www.elsevier.nlrlocateraqua-online
Elimination of the gill worm Urastoma cyprinae
ž
Graff from the eastern oyster Crassostrea
/
ž
/
Õ
irginica Gmelin using different
salinity–temperature combinations
Erick Bataller, Andrew D. Boghen
)Department of Biology, UniÕersite de Moncton, Moncton, New Brunswick, Canada E1A 3E9´
Accepted 7 June 1999
Abstract
Ž .
Urastoma cyprinae Class: Turbellaria is a facultative parasite of the eastern oyster Cras-sostrea Õirginica. Because the worms are clearly visible when present in oysters, they could conceivably have a negative impact on the lucrative half-shell oyster market. Our work examines the salinity and temperature tolerances of U. cyprinae to help formulate ways of eliminating the parasite from oysters prior to marketing. Isolated worms were exposed to salinities of 0‰ to 55‰
at temperatures of 58C, 158C and 208C, and in infected oysters at salinities of 8‰, 28‰ and 55‰
at temperatures of 108C, 158C and 208C. Findings reveal that the salinity tolerance of isolated U.
cyprinae ranges between 12‰ and 40‰. In the case of infected oysters, two scenarios for the
destruction of worms emanated: firstly, to depurate oysters for 2 days at a salinity and temperature of 55‰ and 108C, respectively, or, alternatively, to expose oysters to a salinity–temperature
regime of 8‰ and 208C for 4 days. However, to eliminate all traces of dead worms from oysters,
the optimal salinity–temperature combination was found to be 8‰ at 208C for 8 days.q2000
Elsevier Science B.V. All rights reserved.
Keywords: CrassostreaÕirginica; Oyster; Urastoma cyprinae; Gill worm; Turbellarian; Salinity; Temperature;
Depuration
1. Introduction
Ž Urastoma cyprinae is a Turbellarian belonging to the order Prolecithophora
Holo-. Ž .
coela Cannon, 1986 and has been reported from the gills of various bivalve species
)Corresponding author. Fax:q1-5068584541.
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
( ) E. Bataller, A.D. BoghenrAquaculture 182 2000 199–208
200
ŽGoggin and Cannon, 1989; Noury-Sraıri et al., 1990 . Murina and Solonchenko 1991
¨
. Ž .Ž .
and Teia dos Santos and Coimbra 1995 suggested that the worm may provoke
pathological changes in the mussels Mytilus edulis and Mytilus galloproÕinciallis. This
Ž .
was confirmed by Robledo et al. 1994 who demonstrated that U. cyprinae induces severe gill disruption in M. galloproÕincialis.
Ž
In Canada, U. cyprinae was reported in the blue mussel M. edulis Fleming et al.,
. Ž .
1981 . Burt and Drinnan 1968 described the worm from the gills and mantle of the eastern oyster CrassostreaÕirginica sampled along the Atlantic coast and suggested that
it was in all probability a facultative commensal.
Ž .
In their preliminary investigation, Plourde et al. 1991 raised the possibility that there may exist an inverse relationship between the number of worms and the physio-logical condition of infected oysters. As part of a comprehensive oyster-monitoring
Ž .
program, Boghen et al. 1993 reported high levels of infestation of the eastern oyster by
Ž .
U. cyprinae. More recently, Brun et al. 1999a conclusively showed that the worms
were strongly attracted to oyster gill mucus, dispelling the likelihood that they were simply facultative commensals.
Aside from any potentially negative effects on oysters, the visible presence of the worms on the gills, especially when occurring in the hundreds and even thousands, could contribute to a drop in demand by the lucrative half-shell market. Our work examines the salinity and temperature tolerances of U. cyprinae to gain new insight into the biology of the worm, and help formulate ways of eliminating the parasite from oysters.
2. Material and methods
Ž .
Adult oysters ns435 infected with U. cyprinae were collected from Shippagan
Bay in northeastern New Brunswick, Canada, during August of 1996 and 1997. Average ambient temperature and salinity readings were 208C and 28‰, respectively. Oysters
were placed on ice, transported to the Universite de Moncton, New Brunswick and
´
maintained at 58C for 2–3 days prior to the beginning of the experiments.
2.1. Salinity tolerance of isolated U. cyprinae
U. cyprinae were removed by Pasteur pipette from the gills of oysters under a
dissecting microscope and stored overnight in covered 100-ml beakers filled with
Ž .
artificial sea water Instant Ocean, Aquarium Systems, OH, USA at 22‰ and 58C. The worms were subsequently transferred to petri dishes. Three 25-ml glass petri dishes Žreplicates each containing 10 U. cyprinae were exposed to 21 different salinity–tem-.
Ž .
perature combinations total of 630 worms in 63 dishes . Tolerance of U. cyprinae to salinities of 12‰, 13‰, 14‰, 15‰, 22‰, 31‰ and 40‰ were determined at each of the following temperatures: 58C, 158C and 208C. The dishes were examined daily for up to 65 days and worm mortalities were recorded. After exposing U. cyprinae to a series
Ž .
2.2. Salinity tolerance of U. cyprinae present in oysters
Ten oysters were randomly chosen from the samples collected in Shippagan Bay and examined for parasites under a dissecting microscope. This was done to confirm the
Ž .
presence of U. cyprinae in sufficiently high numbers mean)400 to permit reliable
interpretation of data.
A sample of 135 oysters was divided into 15 groups. The animals were in turn separated and placed into individual, disposable, colour coded, 300 ml plastic bowls. The oysters were maintained at nine different salinity–temperature combinations at the
following salinities, 8‰, 28‰ and 55‰ and temperatures, 108C, 158C and 208C,
respectively. The 28‰ salinity represented the control since it coincided with that which was recorded from the sampling site.
Adequate oxygenation was provided to each bowl by individual plastic air-lines attached to small aquarium pumps. Water was changed every second day, thereby
ensuring that ammonia levels never exceeded 0.2 mgrl. Oysters were unfed throughout
the study.
The bowls, representing each of the three salinities, were uniformly distributed in an even, yet non-symmetrical pattern for each temperature to avoid positional bias in experimental design. Three oysters were selected randomly from each of the salinity– temperature combinations every two days, opened and carefully examined for living or dead U. cyprinae.
3. Results
3.1. Salinity tolerance of isolated U. cyprinae
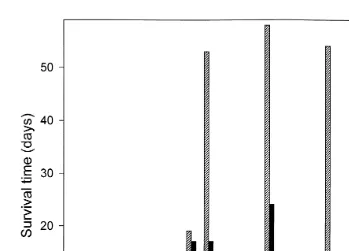
Salinity tolerances of U. cyprinae varied between 12‰ and 40‰ at temperatures of Ž 58C, 158C and 208C, respectively. Worms exposed to salinities exceeding this range i.e.,
. Ž
below 12‰ or above 40‰ generally died within an hour. Best survival i.e., as .
measured by the survival of at least one of the 10 worms was achieved at salinities
Ž .
occurring between 15‰ and 31‰ Fig. 1 . In all instances, with the exception of the
Ž .
lowest tolerable salinities recorded 12‰ and 13‰ , an inverse relationship prevailed between survival time of U. cyprinae and water temperature at any given salinity. Worms survived from a minimum of 6 to a maximum of 58 days, at temperatures of 208C and 58C, respectively.
3.2. Salinity tolerance of U. cyprinae present in oysters
Preliminary findings confirmed that the prevalence of U. cyprinae in oyster sampled
from Shippagan Bay was 100% and that the average intensity was 540"363.
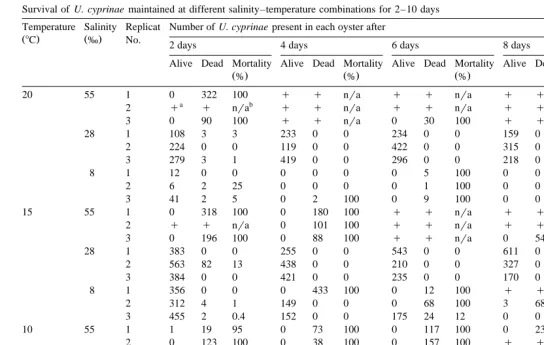
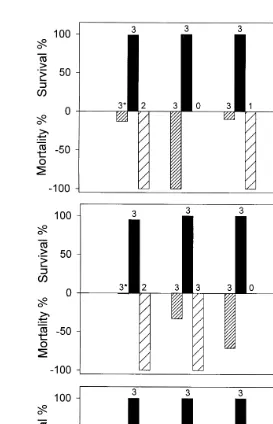
Mortality and survival rates of the worms and oysters for each of the nine salinity– temperature combinations are presented in Table 1 and Fig. 2. At the control salinity
Ž28‰ , none of the oysters died. Despite this, due to unforeseen technical problems,.
( ) E. Bataller, A.D. BoghenrAquaculture 182 2000 199–208
202
Fig. 1. Survival of isolated U. cyprinae maintained at different salinity–temperatures combinations.
and 158C after 4 and 8 days, respectively. In contrast, mortality rates of U. cyprinae ranged between 0% and 100% at all temperatures. Finally, at 55‰, 23 of the 45 oysters
Ž)50% died at different times and temperatures. Heavy mortalities of U. cyprinae,.
exceeding 95%, were detected at all temperatures in surviving oysters.
4. Discussion
In the past, oyster culture has been hampered by a variety of serious diseases,
Ž . Ž .
including, Dermo, SSO Seaside Organism and MSX Ford and Tripp, 1996 . Such conditions contributed to mass mortalities along the Northeast coast of the United States
Ž .
and have been the subject of extensive studies Andrews, 1988 . In contrast, except for Malpeque Disease which first appeared on the Canadian East coast at the beginning of
Ž .
the century and persisted into the 50s Galtsoff, 1964 , the region has been spared of lethal oyster diseases.
Notes to Table 1:
a
q: Dead oyster.
b
nra: Data unavailable.
c
()
Survival of U. cyprinae maintained at different salinity–temperature combinations for 2–10 days Temperature Salinity Replicat Number of U. cyprinae present in each oyster after
Ž8C. Ž‰. No. 2 days 4 days 6 days 8 days 10 days
Alive Dead Mortality Alive Dead Mortality Alive Dead Mortality Alive Dead Mortality Alive Dead Mortality
( ) E. Bataller, A.D. BoghenrAquaculture 182 2000 199–208
204
Fig. 2. Survival of U. cyprinae and the eastern oyster C.Õirginica exposed to temperatures of 108C, 158C and
U Ž .
208C and salinities of 8‰, 28‰ and 55‰. Number of live oysters maximums3 .
U. cyprinae, which is recurrent on the East Coast, has definitely been shown to be
Ž .
destructive to gill tissue in the mussel M. galloproÕincialis Robledo et al., 1994 . Even
Ž .
though Brun et al. 1999a; b conclusively demonstrated that there is a strong attraction
Ž .
the elimination of the gill worm is a prerequisite for an increasingly competitive industry.
Depuration methods for the elimination of microbial organisms or toxic products are Ž
used extensively in aquaculture Haven et al., 1978; Lassus and Berthome, 1987; Kaspar .
and Tamplin, 1993 . Information related to salinity tolerance has previously been combined with depuration techniques to eliminate unwanted organisms from
commer-Ž . Ž
cially important finfish Plumb and Shoemaker, 1995 and shellfish species Haskin et al., 1981; Ford, 1985; Haskin and Ford, 1989; Lewis, 1993; Chu et al., 1994; Fuentes et
.
al., 1995 . The current work is based on the above approach by focusing on salinity tolerance levels of U. cyprinae to eliminate the gill worms from oysters.
Initial findings revealed that the salinity tolerance of isolated worms varied between
Ž .
12‰ and 40‰ Fig. 1 and that within this range, survival was greater at 58C than at either 108C or 208C.
Under natural conditions, as the temperature drops, U. cyprinae leave their host and
Ž .
assume a free-living lifestyle Marcus, 1951; Westblad, 1955; Burt and Drinnan, 1968 . Since this was not achievable under experimental conditions, empirical observations of the development of a mucus-like coating encapsulating the worm at low temperature, may reflect a phenomenon that occurs in nature for free-living phases of parasites. Such adaptations could also take the form of protective cysts or cocoons in other species and
Ž
serve as a protective shield against adverse environmental conditions Klauser, 1986; .
Gyoten, 1991 . While in the case of U. cyprinae, the mucus-like coating might represent a protective adaptation outside the host, it could also serve to facilitate adhesion to macroalgae with which they have been shown to be associated when occurring in a
Ž .
free-living state Marcus, 1951 .
In the second part of the work, infected oysters exposed to a salinity of 28‰ Žcontrol , remained alive for the duration of the experiment. Worms also displayed high.
Ž .
survival after 2 days at 158C and 208C with some exceptions 1%–13% . Such findings
contrasted with those of oysters maintained at 55‰ and 108C and at 8‰ and 208C. All
worms died after 2 and 4 days, respectively, even though the oysters themselves
Ž .
survived Table 1 at these salinities. Infected oysters maintained at 8‰ and 208C and examined after 8 days were free of any traces of worms. This, however, was not the case for oysters maintained at 55‰ and 108C. At this salinity, although U. cyprinae were dead, they could still be observed on the gills.
Good survival of oysters and worms at a salinity of 28‰ is consistent with the natural conditions for oysters found in Shippagan Bay and with findings based on the
Ž .
first experiment Fig. 1 . At 55‰, the salinity level far exceeds the tolerance range of
Ž .
oysters Shumway, 1996 . This not only results in the death of the worms within a short time, but also triggers shell closure by oysters. The result is a total hermetization of the
Ž .
oysters Shumway, 1996; Berger and Kharazova, 1997 , an end to all filtration activities, and therefore, the prevention of expulsion of dead worms.
The situation contrasts with that in which oysters are maintained at 8‰ and 208C. In this instance, while the rearing conditions are also «sub-optimal» and the rate of
Ž .
( ) E. Bataller, A.D. BoghenrAquaculture 182 2000 199–208
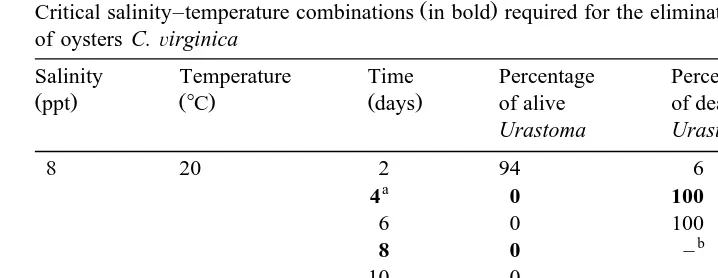
206 Table 2
Ž .
Critical salinity–temperature combinations in bold required for the elimination of U. cyprinae from the gills of oysters C.Õirginica
Salinity Temperature Time Percentage Percentage Percentage
Žppt. Ž8C. Ždays. of alive of dead of alive
Bold letter refers to optimal duration times for the elimination of U. cyprinae for each of the salinity–temper-ature combinations.
b
No trace of U. cyprinae.
oysters continue to filter, albeit at a reduced rate, all the worms are gradually eliminated from the gills by day 8, either due to the dislodging from the gills, or by their removal in a transformed state via the pseudofeces.
One of two possible scenarios for eliminating the parasite from oysters arises from this work: either by immersing oysters in water at 55‰ and 108C for 2 days or by
Ž .
introducing them into a water bath at 8‰ and 208C for 4 days Table 2 . If the grower’s objective is not only to destroy but also to eliminate all traces of the worms, then oysters would have to be immersed in water at 8‰ and 208C for up to 8 days prior to marketing.
Depuration methods do not preclude the fact that infected oysters can also be cleansed by either transferring or culturing them for extended periods at low salinity
Ž .
sites. The transfer of molluscs such as Mya arenaria Coquillage Nordique, 1992 and
Ž .
oysters Haskin and Ford, 1982 to low salinity sites has already been explored for purposes of eliminating undesirable agents. Such an approach, however, might result in lower oyster productivity than if oysters were cultured at sites where salinity conditions
Ž .
were superior Bataller et al., 1999 . Such a decision, however, could only be made following a detailed comparative economic analysis that considers all options.
Whatever method is adopted, the taste of the final product might have been affected in the process. With that possibility in mind, investigations are warranted to determine if a readaptation to ambient salinity conditions might be a prerequisite for ensuring a more marketable product.
Acknowledgements
appreci-ated. The work was partially financed by a Natural Sciences and Engineering Research
Ž .
of Canada NSERC operating grant awarded to Dr. Michael D.B. Burt, by the
Ž .
Environmental Sciences Research Center CRSE of the Universite de Moncton and by
´
the Faculty of Graduate Studies of the University of New Brunswick, Canada. The work is part of the Richibucto Environment and Resource Enhancement Project.
References
Andrews, J.D., 1988. Epizootiology of the disease caused by the oyster pathogen Perkinsus marinus and its effects on the oyster industry. American Fisheries Society Special Publication 18, 47–63.
Bataller, E., 1997. Determination de la zone de tolerance de la salinite du Turbellarie, Urastoma cyprinae, a´ ´ ´ ´ ` l’etat libre et en association avec l’huıtre americaine Crassostrea´ ˆ ´ Õirginica. Aquaculture Canada ’97. Bull.
Aqua. Assoc. Can. 97-2, pp. 60–62.
Bataller, E., Boghen, A.D., Burt, M.D., 1999. Comparative growth of the eastern oyster CrassostreaÕirginica Žgmelin reared at low and high salinities in New Brunswick, Canada. J. Shellfish Res. 18r1, 107–114..
Berger, V.J., Kharazova, A.D., 1997. Mechanisms of salinity adaptations in marine molluscs. Hydrobiologia 355, 115–126.
Boghen, A.D., Allard, J., Bataller, E., 1993. Rapport final et recommandations sur le programme de monitoring pour la cote est du Nouveau Brunswick. Centre de Recherche en Sciences de l’Environnement,ˆ Universite de Moncton, N.B. mars 1993.´
Ž .
Brun, N.T., Boghen, A.D., Allard, J., 1999a. Attraction of Urastoma cyprinae Turbellaria: Urastomidae to
Ž .
the Eastern Oyster Crassostrea virginica. Dis. Aquat. Org. in press .
Brun, N.T., Boghen, A.D., Allard, J., 1999b. Distribution of the turbellarian Urastoma cyprinae on the gills of the Eastern Oyster CrassostreaÕirginica. J. Shellfish Res. 18r1, 175–179.
Burt, M.D.B., Drinnan, R.E., 1968. A microturbellarian found in oyster off the coast of Price Edward Island.
Ž .
J. Fish. Res. Board Can. 25 11 , 2521–2522.
Cannon, L.R.G., 1986. Turbellaria of the World — A Guide to Families and Genera. Queensland. Brisbane, Australia, 132 pp.
Chu, F.-L., Volety, A.K., Constantin, G., 1994. Synergetic effects of temperature and salinity on the response
Ž . Ž .
of oysters CrassostreaÕirginica to the pathogen, Perkinsus marinus. J. Shellfish Res. 13 1 , 293.
Coquillage Nordique, 1992. Purification des myes par depuration a l’ultra-violet ou par reparcage. Fisheries´ ` and Aquaculture Testing and Experimentation Program,a726.
Fleming, L.C., Burt, M.D.B., Bacon, G.B., 1981. On some commensal Turbellarian of the Canadien East Coast. Hydrobiologia 84, 131–137.
Ž
Ford, S.E., 1985. Effects of salinity on survival of the MSX parasite Haplosporidium nelsoni Haskin, Stauber
. Ž .
and Mackin in oysters. J. Shellfish Res. 5 2 , 85–90.
Ford, S.E., Tripp, M.R., 1996. Diseases and defense mechanisms. In: Kennedy, V.S., R.I.E. Newell, A.F. Eble
ŽEds. , The Eastern Oyster Crassostrea. Õirginica. Maryland Sea Grant College, College Park, MD, pp.
581–660.
Fuentes, J., Villalba, A., Zapata, C., Alvarez, G., 1995. Effects of stock and culture environment on infection by Marteilia refringens and Mytilicola intestinalis in the mussel Mytilus galloproÕincialis cultured in
Ž .
Galica NW Spain . Dis. Aquat. Org. 21, 221–226.
Galtsoff, O.S., 1964. The American oyster CrassostreaÕirginica Gmelin. Fish. Bull. 64, 1–480.
Goggin, C.L., Cannon, L.R.G., 1989. Occurrence of a turbellarian from australian tridacnid clams. Int.
Ž .
J. Parasitol. 19 3 , 345–346.
Gyoten, J., 1991. Development, morphology and functions of mucoid glands in cercariae of Paragonimus
Ž .
miyazakii. Jpn. J. Parasitol. 40 3 , 279–286.
Ž .
Haskin, H.H., Ford, S.E., 1982. Haplosporidium nelsoni MSX on Delaware Bay seed oyster beds: a
Ž .
host–parasite relationship along a salinity gradient. J. Invertebr. Pathol. 40 3 , 388–405.
Ž .
Haskin, H.H., Ford, S.E., 1989. Low salinity control of Haplosporidium nelsoni MSX . J. Shellfish Res. 8
( ) E. Bataller, A.D. BoghenrAquaculture 182 2000 199–208
208
Haskin, H.H., Ford, S.E., O’Connor, D., 1981. Environmental influences on MSX infection patterns in
Ž .
Delaware Bay. J. Shellfish Res. 2 1 , 97.
Haven, S.D., Perkins, F.O., Morales-Alamo, R., Rhodes, M.W., 1978. Bacterial depuration by the american
Ž .
oyster CrassostreaÕirginica under controlled conditions. Biological and technical studies, Vol. 1. Special
Scientific Report No. 88, Virginia Institute of Marine Science, Gloucester Point, VA 23062.
Kaspar, C.W., Tamplin, M.L., 1993. Effects of temperature and salinity on the survival of VibrioÕulnificus in Ž .
seawater and shellfish. Appl. Environ. Microbiol. 59 8 , 2425–2429.
Klauser, M.D., 1986. Mucous secretions of the acoel turbellarian ConÕoluta sp. Oersted: an ecological and Ž .
functional approach. J. Exp. Mar. Biol. Ecol. 97 2 , 123–133.
Lassus, P., Berthome, J.P., 1987. Toxicite des moules: etude de la decontamination PSP et DSP in vitro et in´ ´ ´ situ. IFREMERrNantes, Rapport collectif DERO-86.13-MR et DRV-86.01-SR.
Lewis, E.J. Jr., 1993. Preliminary osmoconforming study of the oyster CrassostreaÕirginica. J. Shellfish Res. Ž .
12 2 , 366–367.
Ž .
Marcus, E., 1951. Turbellaria Brasileiros 9 . Bol. Fac. Fil. Cien. Letr. Univ. S. Paulo, Zoologia No. 16, Sao` ` Paulo.
Murina, G.V., Solonchenko, A.I., 1991. Commensals of Mytilus galloproÕincialis in the Black Sea: Urastoma
Ž . Ž .
cyprinae Turbellaria and Polydora ciliata Polychaeta . Hydrobiologia 227, 385–387.
Noury-Sraıri, N., Justine, J.-L., Euzet, L., 1990. Ultrastructure du tegument et des glandes sous-epitheliales de¨ ´ ´ ´
Ž .
Urastoma cyprinae Prolecithophora , Turbellarie parasite de mollusques. Annales de Sciences Naturelles,´
e`me
Zoologie 13 Serie 11, 53–71.´
Plourde, S.M., Boghen, A.D., Allard, J., 1991. Incidence of the Turbellarian, Urastoma cyprinae, in the oyster,
Ž . CrassostreaÕirginica. Bull. Aquacul. Assoc. Can. 91 3 , 1–2.
Plumb, J.A., Shoemaker, C., 1995. Effects of temperature and salt concentration on latent Edwardsiella
ictaluri infections in channel catfish. Dis. Aquat. Org. 21, 171–175.
Robledo, J.A.F., Caceres-Martinez, J., Sluys, R., Figueras, A., 1994. The parasitic turbellarian Urastoma´
Ž .
cyprinae Platyhelminthes: Urastomidae from blue mussel Mytilus galloproÕincialis in Spain: occurrence
and pathology. Dis. Aquat. Org. 18, 203–210.
Ž .
Shumway, S.E., 1996. Natural environmental factors. In: Kennedy, V.S., Newell, R.I.E., Eble, A.F. Eds. , The Eastern Oyster CrassostreaÕirginica. Maryland Sea Grant College, College Park, MD, pp. 467–513.
Teia dos Santos, A.M., Coimbra, J., 1995. Growth and production of raft-cultured Mytilus edulis L., in Ria de Aveiro: gonad symbiotic infestation. Aquaculture 132, 195–211.
Ž .
Westblad, E., 1955. Marine «Alloecoels» Turbellaria from North Atlantic and Mediterranean coast. I. Ark.
Ž .