cranial half of the intestinal tract of T. mossambica.
The weak peptidase reactions in the fourth intestinal segment, and their absence from the brush border in the terminal segment, implies a comparatively smaller role of these regions in peptide hydrolysis. The lower enzyme activities here likely relate to the effect
Ž .
of pH changes along the intestinal tract Moriarty, 1973 , as well as the resorption of the
Ž .
enzymes into the gut mucosa Hofer and Schiemer, 1981 .
The distribution and intensity of peptidases observed in this study correlate well with Ž . the presence and activity of protease enzymes reported in some other tilapia. Fish 1960 reported greater protease activity in the cranial part than in the caudal part of the intestine in T. mossambica. Similarly, T. mossambica demonstrated a higher trypsin
Ž .
activity in the cranial than caudal intestinal regions Nagase, 1964 , while T. shirana Ž
showed pepsin and trypsin activities strictly in the cranial intestinal segment Cockson .
and Bourne, 1972 . Thus, peptidases are present in the same location as protease, where they can immediately act upon the short-chain peptides produced by the proteases.
Interspecific differences have been noted in localization of peptidase activities in the enterocytes. Similar to the results seen here in the Nile tilapia, aminopeptidases in other
Ž
teleost fish generally are present in the brush border Hirji and Courtney, 1982; Vonk .
and Western, 1984; Gawlicka et al., 1995 . However, leucine aminopeptidase in other vertebrates including fish has also been found in cytoplasmic organelles and vesicles of
Ž
the columnar epithelial cells Seligman et al., 1970; Overnell, 1973; Adibi and Kim, .
1981; Hirji and Courtney, 1982 . Complete digestion of peptide in those species apparently involves the enterocytic cytoplasm as well as the brush border.
4.3. Lipase
Early works on lipase activity in the intestinal tract of tilapia have reported varied
Ž . Ž .
results. Keddis 1957 and Moriarty 1973 found no lipase activity in the gut of T.
Ž . Ž .
nilotica. However, Al-Hussaini and Kholy 1953 and Nagase 1964 had previously
and middle parts of the intestine. These previous studies used tributyrin, an ester of butyric acid as the enzyme substrate. However, tributyrin is not specific for lipase, and
Ž
can also be hydrolyzed by esterases. Thus, those reports of lipase activity Al-Hussaini .
and Kholy, 1953; Nagase, 1964 are not conclusive. In our work,a-naphthyl palmitate was used as the substrate. This compound gives a more specific reaction and results in a
Ž .
better enzyme localization, as reported by Knospe and Plendl 1997 . Using this substance, our studies demonstrated positive lipase reactions in the first three gut segments. The staining was most intense in the first two parts, comprising 53.8% of the
Ž .
intestinal length Smith et al., in press . This suggests that lipolytic activity in this fish is indeed present, and occurs mainly in the cranial half of the intestinal tract. The relatively restricted distribution of lipase enzyme in the Nile tilapia concurs with previous reports
Ž .
that lipase activity is lowest in herbivorous fish Opuszynski and Shireman, 1995 , Ž
related to the low fat content in plant materials naturally consumed by tilapia Vonk and .
Western, 1984; Opuszynski and Shireman, 1995 . These earlier reports, together with the observations in this study of the restricted distribution of lipase along the gut length support a low fat level being more appropriate for tilapia diets.
4.4. Non-specific esterases
High levels of non-specific esterases have previously been reported in the intestine of
Ž .
tilapia Li and Fan, 1997 . The results of the present study concur with earlier works and localized esterase activity along the brush border. However, our studies also demon-strated non-specific esterase reactions in the cytoplasm of the enterocytes through the first four intestinal segments. This raises the possibility that lipid metabolism may involve events both intra- and extracellularly in the enterocytes of these gut regions ŽDeimling and Bocking, 1976; Wassmer et al., 1988; Van Lith et al., 1992 ..
4.5. Intestinal alkaline phosphatase
Intestinal alkaline phosphatase is considered to be involved in absorption of nutrients Ž
such as lipid, glucose, calcium and inorganic phosphate Malagelada et al., 1977; . Roubaty and Portmann, 1988; Harris, 1989; Dupuis et al., 1991; Mahmood et al., 1994 . Enzymes localized in the brush border are active in this regard. Detection of the enzyme in the supranuclear cytoplasm indicates the source of its production, which has been
Ž .
shown to be in the Golgi apparatus located in that cellular region Alpers et al., 1995 . The widespread distribution of alkaline phosphatase throughout the first four segments,
Ž .
comprising 93.6% of the intestinal length Smith et al., in press , demonstrates that absorption of numerous forms of nutrients can take place along a tremendous surface area. Furthermore, the general distribution of the enzyme observed in this study correlates well with the localization and intensity of maltase, lipase and peptidases,
Ž
including amylase and proteases, reported in other tilapia Fish, 1960; Nagase, 1964; .
Cockson and Bourne, 1972; Moriarty, 1973 . Thus, this absorptive enzyme is present at the same location as the digestive enzymes, permitting absorption of the smaller particles as soon as they are produced.
Though the greater length of the gut is active in this regard, the stronger IAP activity
Ž . Ž .
The conspicuous lack of extensive enzyme staining in the terminal segment is Ž noteworthy. Only DAP IV was detected here, where it was limited to the basal lamina a
.
site where it was also detected in all other segments . Previous work has suggested that DAP IV localized in the basal lamina is involved with metabolism of inherent cellular structural proteins such as collagen, rather than with digestive processes of foodstuffs ŽSakai and Kawatsu, 1983 . The lack of digestive enzymes in the terminal segment. suggests that this region is inactive in the breakdown of nutrients influenced by these enzymes. Also, the absence of alkaline phosphatase activity in the terminal segment further suggests that nutrient absorption is not an important function of this region. Other activities, such as resorption of electrolytes andror water, likely predominate here.
5. Conclusion
Ž
Results of this study suggest that the first four intestinal segments from the hepatic .
loop through the distal major coil are the major regions in which digestive and absorptive processes catalyzed by these enzymes occur. The first three segments appear to be the regions with the greatest participation in these functions. The decrease and loss of both digestive and absorptive enzyme activities in the distal major coil and terminal portion, respectively, suggest that other functions such as resorption of electrolytes and
Ž
water likely predominate here. The combination of intestinal length Hofer and Schiemer, .
1981; Sugita et al., 1985; Smith et al., in press together with wide distribution of intestinal enzymes along that length enhance the fish’s ability to utilize various food components, and may be one factor contributing to the rapid growth rate of this fish.
Acknowledgements
The authors would like to thank Sandy A. Brown for her assistance in fish culture.
References
Ž .
Agrawal, V.P., Sastry, K.V., Kaushab, S.K.S., 1975. Digestive enzymes of three teleost fishes. Acta Physiol. Acad. Sci. Hung. 46, 93–98.
Al-Hussaini, A.H., Kholy, A.A., 1953. On the functional morphology of the alimentary tract of some omnivorous teleost fish. Proc. Egypt. Acad. Sci. 4, 17–39.
Alpers, D.H., Zhang, Y., Ahnen, D.J., 1995. Synthesis and parallel secretion of rat intestinal alkaline phosphatase and a surfactant-like particle protein. Am. J. Physiol. 268, E1205–E1214.
Ž .
Ash, R., 1980. Hydrolytic capacity of the trout Salmo gairdneri intestinal mucosa with respect of three specific dipeptides. Comp. Biochem. Physiol. B 65, 173–176.
Bancroft, J.D., Hand, N.M., 1987. Enzyme Histochemistry. Oxford Univ. Press, New York, 70 pp.
Boge, G., Rigal, A., Peres, G., 1981. Rates of in vivo intestinal absorption of glycine and glycylglycine by
Ž .
rainbow trout Salmo gairdneri R. . Comp. Biochem. Physiol. A 69, 455–459.
Bowen, S.H., 1981. Digestion and assimilation of periphytic detrital aggregate by Tilapia mossambica. Trans. Am. Fish. Soc. 110, 239–245.
Bowen, S.H., 1982. Feeding, digestion and growth — Qualitative considerations. In: Pullin, R.S.V.,
Ž .
Lowe-McConnell, R.H. Eds. , The Biology and Culture of Tilapias. Proceedings of the International Conference on the Biology and Culture of Tilapias, 2–5 September, 1980, Bellagio, Italy, pp. 141–156. Buddington, R.K., Chen, J.W., Diamond, J., 1987. Genetic and phenotypic adaptation of intestinal nutrient
transport to diet in fish. J. Physiol. 393, 261–281.
Cockson, A., Bourne, D., 1972. Enzymes in the digestive tract of two species of euryhaline fish. Comp. Biochem. Physiol. A 41, 715–718.
Costanzo, G.D.I., Florentz, A., Leray, C., Nonnotte, L., 1983. Structural and functional organization of the brush border membrane in the rainbow trout intestine. Mol. Physiol. 4, 111–123.
Das, K.M., Tripathi, S.D., 1991. Studies on the digestive enzymes of grass carp, Ctenopharyngodon idella
ŽVal. . Aquaculture 92, 21–32..
Deimling, O.V., Bocking, A., 1976. Esterases in histochemistry and ultrahistochemistry. J. Histochem. 8, 215–252.
Dupuis, Y., Tardivel, S., Porembska, Z., Fournier, P., 1991. Effect of some alkaline phosphatase inhibitors on intestinal calcium transfer. Int. J. Biochem. 23, 175–180.
Fang, L.S., Chiou, S.F., 1989. Effect of salinity on the activities of digestive proteases from the tilapia fish, Oreochromis niloticus in different culture environments. Comp. Biochem. Physiol. A 93, 439–443. Fish, G.R., 1960. The comparative activity of some digestive enzymes in the alimentary canal of tilapia and
perch. Hydrobiology 15, 161–178.
Gawlicka, A., Teh, S.J., Hung, S.S.O., Hinton, D.E., de la Noue, J., 1995. Histological and histochemical changes in the digestive tract of white sturgeon larvae during ontogeny. Fish Physiol. Biochem. 14, 357–371.
Harris, H., 1989. The human alkaline phosphatases: what we know and what we don’t know. Clin. Chim. Acta 186, 133–150.
Hirji, K.N., Courtney, W.A.M., 1982. Leucine aminopepidase activity in the digestive tract of perch, Perca fluÕiatilis L. J. Fish Biol. 21, 615–622.
Hofer, R., Schiemer, F., 1981. Proteolytic activity in the digestive tract of several species of fish with different feeding habits. Oecologia 48, 342–345.
Ž .
Horn, M.H., 1998. Feeding and digestion. In: Evans, D.H. Ed. , The Physiology of Fishes. CRC Press LLC, New York, pp. 54–55.
Jobling, M., 1995. Environmental Biology of Fishes. Chapman & Hall, New York, 455 pp.
Kawai, S., Ikeda, S., 1971. Studies of digestive enzymes of fishes: I. Carbohydrases in digestive organ of several fishes. Bull. Jpn. Soc. Sci. Fish. 37, 333–337.
Keddis, M.N., 1957. On the intestinal enzymes of Tilapia niloticus. Boul. Proc. Egypt. Acad. Sci. 12, 21–37. Kim, Y.S., Erickson, R.H., 1985. Role of peptidases of the human small intestine in protein digestion.
Gastroenterology 88, 1071–1073.
Klaren, P.H.M., Flik, G., Lock, R.A.C., Wendelaar Bonga, S.E., 1993. Ca2q transport across intestinal brush border membranes of the cichlid teleost, Oreochromis mossambicus. J. Membr. Biol. 132, 157–166. Knospe, C., Plendl, J., 1997. Histochemical demonstration of lipase activity in the gastric mucosa of the cat.
Anat. Histol. Embryol. 26, 303–304.
Lowe-McConnell, R.H., 1975. Fish Communities in Tropical Freshwater. Longman, New York, 337 pp. Mahmood, A., Yamagishi, F., Eliakim, R., DeSchryver-Kecskemeti, K., Gramlich, T.L., Alpers, D.H., 1994. A
possible role for rat intestinal surfactant-like particles in transepithelial triacylglycerol transport. J. Clin. Invest. 93, 70–80.
Malagelada, J.R., Linscheer, W.G., Fishman, W.H., 1977. The effect of fatty acid perfusion on intestinal alkaline phosphatase: II. Studies on the rat. Am. J. Digest. Dis. 22, 516–523.
Moriarty, D.J.W., 1973. The physiology of digestion of blue-green algae in the cichlid fish, Tilapia nilotica. J.
Ž .
Zool. London 171, 25–39.
Nagase, G., 1964. Contribution to physiology of digestion in Tilapia mossambica Peters: digestive enzymes and the effects of diets on their activity. Z. Vergl. Physiol. 49, 270–284.
Ž .
Opuszynski, K., Shireman, J.V., 1995. Digestive mechanisms. In: Opuszynski, K., Shireman, J.V. Eds. , Herbivorous Fishes: Culture and Use for Weed Management. CRC Press, Boca Raton, FL, pp. 21–31.
Ž
Overnell, J., 1973. Digestive enzymes of the pyloric caeca and of their associated mesentery in the cod Gadus
.
morhua . Comp. Biochem. Physiol. B 46, 519–531.
Pearse, A.G.E., 1985. Histochemistry: Theoretical and Applied, Vol. 2. Livingstone, New York, pp. 441–1055. Roubaty, C., Portmann, P., 1988. Relation between intestinal alkaline phosphatase activity and brush border membrane transport of inorganic phosphate,D-glucose, andD-glucose-6-phosphate. Pfluegers Arch. 412, 482–490.
Sabapathy, U., Teo, L.H., 1993. A quantitative study of some digestive enzymes in the rabbitfish, Siganus canaliculatus and the sea bass, Lates calcarifer. J. Fish Biol. 42, 595–602.
Sakai, T., Kawatsu, H., 1983. Distribution of dipeptidyl aminopeptidase IV in cultured fishes. Bull. Jpn. Soc. Sci. Fish. 49, 683–686.
Seligman, A.M., Wasserkrug, H.L., Plapinger, R.E., Seito, T., Hanker, J.S., 1970. Membrane ultrastructural demonstration of aminopeptidase and glutamyltranspeptidase activities with a diazonium salt that yields a lipophobic, osmiophilic azo dye. J. Histochem. Cytochem. 18, 542–551.
Smith, B.J., Smith, S.A., Tengjaroenkul, B., in press. Gross morphology of the intestinal tract of the tilapia Oreochromis niloticus. Cell. Tissue Organs.
Stevens, C.E., Hume, I.D., 1995. Comparative Physiology of the Vertebrate Digestive System. Cambridge Univ. Press, New York, 400 pp.
Sugita, H., Tokuyama, K., Deguchi, Y., 1985. The intestinal microflora of carp Cyprinus carpio, grass carp Ctenopharyngodon idella and tilapia Sarotherodon niloticus. Bull. Jpn. Soc. Sci. Fish. 51, 1325–1329. Trewavas, E., 1983. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis and Danakilia. British
Ž .
Museum Natural History , London, 583 pp.
Van Lith, H.A., Meijer, G.W., Van Der Wouw, M.J.A., Den Bieman, M., Van Tintelen, G., Van Zutphen,
Ž .
L.F.M., Beynen, A.C., 1992. Influence of amount of dietary fat and protein on esterase-1 ES-1 activities of plasma and small intestine in rats. Br. J. Nutr. 67, 379–390.
Vonk, H.J., Western, J.R.H., 1984. Comparative Biochemistry and Physiology of Enzymatic Digestion. Academic Press, London, 501 pp.
Wassmer, B., Augenstein, U., Ronai, A., De Looze, S., Von Deimling, O., 1988. Lymph esterases of the house
Ž .
mouse Mus musculus : II. The role of esterase-2 in fat resorption. Comp. Biochem. Physiol. B 91, 179–185.