www.elsevier.com / locate / livprodsci
Preventing disease transmission through the transfer of
in-vivo-derived bovine embryos
*
D.A. Stringfellow , M.D. Givens
Department of Pathobiology, College of Veterinary Medicine, Auburn University, Auburn, AL 36849-5519, USA
Abstract
Investigation and experience have demonstrated that movement of in-vivo-derived bovine embryos can be accomplished while effectively limiting spread of infectious disease between populations of cattle. Experimental and theoretical justifications of current strategies for production of specific-pathogen-free, in-vivo-derived embryos are reviewed. Hazards of spreading bovine viral diarrhea virus via in-vivo-derived embryos are dealt with specifically. It is concluded that established sanitary procedures for producing pathogen-free, in-vivo-derived embryos are efficacious if the ethical and technical excellence of those performing the procedures can be assured. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Bovine embryo; Specific-pathogen-free embryo; Bovine viral diarrhea virus
1. Introduction might provide a new mode for transmission of disease. Early concerns about transfer of infectious
Introduction of postnatal animals and semen were agents with embryos were based on
embryo–patho-traditional methods used to replenish and improve gen observations that had been made in laboratory
bloodlines in populations of cattle. Then, in the animals (see review by Wrathall and Sutmoller,
1970s, technological advances provided efficient 1998). However, after consideration of
epi-methods for nonsurgical collection, cryopreservation demiologic factors associated with production and
and nonsurgical transfer of pre-implantation bovine transfer of in-vivo-derived bovine embryos, it was
embryos. Thereafter, an alternative with obvious hypothesized that embryo transfer in this species
economic and humane advantages was available for would prevent transmission of disease if appropriate
movement of germ plasm between populations of precautions were taken. Subsequent research on
cattle. specific bovine pathogens within the context of
When embryo transfer became an option, there embryo production technology supported the
hypoth-was understandable concern that the technology esis and provided a foundation for today’s
safe-embryo-handling recommendations.
The aim of this paper is to review epidemiologic
*Corresponding author. Tel.: 11-334-844-2667; fax:1
1-334-aspects of bovine embryo transfer and results of
844-4955.
early research that provided the basis for sanitary
E-mail address: [email protected] (D.A.
Stringfel-low) guidelines found in the Manual of the International
Embryo Transfer Society. In addition, results of of limiting factors work to prevent the occurrence of
more recent research are summarized with emphasis this complete sequence of events.
on bovine viral diarrhea virus. Finally, we discuss
the impact that results of more recent studies and 2.1. Factors limiting exposure of embryos to
accumulated experience with commercial embryo pathogens
transfer have had on our current view of strategies
for pathogen-free-embryo production. Under ordinary conditions, numerous factors tend
to restrict exposure of embryos to pathogens. Espe-cially important are, limited mobility of embryos,
2. Epidemiological view of embryo transfer limited distribution of pathogens and precautions implicit in or applied to management of donor
Details of agent, host and environmental factors animals.
which collectively support the use of embryo transfer Between conception and collection, embryos are
as a method for controlling the spread of pathogens restricted to the uterine tubes and uterus of the donor
were reviewed previously (Stringfellow, 1985; String- cow. Consequently, a cow that is uninfected serves
fellow and Wright, 1987; Stringfellow et al., 1991) as an isolation unit for the preimplantation embryo
and are only summarized here. prior to collection. If herds or regions of origin of
If a pathogen was to be transmitted by transfer of donor cattle are free from specific pathogens, the
in-vivo-derived bovine embryos, an uninterrupted security of the isolation is enhanced. If a donor cow
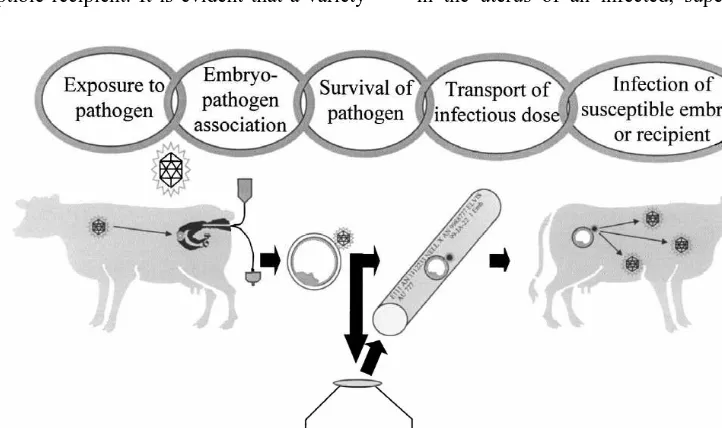
sequence of events would have to occur (Fig. 1). is infected with an agent of disease, it is still possible
Key elements in this hypothetical chain of events that embryos might remain unexposed. The classic
include: (1) exposure of embryos to the pathogen, example is a cow infected with Brucella abortus.
(2) continued association of pathogen with the While this bacterium is a reproductive pathogen, an
embryos, (3) maintenance of infectivity of pathogen accumulation of evidence from studies in the 1980s
throughout embryo manipulation and processing, and (see review by Stringfellow and Wright, 1989)
finally, (4) delivery of an infective dose of pathogen indicated that exposure of preimplantation embryos
to a susceptible recipient. It is evident that a variety in the uterus of an infected, superovulated cow is
highly unlikely. The primary reason is that brucella (Riddell et al., 1993c; Betteridge, 1995) when
com-do not remain in the postpartum uterus after multiple pared to the murine zona pellucida (about 5 mm)
estrous cycles and will only return to the uterus long (Gwatkin, 1967), and there is no current proof of
after conception when the fetus and placenta are well true vertical transmission (via hereditary
incorpora-developed. tion) of retroviruses or other pathogens in cattle. To
Donor cattle, representing the highest genetic illustrate the latter, a retrovirus of cattle, bovine
merit, are normally afforded the best available health leukemia virus, is not incorporated into the genome
care. The herds of origin are often free of many of gametes (Divers et al., 1995). Without concern for
pathogens due to participation in disease-control infectious disease transmission via the gametes, it
programs and enforcement of judicious herd-replace- was especially important to confirm that the
rela-ment policies. The extent to which preventive man- tively thick bovine zona pellucida could serve as an
agement and medicine is practised can be evaluated effective barrier. In various studies, summarized
by the veterinarian who is a member of an official below, it was confirmed that pathogens would not
embryo collection team (Evans, 1998). The team penetrate this barrier, and only a few would adhere
veterinarian also can clinically assess the health of to it. Still, an important concern was that pathogens
the donor cow at or around the time of embryo found in body fluids or as contaminants in media
collection. Finally, some national regulatory au- might remain in close proximity to the embryo until
thorities may request specific tests of donor cattle to the time of transfer.
further enhance safety when embryos are to be Techniques for collection, processing and transfer
moved internationally. of in-vivo-derived embryos vary, but in each
situa-tion, there are dilution factors associated with the
2.2. Factors limiting continued association of sheer volume of recovery and holding medium that
pathogens with embryos would serve to dilute any pathogen that might be
present in the embryo’s environment. Also,
pre-If embryos were to be exposed to pathogens, scribed procedures for washing, with or without
several factors tend to prevent continued association. trypsin, ensure that certain pathogens will be
elimi-These include inherent resistance of the embryo nated by dilution, dislodging, or inactivation
(String-provided by the zona pellucida and natural or fellow, 1998). Finally, the use of antibiotics in
prescribed cleansing associated with embryo collec- media for recovery, culture and storage of embryos
tion and transfer. effectively deters the spread of some prokaryotic
The mammalian zona pellucida is a distinctive pathogens and suppresses nonpathogenic microbial
extracellular matrix that ensures species specificity of contaminants (Riddell and Stringfellow, 1998).
fertilization, block to polyspermy and protection of
the embryo during very early stages of development 2.3. Factors limiting infectivity and delivery of an
(Dunbar, 1983). However, it is well known that the infectious dose of pathogens to recipients
zona pellucida functions as a microporous
mem-brane, allowing traffic of relatively large molecules Media and techniques for collection, storage and
(Sellens and Jenkins, 1975). This permeability and transfer of bovine embryos are intended to ensure
results of several early studies in laboratory animals that embryos maintain their developmental
compe-led to concerns that the zona pellucida might not tence, but they do not necessarily ensure that
infec-provide a significant barrier to pathogens. As exam- tivity of associated pathogens is maintained. An
ples, in one report, the passage of Mengo virus example of a negative impact of embryo processing
through the murine zona pellucida was demonstrated on viability of pathogen was illustrated in an early
(Gwatkin, 1967), and in another report, the genetic study in which a standard embryo-cryopreservation
spread of the retrovirus that caused murine mammary procedure resulted in 64% or 99.9% reduction in
tumor was reported (Bentivelzen et al., 1970). viability of Brucella abortus in the absence or
However, it is important to note that the bovine zona presence of antibiotics, respectively (Stringfellow et
The last link in the hypothetical chain of infection recipient cows resulting in viable pregnancy with a
is delivery of an infectious dose of pathogen along high degree of consistency. The potential usefulness
with a viable embryo into the uterus of a susceptible of these procedures in international trade led to
recipient (Fig. 1). It should not be presumed that all questions by regulatory authorities and scientists
recipients are susceptible via the intrauterine or any about the role of embryo transfer in transmission of
other route. Some may be resistant to infection based infectious diseases. Consequently, embryo–pathogen
on naturally acquired immunity (through prior expo- interactions became an important topic of
inves-sure) or induced immunity (through vaccination). tigation.
Even if recipients are susceptible to infection with a Selection of infectious agents for study was
some-specific pathogen, the amount of infectious agent that what serendipitous; but usually, the pathogens were
is associated with a single embryo may not constitute either objects of national prevention, control and
an infective dose. The natural and prescribed obsta- eradication efforts, or there was some reason to
cles such as dilution factors and washing that were believe that embryos might be exposed to them.
described above would all tend to reduce the in- Most studies utilized one of four standard
ex-oculum. In reality, there has been no report of a perimental approaches (Bielanski and Hare, 1998).
comprehensive study to deal specifically with in- In one of these approaches, zona pellucida intact
trauterine, day 7 inoculations with any bovine patho- embryos were exposed to pathogen in vitro, washed
gen. and then assayed in vitro to determine if infectious
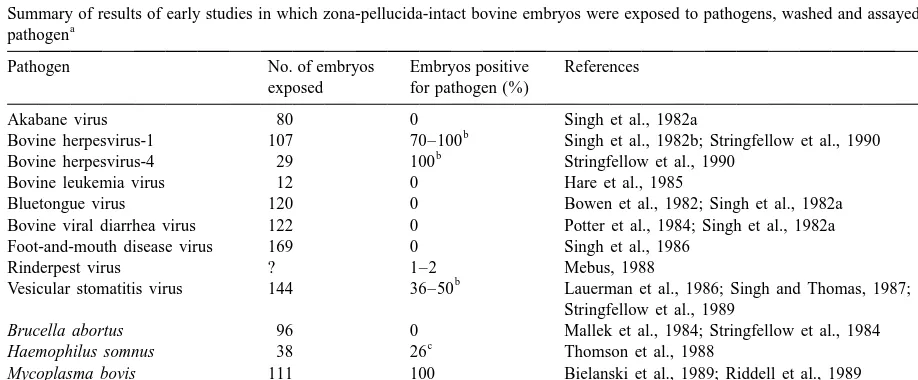
agent was present. In Table 1, collective results are presented for some initial studies of this type. As
3. Early research providing the basis for regards the studies summarized in this table, it is
sanitary guidelines important to note that the artificial exposure general-ly was to high concentrations of pathogen to mimic a
By the end of the 1970s, embryos could be worse case scenario. Also, washing procedures
gen-collected nonsurgically from superovulated cows, erally conformed to a protocol that has since been
cryopreserved for extended periods and transferred to adopted as the recommended procedure of the
Inter-Table 1
Summary of results of early studies in which zona-pellucida-intact bovine embryos were exposed to pathogens, washed and assayed for the a
pathogen
Pathogen No. of embryos Embryos positive References exposed for pathogen (%)
Akabane virus 80 0 Singh et al., 1982a
b
Bovine herpesvirus-1 107 70–100 Singh et al., 1982b; Stringfellow et al., 1990 b
Bovine herpesvirus-4 29 100 Stringfellow et al., 1990
Bovine leukemia virus 12 0 Hare et al., 1985
Bluetongue virus 120 0 Bowen et al., 1982; Singh et al., 1982a Bovine viral diarrhea virus 122 0 Potter et al., 1984; Singh et al., 1982a Foot-and-mouth disease virus 169 0 Singh et al., 1986
Rinderpest virus ? 1–2 Mebus, 1988
b
Vesicular stomatitis virus 144 36–50 Lauerman et al., 1986; Singh and Thomas, 1987; Stringfellow et al., 1989
Brucella abortus 96 0 Mallek et al., 1984; Stringfellow et al., 1984
c
Haemophilus somnus 38 26 Thomson et al., 1988
Mycoplasma bovis 111 100 Bielanski et al., 1989; Riddell et al., 1989
Mycoplasma bovigenitalium 49 100 Riddell et al., 1989
Mycobacterium paratuberculosis 20 30 Rohde et al., 1990
Ureaplasma diversum 26 100 Britton et al., 1988
a
This table is adapted from tabulated material in Appendix B, Manual of the International Embryo Transfer Society (Anonymous, 1998b). b
Trypsin treatment was thought to be effective for removal of this pathogen. c
Table 2
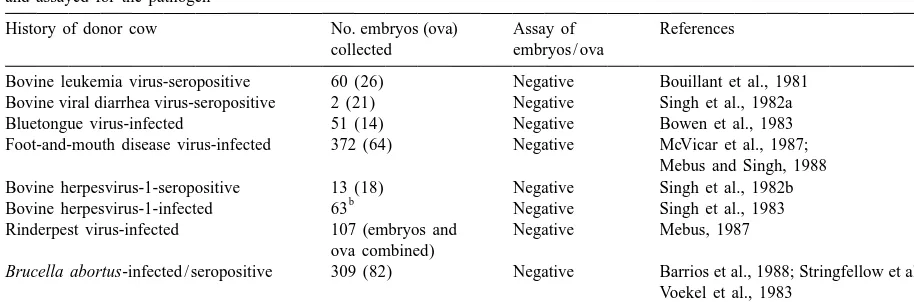
Summary of results of early studies in which zona-pellucida-intact bovine embryos from infected or seropositive donor cows were washed a
and assayed for the pathogen
History of donor cow No. embryos (ova) Assay of References collected embryos / ova
Bovine leukemia virus-seropositive 60 (26) Negative Bouillant et al., 1981 Bovine viral diarrhea virus-seropositive 2 (21) Negative Singh et al., 1982a Bluetongue virus-infected 51 (14) Negative Bowen et al., 1983 Foot-and-mouth disease virus-infected 372 (64) Negative McVicar et al., 1987;
Mebus and Singh, 1988 Bovine herpesvirus-1-seropositive 13 (18) Negative Singh et al., 1982b
b
Bovine herpesvirus-1-infected 63 Negative Singh et al., 1983 Rinderpest virus-infected 107 (embryos and Negative Mebus, 1987
ova combined)
Brucella abortus-infected / seropositive 309 (82) Negative Barrios et al., 1988; Stringfellow et al., 1988; Voekel et al., 1983
Chlamydia psittaci-infected 5 Negative Bowen et al., 1978
a
This table is adapted from tabulated material in Appendix B, Manual of the International Embryo Transfer Society (Anonymous, 1998b). b
These embryos were treated with trypsin as part of the washing procedure prior to assay.
national Embryo Transfer Society (Stringfellow, agents with subsequent carriage from the in vivo
1998). For five of the nine viral pathogens (akabane environment (donor cow) has not been shown.
virus, bovine leukemia virus, bluetongue virus, To determine if pathogens might associate
differ-bovine viral diarrhea virus and foot-and-mouth dis- ently with embryos if exposure occurred in vivo, a
ease virus), embryos were free of infectious agent number of studies were conducted in which
zona-after artificial exposure and washing. For three other pellucida-intact bovine embryos or ova and uterine
viral pathogens (bovine herpesvirus-1, bovine recovery media from infected or seropositive donor
herpesvirus-4, and vesicular stomatitis virus) wash- cows were assayed for pathogen. The collective
ing was not totally effective, but washing with results of these studies were that pathogen could
trypsin was considered to be effective for producing often be found in the recovery media when donors
virus-free embryos. The report that rinderpest virus were known to be infected (Table 3), but after proper
was isolated from a small proportion (1–2%) of washing or trypsin treatment, pathogen was never
embryos after artificial exposure and washing was a isolated from embryos or ova (Table 2).
preliminary report that was never confirmed, and it Finally, more expensive studies and field trials
was later shown that zona-pellucida-intact embryos were conducted in which embryos were collected
and ova collected from cattle infected with rinderpest from infected or seropositive donor cattle and
trans-were free of virus after washing (Table 2). Thus, ferred to uninfected recipients. Afterward, the
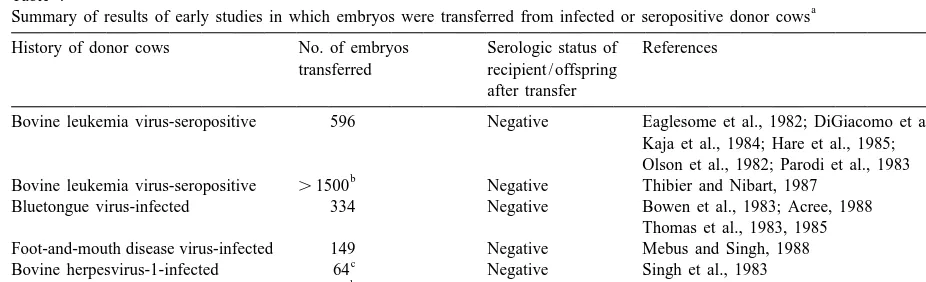
recipi-experimental evidence had accumulated to indicate ents and offspring were monitored for infection. For
that viral pathogens were not likely to penetrate the the six viral and one bacterial pathogen evaluated in
bovine zona pellucida, and the few that adhered to this way, no recipient or calf sero-converted (Table
the zona pellucida could be effectively removed by 4).
washing with trypsin.
Likewise, applied treatments were effective for
removal of the bacterial pathogens listed in Table 1. 4. Impact of the early research on approaches
Brucella abortus was effectively removed by wash- to health certification of embryos
ing and Haemophilus somnus was sensitive to
treat-ment with antibiotics. The remaining mycoplasmal Three strategies that have been used to produce
and mycobacterial pathogens were not removed by specific-pathogen-free embryos are testing of donor
any treatment, after artificial exposure, but the cattle, embryo treatment or a combination of donor
Sutmol-Table 3
Summary of results of early studies in which uterine recovery media from infected or seropositive donor cows were assayed for the a
pathogen
History of donor cow Uterine recovery medium Percent References (no. positive / total) positive
Bovine leukemia virus-seropositive 4 / 25 16% Bouillant et al., 1981 Bluetongue virus-infected 12 / 30 40% Bowen et al., 1983;
Thomas et al., 1985 Foot-and-mouth disease virus-infected 15 / 22 68% McVicar et al., 1987;
Mebus and Singh, 1988 Bovine herpesvirus-1-infected 9 / 33 27% Singh et al., 1983
b
Brucella abortus-infected / seropositive 9 / 116 8% Stringfellow et al., 1982, 1983, 1988; Voekel et al., 1983
a
This table is adapted from tabulated material in Appendix B, Manual of the International Embryo Transfer Society (Anonymous, 1998b). b
Positives were not cycling / superovulated cows.
Table 4
a Summary of results of early studies in which embryos were transferred from infected or seropositive donor cows
History of donor cows No. of embryos Serologic status of References transferred recipient / offspring
after transfer
Bovine leukemia virus-seropositive 596 Negative Eaglesome et al., 1982; DiGiacomo et al., 1986; Kaja et al., 1984; Hare et al., 1985;
Olson et al., 1982; Parodi et al., 1983 b
Bovine leukemia virus-seropositive .1500 Negative Thibier and Nibart, 1987 Bluetongue virus-infected 334 Negative Bowen et al., 1983; Acree, 1988
Thomas et al., 1983, 1985 Foot-and-mouth disease virus-infected 149 Negative Mebus and Singh, 1988
c
Bovine herpesvirus-1-infected 64 Negative Singh et al., 1983 b
Bovine herpesvirus-1-seropositive .1500 Negative Thibier and Nibart, 1987
Brucella abortus-seropositive 39 Negative Del Campo et al., 1987
a
This table is adapted from tabulated material in Appendix B, Manual of the International Embryo Transfer Society (Anonymous, 1998b). b
Embryos were collected from donor herds in which most of the cows were bovine leukemia virus- and bovine herpesvirus-1-seropositive.
c
Embryos treated with trypsin between collection and transfer.
ler, 1998; Givens and Stringfellow, 1999). The their International Animal Health Code (Anonymous,
cautious approach of donor testing was applied 1994).
initially to document the health of embryos that Standardized embryo washing and trypsin
treat-moved between countries. However, by the mid ments are detailed in the Manual of the International
1980s much of the early research (Tables 1–4) had Embryo Transfer Society (Stringfellow, 1998).
been completed and published. It had become clear Briefly, essential requirements for embryo washing
that risks of transmission of diseases by embryo are as follows: only groups of 10 or fewer,
zona-transfer were low, and the value of embryo treat- pellucida-intact embryos from a single donor should
ments that had been used so effectively in research be washed together. A minimum of 10 washes are
was recognized. Accordingly, the research protocols applied, using separate sterile micropipets between
for washing and trypsin treatment were standardized each two washes such that each wash is a 100-fold
by the International Embryo Transfer Society (String- dilution of the previous wash. After washing,
em-fellow, 1998) and the value of their use was bryos should be free of observable adherent material
at 503 magnification. Trypsin treatment has the not associate with zona-pellucida-intact embryos
same general requirements as washing except that after in vitro exposure when the 10-wash protocol
there are 12 washes. Embryos are pre-washed five described above was used (Bowen et al., 1982; Singh
times, exposed to trypsin in a 6th and 7th wash for a et al., 1982a). In comparing this 1990 report to
total of 60 to 90 s and then washed five more times previous reports, the logical conclusion would seem
without trypsin. to be that inadequate washing allows a greater
Depending on circumstances, donor testing might potential for the transmission of disease with
em-still be used in conjunction with or in lieu of embryo bryos.
treatment to certify health of transported embryos The reputed value of the 10-wash protocol for
(Anonymous, 1994). However, it is noteworthy that ensuring freedom from foot-and-mouth disease virus
the Research Subcommittee of the IETS has con- was reaffirmed in a report by Camaano et al. (1993).
cluded that sufficient evidence has accrued to show In their study, 94 zona-pellucida-intact bovine
em-7.5
that the risk of transmission of certain diseases is bryos and ova were exposed to high titers (10 ) of
negligible if embryos are properly treated between foot-and-mouth disease virus for 16 h and washed as
collection and transfer. These diseases are blueton- recommended in the IETS protocol. Then 79
em-gue, Brucella abortus, enzootic bovine leukosis, bryos / ova were assayed immediately for infectious
foot-and-mouth disease, and infectious bovine virus while 15 embryos were cultured for 24 h before
rhinotracheitis (IETS to the OIE; Anonymous, testing. No virus was isolated from any of the
1998a). properly washed embryos / ova, and exposure to the
virus had no apparent effect on embryonic develop-ment in the cultured group.
5. Embryo–pathogen research in the 1990s
5.2. Testing novel treatments for bovine embryos
The emphasis of embryo–pathogen research in the after artificial exposure to pathogens
1990s has shifted to deal more with the newer
in-vitro-derived embryo technologies. However, Since early studies identified prokaryotic
patho-there have been some additional reports with rele- gens that adhered to the zona pellucida after artificial
vance to in-vivo-derived bovine embryo production, exposure and washing or trypsin treatment (e.g.
and some questions still remain to be answered. mycoplasmas; Britton et al., 1988; Bielanski et al.,
1989; Riddell et al., 1989) there has been only slight
5.1. Additional ‘in vitro exposure and in vitro progress towards development of effective treatments
assay’ studies with bovine viruses to deal with these pathogens. Use of antibiotics in
embryo culture and wash media has continued to be
In a report by Gillespie et al. (1990), the infective based on conventional recommendations for their use
status of bovine embryos after artificial exposure to in cell culture media (Riddell and Stringfellow,
seven viruses was described with results seeming to 1998), but some insight has been gained into how
contradict those in previous studies. They declared antibiotics could be applied to combat prokaryotes.
that pseudorabies virus, bovine herpesvirus-1, ves- Increasing the concentration and time of exposure to
icular stomatitis virus, bluetongue virus, and bovine currently used antibiotics as well as increasing the
viral diarrhea virus all adhered to the bovine zona temperature of treatment media have all been tried.
pellucida after in vitro exposure but that bovine Riddell et al. (1993a) reported that treatment with
enterovirus and parainfluenza-3 virus did not adhere. kanamycin (1000 mg / ml) or tylosin (200 mg / ml)
Unfortunately, embryos were either not washed or was effective for producing Mycoplasma bovis-free
washed only five times between exposure and assay, bovine embryos when the artificially exposed
em-indicating that their methodology may have been bryos were washed 10 times and then incubated
responsible for results that conflicted with those in (378C) for an additional 4 h in media containing the
previous reports. For example, in early studies antibiotic. The concentrations of antibiotics in these
suppliers, but treatments had no apparent detrimental were effectively removed when embryos were
col-effects on the embryos. In a similar study, Otoi et al. lected from bovine leukemia-infected donor cows.
(1993) demonstrated that bacteria-free bovine em- Subsequently, 585 embryos collected from naturally
bryos could be produced after artificial exposure to infected donors were washed four times and
trans-Escherichia coli if they were incubated (38.58C) for ferred, resulting in 278 pregnancies. Four calves
2 h in medium with gentamicin (50mg / ml) prior to dying at parturition were not tested, but the other 274
washing 10 times. In an attempt to develop a novel were free of bovine leukemia virus.
treatment, Riddell et al. (1993b) tried to use organic In a small study, Schlafer et al. (1990) incubated
halamines to inactivate Mycoplasma bovis adhering day 6 embryos in bluetongue virus-infected cell
to bovine embryos. The mycoplasma were effective- cultures for 24 h, washed them three times and then
ly inactivated, but there was insufficient margin for transferred two embryos into each of three
seronega-error between mycoplasmacidal and embryocidal tive recipients. None of the three heifers became
concentrations to recommend regular use of these pregnant, but virus was isolated from blood and a
chemicals. vaginal swab from one of the three heifers taken on
In a novel approach for inactivation of viruses the 7th day after the embryos were transferred.
associated with embryos, Bielanski et al. (1992) Because of the experimental procedures used, it is
successfully used photosensitive agents (hemato- impossible to determine if adherence of the virus to
porphrin and hematoporphrin derivative) to inacti- embryos or inadequate washing was responsible for
vate bovine herpesvirus-1 and bovine viral diarrhea transmission of the virus.
virus. Their treatment had no apparent negative In a large scale study reported by Acree et al.
effect on the viability of embryos, but there is a need (1991), 60 heifers were artificially exposed to
to determine if normal rates of pregnancy with birth bluetongue virus. Embryos were collected from 59 of
of normal calves could be achieved after transfer of these heifers during either the acute or convalescent
embryos treated in this way. stage of the disease. Embryos also were collected
Certainly, added security could be provided by from serologically positive donors that were
pre-new broad-spectrum treatments for embryos, but sumed to have been naturally infected during the
there appears to be little current interest in research previous year. Thus, they were considered to be
in this area. Presumably, this is because current recovered donors. A total of 169, 141 and 52
protocols for certifying the health of embryos have embryos were collected from acute, convalescent and
been efficient and effective. recovered donors, respectively, and washed 10 times
according to the IETS standard procedure. In vitro
5.3. Collection and assay of embryos from infected assays (cell culture and embryonating chicken eggs)
donor cows or transfer of embryos after in vitro of 57, 20 and 25 of the embryos, respectively, were
exposure to pathogen negative for bluetongue virus. Furthermore, 248
embryos (110 from acute, 121 from convalescent and
In additional investigations in the 1990s, embryos 17 from recovered donors) were transferred to
were collected from donors that were artificially or seronegative recipients. A total of 95 calves were
naturally infected with bovine leukemia virus, born (36 from acute, 52 from convalescent and seven
bluetongue virus, foot-and-mouth disease virus or from recovered donors). There was no evidence of
bovine spongiform encephalopathy. Also, in one transmission of bluetongue to any recipients (or
study, embryos were subjected to long term exposure offspring) with embryos from acute or convalescent
in vitro to bluetongue virus. Then, as in earlier stage donors. However, two recipients of embryos
studies, the embryos were evaluated for their po- from recovered donors (and subsequently their
off-tential to transmit the pathogens by direct assay in spring) seroconverted between the 5th and 9th month
vitro or by transfer to recipients with subsequent after transfer of embryos. Subsequent investigation
evaluation of recipients and offspring for pathogen. yielded evidence that these recipients were naturally
Krolinski et al. (1994) evaluated a four-step, exposed to bluetongue virus in mid to late pregnancy
adequately protect the latter recipients from insect birth and two fetuses aborted at 5 months of
gesta-vectors is unfortunate, but failure to transmit virus tion. Sera from normal calves and one set of
with embryos from all of the acutely and convales- premature twins were negative for
anti-foot-and-cently infected donors is still a strong endorsement mouth-disease virus antibody. Thus, collective
re-for the IETS washing protocol. sults showed that the IETS protocol for washing was
There were two reports of studies evaluating effective for producing embryos without detectable
potential for use of embryo transfer to salvage amounts of associated virus regardless of whether in
genetic material from foot-and-mouth virus-infected vitro or in vivo assays were used.
cattle. Villar et al. (1990) reported on a field trial in Finally, the enormous task of determining if
Argentina in which 253 embryos were recovered properly washed embryos from donor cows with
from 48 foot-and-mouth virus seropositive (convales- clinical bovine spongiform encephalopathy (BSE)
cent) cows and washed according to IETS pro- might carry the disease to recipients or their embryo
cedures. The flushing fluids and 171 of the embryos transfer offspring was begun in 1990. Because of the
were tested for presence of virus by isolation in cell long incubation period of the disease, this study is
culture or intradermal lingual inoculation of negative not scheduled to conclude until the year 2001. A
cattle. All results were negative. In addition, the current update of results is found elsewhere in this
remaining 82 embryos were cryopreserved and 42 issue (see BSE update by Wrathall). Preliminary
were transferred to seronegative recipients in a foot- progress reported by (Wrathall et al., 1997) indicated
and-mouth-disease-free area of the country. Fourteen that bioassays of degenerate embryos / ova and
live calves were born. The calves and recipients uterine recovery medium from BSE affected cows
remained seronegative. Thus, the washing procedure were all negative.
and embryo transfer procedures were confirmed to be useful for preservation of germ plasm from infected
populations of cattle. 6. Bovine viral diarrhea virus (BVDV)
Mebus and Singh (1991) reported results of a
comprehensive evaluation of infective potential of Bovine viral diarrhea virus is an economically
embryos collected from donors acutely infected with significant pathogen that has worldwide distribution
foot-and-mouth disease virus. In this study, 436 among populations of cattle (Baker, 1995). The virus
embryos / ova were collected at slaughter from 30 is known to be associated with semen (Guerin et al.,
superovulated cows that had been exposed by in- 1992), ovaries (Booth et al., 1992) and serum
travenous inoculation of foot-and-mouth disease (Brock, 1998) from infected cattle. Programs to
virus 22 h prior to collection of embryos. All except eradicate the virus in cattle have been considered in
two of the donors had a detectable viremia at the several countries. In two early studies (Singh et al.,
time of embryo collection, and virus was isolated 1982a; Potter et al., 1984) zona pellucida-intact,
from the uterine recovery medium from seven in-vivo-derived, bovine embryos were reported to be
donors. All embryos and ova were washed according free of virus after artificial exposure to cytopathic
to IETS protocols prior to assay, cryopreservation or isolates of BVDV, washing and in vitro assay for
transfer. Two hundred and four washed embryos and virus (Table 1). Several more recent studies have
ova were sonicated and injected (via intradermal confirmed the value of IETS treatments for
produc-lingual route) into steers that remained clinically ing BVDV-free embryos. However, results of other
normal and seronegative. Thirty-two embryos / ova research have created some doubt.
with defects in the zona pellucida were assayed in
cell culture and no virus was isolated. One hundred 6.1. Association of BVDV with oocytes
and six fresh embryos and 43 cryopreserved embryos
were transferred to 80 and 31 recipients, respective- While it has been known for some time that
ly. All recipients remained seronegative and clinical- noncytopathic BVDV can result in early embryonic
ly normal. The outcome of these transfers were 15 death or be transmitted vertically from cow to fetus
(Brownlie, 1990), presence of pathogens within the Comparing the results of those studies with
persis-female gamete had not been considered a serious tently and acutely infected animals, it is tempting to
concern relative to in-vivo-derived embryo product- speculate that oocytes are less likely to contain
ion in cattle. Recently, evidence for presence of BVDV in acutely infected animals; nevertheless,
BVDV in oocytes of developing follicles of cattle oocyte infection in acutely infected animals cannot
was reported by Fray et al. (1998). Their study was be discounted. Besides, regardless of the type of
designed to investigate the cellular tropism of BVDV infection, we now have information that the female
in the ovaries of three persistently infected heifers. gamete could be infected with BVDV and the
Ovaries were collected from these heifers at post relevant epidemiologic question remains: are infected
mortem and assessed for presence (by virus isola- oocytes developmentally competent?
tion) and localization (by immunohistochemistry) of
7
BVDV. Samples from each ovary contained |10 6.2. Developmental competence of oocytes from
tissue culture infective doses50/ ml of BVDV. BVDV-infected cows
Immunofluorescence observed in cryosections of
each ovary indicated the presence of viral proteins in Whether oocytes containing BVDV are capable of
ovarian stroma, thecal cells in the walls of develop- final maturation, participation in conception and
ing follicles, cumulus cells and in a percentage of the development to transferrable stage embryos is a
oocytes examined. Approximately 2000 oocytes question that remains to be answered, yet a little
were viewed in sections from all of the ovaries with insight into the potential for this occurring is
pro-the observation that about 18.7% contained viral vided in some reports. For example, a negative
antigen. Further, there was no difference in per- impact of BVDV infection on ovarian function has
centage of BVDV-infected oocytes between those in been demonstrated. In a controlled study by Grooms
primordial (18.2%), primary (19.4%) and secondary et al. (1998c), acute infection with BVDV was
(21.2%) follicles. While their conclusion was that associated with a reduction in maximum diameter
oocytes and cumulus in developing follicles of and rate of growth of anovulatory and ovulatory
persistently infected cows are infected with BVDV, dominate follicles as well as a reduction in the
the epidemiologically relevant question that remains number of subordinate follicles.
unanswered is: are the infected oocytes developmen- Other investigations within the context of normal
tally competent? embryo production also demonstrated that BVDV
In two similar studies, immunohistochemical tech- infections reduced reproductive efficiency. The effect
niques were used in an attempt to identify ovarian of acute infections on superovulatory responses was
cell types that might be infected with BVDV follow- examined by Kafi et al. (1997). In their trial,
ing acute infection (Grooms et al., 1998a) and approximately equal groups of uninfected (n512)
following immunization with modified live BVDV and acutely infected (n513) Friesian heifers were
vaccine (Grooms et al., 1998b). Viral antigen was treated for superovulation, observed for estrus,
in-detected between 6 and 60 days after acute infections seminated, and underwent nonsurgical embryo
re-in re-interstitial stroma cells and macrophage-like cells covery. The infected heifers were inoculated
in-that were associated with primary follicles, secon- tranasally with virus on the 9th day preceding the
dary follicles, antral follicles, corpus luteum and planned day of insemination. Only three of 13
corpus albicans. However, specific staining of luteal, infected heifers displayed signs of estrus compared
thecal or granulosa cells was not observed. After to 10 of 12 control heifers. Also, the mean numbers
administration of modified live BVDV vaccines, viral of transferrable embryos from control and infected
antigen was detected in ovarian sections taken on animals were 4.0 and 0.2, respectively.
days 10, 20 and 30 after vaccination. Again stained In another report by Brock et al. (1997) the
cells were stromal and macrophage-like cells in the reproductive inefficiency of seven persistently
infec-ovarian cortex. The authors concluded in each study ted donors was evident by their consistent failure to
that the observed changes could lead to reduced respond to superstimulation and by their consistent
embryos was produced from these persistently infec- failed to identify BVDV RNA with PCR assay after
ted heifers. Most of these embryos and nonfertilized ova were washed 10 times using the IETS protocol.
and degenerated ova were washed according to IETS
guidelines and found to be free of BVDV as de- 6.3. Conclusions about potential spread of BVDV
termined by virus isolation and PCR assay. Also, a through transfer of in-vivo-derived embryos
BVDV-free calf was born after transfer of an embryo
from one of the persistently infected donors to a Finding BVDV antigen in developing oocytes is a
seronegative donor. concern, yet, to date, there is no report of BVDV
Two other reports have described the birth of associated with zona pellucida-intact embryos that
BVDV-free calves after transfer of embryos from have been washed according to the IETS protocol.
persistently infected donors. Wentink et al. (1991) Further, the few calves (n54) produced from
infec-reported the collection of one viable and five degen- ted cows have been BVDV-free. Also, it is logical to
erated embryos from a persistently infected cow. speculate that reduced reproductive efficiency of
Bovine viral diarrhea virus was isolated from re- infected cows might be partially due to incompetence
covery medium. The viable embryo was washed 10 of oocytes that are infected, oocytes that are from
times, treated with trypsin and transferred to a follicles with infected cells or oocytes from ovaries
BVDV-immune recipient, resulting in the birth of a that are impaired by stromal infection.
BVDV-free calf. Similarly, Bak et al. (1992) Of course, many questions remain to be answered.
superovulated and inseminated two persistently in- Among other concerns, future research should
ad-fected heifers and recovered embryos nonsurgically dress (1) the competence of oocytes from infected
from the uterus. Uterine recovery medium from both cows and (2) the efficiency of IETS washing
pro-animals contained BVDV. Eight embryos were re- cedures after in vitro exposure of embryos to
repre-covered from these two heifers and washed 10 times. sentative field isolates of type I and type II
Then six embryos were transferred to six recipients. noncytopathic BVDV.
Four recipients were pregnant at 35 days and two calved. Both calves were free of BVDV.
The only evidence that BVDV might be associated 7. Experience with natural disease transmission
with transferrable embryos from persistently infected via in-vivo-derived bovine embryos
cows was reported by Tsuboi and Imada (1998).
They superstimulated, artificially inseminated, and To date there has been no documented case of an
nonsurgically collected embryos from persistently infectious agent being transmitted through transfer of
infected Friesian heifers. A total of eight normal an in-vivo-derived bovine embryo. This is despite
embryos and four degenerated ova were recovered in the enormous number of embryos that have been
two collections from the first heifer while five transferred world wide over the past 20 years.
degenerated ova were recovered in a single collec- Statistics were not compiled until recently, so the
tion from the second heifer. The zona-pellucida- actual number of embryos transferred in the 1970s
intact embryos and ova were washed three times and and 1980s is not known. Some insight into 1980s
frozen prior to RNA extraction and assay by RT- activity was provided by a survey conducted by the
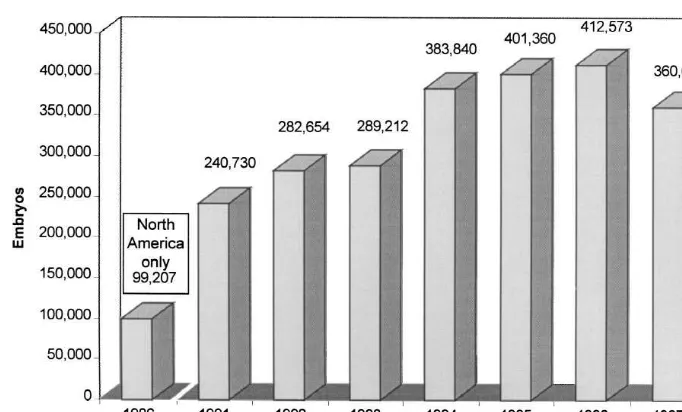
PCR. Of 17 ova and embryos, BVDV RNA was IETS in which nearly 100,000 embryos were
re-detected in association with one compact morula and portedly transferred in North America alone in 1986
one blastocyst from the first heifer. Results of this (Kraemer, 1987). Since 1991, annual data have been
study would have had greater significance if the retrieved by the IETS through a worldwide survey of
embryos and ova had been washed using IETS the embryo transfer industry. According to the most
guidelines prior to the RT-PCR assay. As it is, the recent report 360,656 in-vivo-derived embryos were
virus-positive embryos might have been the result of transferred worldwide in 1997 (Thibier, 1998). The
infections of the embryo, adherence of the virus to statistics for 1991 to 1997 are illustrated in Fig. 2.
the zona pellucida, or inadequate washing. It is It is likely that tens of thousands, if not hundreds
Fig. 2. Number of in-vivo-derived bovine embryos transferred worldwide (per IETS Data Retrieval Committee).
value of current washing and trypsin treatments were maintaining our current record of safety is the ethical
recognized and the techniques were consistently and technical excellence of those who apply the
used. Worldwide implementation of the concept of technology of embryo transfer.
approved embryo collection teams just began within the last few years (Evans, 1998). Thus, many of the embryos transferred, especially domestic transfers, in
References
early years were performed without knowledge of the benefit of current sanitary procedures. Despite
Acree, J.A., 1988. Embryo transfer in the control of bluetongue in
this circumstance, transmission of infectious agents
cattle. In: Proceedings 92nd Annual Meeting U.S.A.H.A., Little
was not observed. This early record of freedom from
Rock, Arkansas, Oct. 16–21, p. 124.
disease transmission with what might be considered Acree, J.A., Echternkamp, S.E., Kappes, S.M., Luedke, A.J.,
inadequate precautions serves to reinforce the view Holbrook, F.R., Pearson, J.E., Ross, G.S., 1991. Failure of
of innate safety of the procedures. That many embryos from bluetongue infected cattle to transmit virus to
susceptible recipients or their offspring. Theriogenology 36,
hundreds of thousands of embryos have been
trans-689–698.
ferred annually in the 1990s without transmitting
Anonymous, 1994. In: 6th ed, International Animal Health Code,
disease, supports the value of current sanitary proto- Office International des Epizooties, Paris.
cols. Anonymous, 1998a. Conclusions of the research subcommittee of
Of course, circumstances could change. It is the International Embryo Transfer Society (IETS) Import /
Export Committee. Rev. Sci. Tech. Off. Int. Epiz 17, 839.
conceivable that changes in technology, emerging
Anonymous, 1998b. Appendix B. Data on embryo-pathogen
pathogens, altered patterns of movement of embryos
studies in cattle, sheep, goats and swine, from tables in the
or some other factors might increase the potential for nd
IETS Manual, 2 Edition (1990). In: Stringfellow, D.A., rd
transmission of infectious agents through embryo Seidel, S.M. (Eds.), 3 Edition, Manual of the International
transfer. Hence, it is important to maintain an Embryo Transfer Society, IETS, Savoy, IL, pp. 151-163.
Bak, A., Callesen, H., Meyling, A., Greve, T., 1992. Calves born
awareness of changing technologies and their
appli-after embryo transfer from donors persistently infected with
cations within the context of the health of our cattle
BVD virus. Vet. Rec. 131, 37.
populations. Continued conscientious application of Baker, J.C., 1995. The clinical manifestation of bovine viral
our time-tested procedures for health certification of diarrhea virus infection. Vet. Clin. North Am.: Food Anim.
Barrios, D.R., Kraemer, D.C., Bessoudo, E., Adams, L., 1988. Del Campo, M.R., Tamayo, R., Del Campo, C.H., 1987. Embryo Failure to isolate Brucella abortus from embryos or ova from transfer from brucellosis-positive donors: a field trial. culture-positive superovulated cows. Theriogenology 29, 353– Theriogenology 27, 221, Abstract.
361. DiGiacomo, R.F., Studer, E., Evermann, J.E., Evered, J., 1986. Bentivelzen, P., Daama, J.H., Hayeman, P., Calafat, J., 1970. Embryo transfer and transmission of bovine leukosis virus in a
Genetic transmission of viruses that incite mammary tumors in dairy herd. J. Am. Vet. Med. Assoc. 188, 827–828.
mice. Proc. Natl. Acad. Sci. USA 67, 377–384. Divers, T.J., Bartholomew, R.C., Galligan, D., Littel, C., 1995. Betteridge, K.J., 1995. Phylogeny, ontogeny and embryo transfer. Evidence for transmission of bovine leukemia virus by rectal Theriogenology 44, 1061–1098. palpation in a commercial dairy herd. Prev. Vet. Med. 23, Bielanski, A., Eaglesome, M.D., Ruhnke, H.L., Hare, W.C.D., 133–141.
1989. Isolation of Mycoplasma bovis from intact and microin- Dunbar, B.S., 1983. Morphological, biochemical and immuno-jected preimplantation bovine embryos washed or treated with chemical characterization of the mammalian zona pellucida. In: trypsin or antibiotics. J. In Vitro Fert. Emb. Trans. 6, 236–241. Hartman, J.F. (Ed.), Mechanisms and Control of Animal Bielanski, A., Dubuc, C., Hare, W.C.D., Myers, D.J., Eaglesome, Fertilization, Academic Press, New York, pp. 139–175.
M.D., 1992. Inactivation of bovine herpesvirus-1 and bovine Eaglesome, M.D., Mitchell, D., Betteridge, K.J., Randall, G.C.B., virus diarrhea virus in association with preimplantation bovine Singh, E.L., Samagh, B.S., Hare, W.C.D., 1982. Transfer of embryos using photosensitive agents. Theriogenology 38, 633– embryos from bovine leukemia virus-infected (BLV-positive) 664. cattle to BLV-negative recipients. Preliminary results. Vet. Rec. Bielanski, A., Hare, W.C.D., 1998. Procedures for design and 111, 122–123.
analysis of research on transmission of infectious disease by Evans, B.R., 1998. Practical and ethical considerations for the embryo transfer. In: Stringfellow, D.A., Seidel, S.M. (Eds.), international certification and marketing of embryos. In: Strin-3rd Edition, Manual of the International Embryo Transfer gfellow, D.A., Seidel, S.M. (Eds.), 3rd ed, Manual of the Society, IETS, Savoy, IL, pp. 143–149. International Embryo Transfer Society, IETS, Savoy, IL, pp. Booth, P.J., Stevens, D.A., Collins, M.E., Brownlie, J., 1992. 7–15.
Detection of bovine viral diarrhoea virus (BVDV) in ovarian Fray, M.D., Prentice, H., Clarke, M.C., Charleston, B., 1998. and oviductal tissue. J. Reprod. Fertil., 928, Abstract. Immunohistochemical evidence for the localization of bovine Bouillant, A.M.P., Rukerbauer, G.M., Eaglesome, M.D., Samagh, virus diarrhea virus, a single-stranded RNA virus, in ovarian
B.S., Singh, E.L., Hare, W.C.D., Randall, G.C.B., 1981. At- oocytes in the cow. Vet. Path. 35, 253–259.
tempts to isolate bovine leukemia and bovine syncytial viruses Gillespie, J.H., Schlafer, D.H., Foote, R.H., Quick, S., Dougherty, from blood, uterine flush fluid, unfertilized ova and embryos E., Schiff, E., Allen, S., 1990. Comparison of persistence of from infected donor cattle. Ann. Rec. Vet. 12, 385–395. seven bovine viruses on bovine embryos following in vitro Bowen, R.A., Howard, T.H., Pickett, B.W., 1982. Interaction of exposure. Dtsch. Tierarztl. Wschr. 97, 65–68.
bluetongue virus with preimplantation embryos from mice and Givens, M.D., Stringfellow, D.A., 1999. Potential of embryo cattle. Am. J. Vet. Res. 40, 1907–1911. transfer for infectious disease control. In: Howard, J.L., Smith, Bowen, R.A., Howard, T.H., Elsden, R.P., Seidel, Jr. G.E., 1983. R.A. (Eds.), Current Veterinary Therapy 4: Food Animal
Embryo transfer from cattle infected with bluetongue virus. Practice, W.B. Saunders, Philadelphia, pp. 592–595. Am. J. Vet. Res. 44, 1625–1628. Grooms, D.L., Brock, K.V., Pate, J.L., Day, M.L., 1998a. Changes Bowen, R.A., Spears, P., Storz, J., Seidel, Jr. G.E., 1978. Mecha- in ovarian follicles following acute infections with bovine viral
nism of infertility in genital tract infections due to Chlamydia diarrhea virus. Theriogenology 49, 595–605.
psittaci transmitted through contaminated semen. J. Infect. Dis. Grooms, D.L., Brock, K.V., Ward, L.A., 1998b. Detection of 138, 95–98. bovine viral diarrhea virus in the ovaries of cattle acutely Britton, A.P., Miller, R.B., Ruhnke, H.L., Johnson, W.M., 1988. infected with bovine viral diarrhea virus. J. Vet. Diagn. Invest.
The recovery of ureaplasmas from bovine embryos following 10, 125–129.
in vitro exposure and ten washes. Theriogenology 30, 997– Grooms, D.L., Brock, K.V., Ward, L.A., 1998c. Detection of 1003. cytopathic bovine viral diarrhea virus in the ovaries of cattle Brock, K.V., 1998. Quality control for materials of animal origin following immunization with a modified live virus vaccine. J.
used in embryo production and transfer. In: Stringfellow, D.A., Vet. Diagn. Invest. 10, 130–134.
Seidel, S.M. (Eds.), 3rd ed, Manual of the International Guerin, B., Chaffaux, S., Marquant-LeGuienne, B., Allietta, M., Embryo Transfer Society, IETS, Savoy, IL, pp. 135–140. Thibier, M., 1992. IVF and IV culture of bovine embryos using Brock, K.V., Lapin, D.R., Skrade, D.R., 1997. Embryo transfer semen from a bull persistently infected with BVD.
from donor cattle persistently infected with bovine viral Theriogenology 37, 217, Abstract.
Kafi, M., McGowan, M.R., Kirkland, P.D., Jillella, D., 1997. The Riddell, K.P., Stringfellow, D.A., 1998. The use of antibiotics in effect of bovine pestivirus infection on the superovulatory media for recovery, culture and storage of embryos. In: response of Friesian heifers. Theriogenology 48, 985–996. Stringfellow, D.A., Seidel, S.M. (Eds.), 3rd ed, Manual of the Kaja, R.W., Olson, C., Rowe, R.F., Stauffacher, R.H., Strozinski, International Embryo Transfer Society, IETS, Savoy, IL, pp.
L.L., Hardie, A.R., Bause, I., 1984. Establishment of a bovine 85–91.
leukosis virus-free dairy herd. J. Am. Vet. Med. Assoc. 184, Riddell, K.P., Stringfellow, D.A., Gray, B.W., Riddell, M.G., 184–185. Galik, P.K., 1993a. Antibiotic treatment of bovine embryos. J. Kraemer, D.C., 1987. Current status, advantages and potential Assist. Reprod. Genet. 10, 488–491.
applications of embryo transfer in relation to animal production Riddell, K.P., Stringfellow, D.A., Gray, B.W., Riddell, M.G., and / or species preservation in North America. In: Hare, Galik, P.K., 1993b. Antimicrobial treatment of bovine embryos. W.C.D., Seidel, S.M. (Eds.), Proceedings of the International Theriogenology 39, 297, Abstract.
Embryo Movement Symposium, IETS, Montreal, pp. 63–73. Riddell, K.P., Stringfellow, D.A., Gray, B.W., Riddell, M.G., Krolinski, J., Marcinkowski, K., Lukaszewski, Z., Otachel-Haw- Wright, J.C., Galik, P.K., 1993c. Structural and viral associa-ranek, J., 1994. Bovine embryo transfer as a method of tion comparisons of bovine zonae pellucidae from follicular obtaining healthy offspring from cows infected with bovine oocytes, day-7 embryos and day-7 degenerated ova. leukosis virus (BLV). Medycyna Weterynaryjna 48, 79–81. Theriogenology 40, 281–291.
Lauerman, L.H., Stringfellow, D.A., Sparling, P.H., Kaub, L.M., Riddell, K.P., Stringfellow, D.A., Panangala, V.S., 1989. Inter-1986. In vitro exposure of preimplantation bovine embryos to action of Mycoplasma bovis and Mycoplasma bovigenitalium vesicular stomatitis virus. J. Clin. Microbiol. 24, 380–383. with preimplantation bovine embryos. Theriogenology 32, Mallek, Z., Guerin, B., Nibart, M., Parez, M., Thibier, M., 1984. 633–641.
Consequences de la contamination in vitro des embryons de Rohde, R.F., Shulaw, W.P., Hueston, W.D., Bech-Nielsen, S., souris et de vaches par Brucella abortus. Bull. Acad. Vet. Haibel, G.K., Hoffsis, G.F., 1990. Isolation of Mycobacterium France 57, 479–490. paratuberculosis from washed bovine ova after in vitro
expo-McVicar, J.W., Singh, E.L., Mebus, C.A., Hare, W.C.D., 1987. sure. Am. J. Vet. Res. 51, 708–710.
Embryo transfer as a means of controlling viral infections. VIII. Schlafer, D.H., Gillespie, J.H., Foote, R.H., Quick, S., Pennow, Failure to detect foot-and-mouth disease viral infectivity asso- N.N., Dougherty, E.P., Schiff, E.I., Allen, S.E., Powers, P.A., ciated with embryos collected from infected donor cattle. Hall, C.E., Voss, H., 1990. Experimental transmission of bovine Theriogenology 26, 595–601. viral diseases by insemination with contaminated semen during Mebus, C.A., 1987. Report to the Infectious Diseases of Cattle embryo transfer. Dt. Tierarztl. Wschr. 97, 68–72.
Committee. In: Proceedings 91st Annual Meeting U.S.A.H.A., Sellens, M.H., Jenkins, E.J., 1975. Permeability of the mouse zona Louisville, KY, Oct. 25–30, p. 10. pellucida to immunoglobulin. J. Reprod. Fertil. 42, 153–157. Mebus, C.A., 1988. Report to the Embryo Movement Subcommit- Singh, E.L., Eaglesome, M.D., Thomas, F.C., Papp-Vid, G., Hare,
tee of the U.S.A.H.A. Import–Export Committee. In: Proceed- W.C.D., 1982a. Embryo transfer as a means of controlling the ings 92nd Annual Meeting U.S.A.H.A., Little Rock, AR, Oct. transmission of viral infections. I. The in vitro exposure of 16–21, p. 246. preimplantation bovine embryos to akabane, bluetongue, and Mebus, C.A., Singh, E.L., 1988. Failure of bovine embryos from bovine viral diarrhea viruses. Theriogenology 17, 437–444.
viremic donors to transmit foot-and-mouth disease. In: Singh, E.L., Thomas, F.C., Eaglesome, M.D., Papp-Vid, G., Hare, Proceedings 92nd Annual Meeting U.S.A.H.A., Little Rock, W.C.D., 1982b. Embryo transfer as a means of controlling the AR, Oct. 16–21, pp. 183–185. transmission of viral infections. II. The in vitro exposure of Mebus, C.A., Singh, E.L., 1991. Embryo transfer as a means of preimplantation bovine embryos to infectious bovine
rhinot-controlling the transmission of viral infections. XIII. Failure to racheitis virus. Theriogenology 18, 133–140.
transmit foot-and-mouth disease virus through the transfer of Singh, E.L., Hare, W.C.D., Thomas, F.C., Bielanski, A., 1983. embryos from viremic donors. Theriogenology 35, 435–441. Embryo transfer as a means of controlling viral infections. IV. Olson, C., Rowe, R.F., Kaja, R., 1982. Embryo transplantation Non-transmission of infectious bovine rhinotracheitis / infecti-and bovine leukosis virus. Preliminary Report. In: Straub, O.C. ous pustular vulvovaginitis virus from donors shedding virus. (Ed.), Fourth International Symposium On Bovine Leukosis, Theriogenology 20, 169–176.
Martinus Nijhoff, The Hague, pp. 361–370. Singh, E.L., McVicar, J.W., Hare, W.C.D., Mebus, C.A., 1986. Otoi, T., Tachikawa, S., Kondo, S., Suzuki, T., 1993. Effect of Embryo transfer as a means of controlling transmission of viral washing, antibiotics and trypsin treatment of bovine embryos infections. VII. The in vitro exposure of bovine and porcine on the removal of adhering k99 Escherichia coli. J. Vet. Sci. 55, embryos to foot-and-mouth disease virus. Theriogenology 26,
1053–1055. 587–593.
Parodi, A., Manet, G., Vaillaume, A., Crespau, F., Toma, B., Levy, Singh, E.L., Thomas, F.C., 1987. Embryo transfer as a means of D., 1983. Transplantation embryonnaire et transmission de controlling the transmission of viral infections. XI. The in vitro l’agent de la leucose bovine enzootique. Bull. Acad. Vet. France exposure of bovine and porcine embryos to vesicular stomatitis
56, 183–189. virus. Theriogenology 28, 691–697.
Stringfellow, D.A., 1998. Recommendations for the sanitary Thibier, M., 1998. The 1997 embryo transfer statistics from handling of in-vivo-derived embryos. In: Stringfellow, D.A., around the world: a data retrieval committee report. Emb. Seidel, S.M. (Eds.), 3rd ed, Manual of the International Trans. News. 16, 17–20.
Embryo Transfer Society, IETS, Savoy, IL, pp. 79–84. Thibier, M., Nibart, M., 1987. Disease control and embryo Stringfellow, D.A., Howell, V.L., Schnurrenberger, P.R., 1982. importation. Theriogenology 27, 37–47.
Investigations into the potential for embryo transfer from Thomas, F.C., Singh, E.L., Hare, W.C.D., 1983. Embryo transfer
Brucella abortus infected cows without transmission of in- as a means of controlling viral infections. III. Non-transmission fection. Theriogenology 18, 733–743. of bluetongue virus from viremic cattle. Theriogenology 19, Stringfellow, D.A., Scanlan, C.M., Hannon, S.J., Panangala, V.S., 425–431.
Gray, B.W., Galik, P.K., 1983. Culture of uterine flushings, Thomas, F.C., Singh, E.L., Hare, W.C.D., 1985. Embryo transfer cervical mucus and udder secretions collected post-abortion as a means of controlling viral infections. VI. Bluetongue from heifers artificially exposed to Brucella abortus. virus-free calves from infectious semen. Theriogenology 24,
Theriogenology 20, 77–83. 345–350.
Stringfellow, D.A., Scanlan, C.M., Brown, R.R., Meadows, G.B., Thomson, M.S., Stringfellow, D.A., Lauerman, L.H., 1988. Gray, B.W., Young-White, R.R., 1984. Culture of bovine Adherence of Haemophilus somnus to bovine embryos after in embryos after in vitro exposure to Brucella abortus. vitro exposure. Am. J. Vet. Res. 49, 63–66.
Theriogenology 21, 1005–1012. Tsuboi, T., Imada, T., 1998. Detection of BVDV in bovine Stringfellow, D.A., Wolfe, D.F., McGuire, J.A., Lauerman, L.H., embryos derived from persistently infected heifers by PCR. Vet.
Gray, B.W., Sparling, P.H., 1986. Effects of embryo-freezing Rec. 142, 114–115.
and thawing techniques on the survivability of Brucella Voekel, S.A., Stuckey, K.W., Looney, C.R., Enright, F.M., Humes,
abortus. Theriogenology 26, 553–559. P.E., Godke, R.A., 1983. An attempt to isolate Brucella abortus Stringfellow, D.A., Wright, J.C., 1987. Viewing the scientific from uterine flushings of superovulated donor cattle.
evidence for the health status of embryos for international Theriogenology 19, 255–366.
movement. In: Hare, W.C.D., Seidel, S.M. (Eds.), International Villar, J.A., Munar, C., Salomone, D., Caamano, J.N., Laporte, O., Embryo Movement Symposium, IETS, Ottawa, pp. 134–141. Burry, E., Vautier, R., Sadir, A., Singh, E.L., Acree, J.A., Stringfellow, D.A., Panangala, V.S., Galik, P.K., 1988. Recovery Carrillo, B., 1990. Transferencia de embriones bovinos libres and culture of ova from Brucella abortus infected cows. del virus de la fiebre aftosa. Revista de Medicina Veterinaria
Theriogenology 29, 1105–1112. 71, 268–276.
Stringfellow, D.A., Lauerman, L.H., Thomson, M.S., 1989. Wentink, G.H., Aarts, J., Mirck, M.H., vanExsel, A.C.A., 1991. Trypsin treatment of bovine ova after in vitro exposure to Calf from a persistently infected heifer born after embryo vesicular stomatitis virus. Am. J. Vet. Res. 50, 990–992. transfer with normal immunity to BVDV. Vet. Rec. 129, 449– Stringfellow, D.A., Wright, J.C., 1989. A review of the epi- 450.
demiologic aspects of embryo transfer from Brucella abortus- Wrathall, A.E., Brown, K.F.D., Fraser, H., Chree, A., Ferguson, infected cows. Theriogenology 31, 997–1006. C.A., 1997. Embryos and uterine flush fluids from cattle with Stringfellow, D.A., Lauerman, L.H., Nasti, K.B., Galik, P.K., bovine spongiform encephalopathy are not infective for mice.
1990. Trypsin treatment of bovine ova after in vitro exposure Theriogenology 47, 384, Abstract.
to infectious bovine rhinotracheitis virus or bovine herpesvirus- Wrathall, A.E., Sutmoller, P., 1998. Potential of embryo transfer to 4. Theriogenology 34, 427–434. control transmission of disease. In: Stringfellow, D.A., Seidel, Stringfellow, D.A., Riddell, K.P., Zurovac, O., 1991. The potential S.M. (Eds.), 3rd ed, Manual of the International Embryo