Control of postharvest decay of apple fruit by
Aureobasidium pullulans
and induction of defense responses
Antonio Ippolito
a,*, Ahmed El Ghaouth
b,c, Charles L. Wilson
c,
Michael Wisniewski
caDipartimento di Protezione delle Piante e Microbiologia Applicata,Uni6ersita` degli Studi di Bari, Via Amendola 165/A-70126 Bari, Italy
bFaculte des Sciences et Techniques,Uni6ersite´ de Nouakchott,Nouakchott BP5026, Mauritania cUSDA-ARS Appalachian Fruit Research Station,45 Wiltshire Road,Kearneys6ille,WV 25430, USA
Received 14 September 1999; accepted 17 April 2000
Abstract
The biocontrol activity of Aureobasidium pullulans on decay of apple fruit caused by Botrytis cinerea and
Penicillium expansum, and its ability to induce biochemical defense responses in apple tissue, were investigated. In apple wounds,A.pullulansmultiplied rapidly and controlled decay caused by eitherB.cinereaorP.expansum. At the end of the storage period, A. pullulans reduced the incidence of gray and blue mold of apple by 89 and 67%, respectively, compared to the water-treated control. In addition to controlling decay,A.pullulanscaused a transient increase inb-1,3-glucanase, chitinase, and peroxidase activities starting 24 h after treatment and reaching maximum levels 48 and 96 h after treatment. An increase inb-1,3-glucanase, chitinase, and peroxidase activity was also triggered by wounding, although, the level of increase was markedly lower than that detected in treated fruit. The ability ofA.
pullulans to increase activities of b-1,3-glucanase, chitinase, and peroxidase in addition to its known capacity to out-compete pathogen for nutrients and space, may be the basis of its biocontrol activity. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Apple fruit; Aureobasidium pullulans;b-1,3-glucanase; Biocontrol; Blue mold; Botrytis cinerea; Chitinase; Gray mold; Microbial antagonist;Penicillium expansum; Peroxidase
www.elsevier.com/locate/postharvbio
1. Introduction
Due to the development of fungicide-resistant isolates of postharvest pathogens, and the possible
deregistration of some of the more effective postharvest fungicides (Eckert et al., 1994), the development of microbial antagonists for the con-trol of postharvest decay of fruit has been pur-sued actively in several laboratories (Roberts, 1990; Wilson and Wisniewski, 1994; Bull et al., 1997; Chand-Goyal and Spotts, 1997; Droby et al., 1998; El Ghaouth et al., 1998; Janisiewicz,
* Corresponding author. Tel.:+39-080-5443053; fax:+ 39-080-5442911.
E-mail address:[email protected] (A. Ippolito).
1998; Ippolito and Nigro, 2000). Currently, at least three formulated microbial antagonists As-pire, Biosave-100, and Biosave-110 are now com-mercially available for use as a postharvest treatment. The main mode of action of the yeast biocontrol agents is believed to be competition for nutrients and space (Droby and Chalutz, 1994). Additional modes of action such as mycopara-sitism, induced resistance and the production of lytic enzymes such as b-1,3-glucanase have been suggested (Wisniewski et al., 1991; El Ghaouth et al., 1998).
Recently, we have isolated the yeast-like fun-gus, Aureobasidium pullulans (de Bary) Arnaud, from the surface of table grape berries and have shown that it was effective in controlling decay of table grape, kiwifruit, strawberry and sweet cherry (Ippolito et al., 1997; Lima et al., 1997; Nigro et al., 1997; Schena et al., 1999). Isolates of A. pullulans were shown also to be effective in pro-tecting onion leaf surfaces (Fokkema and
Lor-beer, 1974), and moderately suppressed
sporulation ofBotrytis acladaFresen andBotrytis cinerea Pers.:Fr. on dead leaves (Kohl et al., 1997). In vitro studies revealed that A. pullulans was capable of secreting antibiotic compounds (McCormack et al., 1994) and this was indirectly supported by the cytoplasmic alterations dis-played by B. aclada cells in intimate contact with A. pullulans cells (Kohl et al., 1997). In order to maximize the potential use ofA. pullulansfor the control of postharvest decay of fruit, an under-standing of its mode of action is necessary. Little has been published regarding its ability to induce host defense responses. In the present study, we report on the biocontrol activity of A. pullulans against major postharvest pathogens of apple fruit and also delineate some of the biochemical defense reactions that occur during the interaction between A. pullulans and apple tissue.
2. Materials and methods
2.1. Microorganisms and fruit materials
Isolates of Penicillium expansum Link and B. cinerea were isolated from infected fruit and
maintained on potato dextrose agar (PDA). A spore suspension was obtained by flooding 2-week-old PDA cultures of B. cinerea and P. ex -pansumwith sterile distilled water containing 0.1% (v/v) Tween 80. Spore concentration of the patho-gens was determined with a hemacytometer and adjusted with sterile distilled water to obtain 106
spores ml−1ofB.cinereaand 104 spores ml−1of
P. expansum.
A.pullulansisolate L47, was grown at 24°C for 48 h in shake-flask cultures of nutrient-yeast broth (NYB). Yeast cells were pelleted by cen-trifugation with a Sorval RC-58 centrifuge (Du-pont Instruments, Wilmington, DE) at 3000×g for 20 min, resuspended in sterile distilled water, and centrifuged again. The resulting pellets were dispersed in sterile distilled water and the concen-tration of the antagonist was adjusted to 6×107
colony-forming units (CFU) ml−1 using a
hemacytometer.
Tree-ripe apples (Malus domestica Borkh. cv Red Delicious) were hand-harvested at commer-cial maturity at the USDA-ARS, Appalachian Fruit Research Station, Kearneysville, WV. The fruit were sorted on the basis of size and the absence of physical injuries or infections and stored at 4°C under regular air for 2 months before their use in biocontrol tests.
2.2. Biocontrol acti6ity of A. pullulans
Apple fruit were wounded (3 mm×5 mm
deep), treated with either 35 ml of a cell suspen-sion of A. pullulans at 6×107 CFU ml−1 or
sterile distilled water, and challenge-inoculated with 20 ml of a conidial suspension of B. cinerea or P. expansum at 106 and 104 spores ml−1,
ho-mogeneity of variances. Treatment means were separated by Duncan’s multiple range test.
2.3. Wound colonization by A. pullulans
Apples were wounded, treated with 35 ml of antagonist suspension, challenge-inoculated with a conidial suspension of B. cinerea or treated with sterile distilled water, and stored at 24°C as described above. For each treatment, 40
fruits were arranged in a randomized
complete block and the experiment was done twice, with similar results combined for statistical analysis.
Tissue samples from the different treatments were collected at various times (0, 6, 12, 24, 48, 72, and 96 h) after treatment. At each sampling time, tissue samples containing the wounds were removed with a No. 7 cork borer from four apples randomly selected from each treatment. Tissue samples were homogenized in 5 ml of sterile wa-ter, vortexed, dilution-plated in triplicate on yeast – maltose agar medium, and the plates were incubated at 24°C. Colonies were counted after 48 h and the results are expressed as the mean num-ber of CFU per wound.
2.4. Induction of defense responses by A. pullulans
Apple fruit were individually wounded, treated with a yeast-like fungus suspension at 6×107
CFU ml−1 or sterile distilled water and stored at
20°C as described above. For each treatment, four replicates of ten fruit each were arranged in a randomized complete block design. The experi-ment was maintained for 1 week and repeated twice. Tissue samples were collected at various time intervals (0, 24, 48 and 96 h) after treatment from six fruit randomly selected from each treatment. From each fruit, tissue samples were taken from the wounds and individually homogenized at 4°C in two volumes (w/v) of 50 mM sodium acetate buffer, pH 5.0, and the ho-mogenate was centrifuged at 4°C (15 min, 10 000×g). Proteins in the supernatant were pre-cipitated in 60% acetone (v/v) at −20°C and the resulting pellet, following centrifugation
(30 min, 12 000×g at 4°C), was washed three times with 60% acetone. The pellet was suspended in 2 ml of 50 mM sodium acetate buffer (pH 5.0) and assayed for chitinase, b-1,3-glu-canase, and peroxidase activities. Protein concen-trations were determined with the Bio – Rad protein assay kit.
2.5. Enzyme assays
Chitinase activity was assayed using dye-labeled carboxymethylchitin following the method of Wirth and Wolf (1990). Chitinase activity was
measured by mixing 100 ml of crude enzyme
solution with 200 ml of 2% dye-labeled car-boxymethylchitin in 50 mM sodium acetate
buffer (pH 5.0). After 1 h of incubation
at 37°C, the reaction was stopped by adding 100 ml of 1.0 M HCl, the reaction mixture cooled,
centrifuged, and the absorbance of the
supernatant was measured at 550 nm. Chitinase activity was calculated according to Wirth and Wolf (1990) and expressed in international units (U) mg−1 of total protein. One unit (U) was
defined as the amount of enzyme required to catalyze the formation of 1 nmol min−1
of product.
b-1,3-glucanase was determined following the method of Abeles and Forrence (1979). b-1,3-glu-canase activity was assayed by incubating 62.5 ml of enzyme preparation for 2 h at 40°C in 62.5 ml of 4% laminarin. The reaction was terminated by heating the sample in boiling water for 10 min and the amount of reducing sugars was measured spectrophotometrically at 492 nm after reaction with 372 ml of 3,5-dinitrosalicilate. Final activity values are reported as nktalmg−1of total protein.
One nktal was defined as the enzyme activity catalyzing the formation of 1 nmol s−1
glucose equivalents.
Peroxidase activity was determined using guaia-col as substrate (Hammerschmidt et al., 1982). The reaction mixture consisted of a (1:1) mixture of crude extract and the following solution: 10 mM guaiacol and 10 mM H2O2as substrate in 50
3. Results
3.1. Biocontrol acti6ity
A.pullulanswas effective in controlling decay of apple fruit caused by B.cinerea andP. expansum (Fig. 1 A and B). Fruit treated withA. pullulans and then inoculated with either pathogen showed symptoms of infection only after 5 days of storage at 24°C, while among control fruit, lesions were
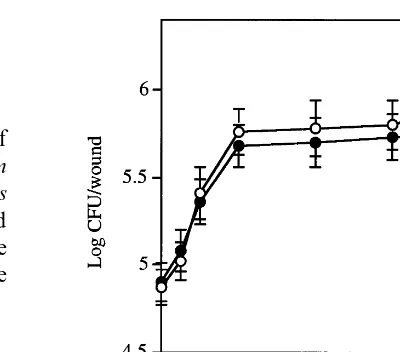
Fig. 2. Population dynamics of Aureobasidium pullulans in apple wounds in the presence ( ) and absence of Botrytis cinerea (). Bars represent standard deviations.
Fig. 1. Biocontrol activity ofAureobasidium pullulanson decay of ‘Red Delicious’ apples caused byBotrytis cinerea(A) and Penicillum expansum(B). Apple wounds were treated with 35 ml aliquots of A. pullulans (6×107 CFU ml−1) or sterile distilled water, and challenge-inoculated with 30 ml of a conidial suspension ofB.cinereaorP.expansum. The percent-age of decay was based on four replicates of 20 fruit each. Incidence of decay on apples was determined after 7 and 14 days of storage at 24°C. Columns with the same letter within the same time interval are not significantly different according to Duncan’s multiple range test,P=0.05.
visible by the 3rd day of storage (data not shown). By day 7, over 80% of the control fruit were diseased, while 1 and 11% of fruit treated withA. pullulans developed lesions caused by B. cinerea and P. expansum, respectively. By the end of the 14-day storage period, decay incidence among apple fruit treated with A. pullulans and then inoculated with B. cinerea and P. expansum was 11 and 33%, respectively, while 100% of the con-trol fruit were diseased (Fig. 1 A and B). In apple wounds, A. pullulans multiplied similarly in the presence or absence of B. cinerea (Fig. 2). An initial increase in the population of A. pullulans occurred after 6 h at 24°C. Within 24 h, the A. pullulans population size increased nearly seven-fold and remained elevated for 4 days.
3.2. Induction of antifungal hydrolases
respectively, above that observed in wounded, non-inoculated control tissue. Comparison of the time-dependent increase in chitinase and
b-1,3-glucanase in response to treatment with A.pullu -lans showed that the overall increase in chitinase was lower than that of b-1,3-glucanase (Fig. 3 A vs. B). An increase in chitinase and b-1,3-glu-canase activity with time was observed also in wounded control fruit, but the level of increase was markedly lower than that detected in treated fruit. By 48 h after treatment,b-1,3-glucanase and chitinase activity in wounded fruit was over one-fold higher than non-wounded control levels (Fig. 3 A and B). In non-wounded control fruit, a comparatively small increase in b-1,3-glucanase and chitinase activity with time was detected.
Analysis of the induction of peroxidase activity also showed a pattern similar to that for chitinase and b-1,3-glucanase in apples treated with A. pullulans (Fig. 3 C). Peroxidase activity increased in treated apples with time, reaching a maximum 48 h after treatment. Peroxidase activity then slightly decreased by 96 h after treatment but remained over one-fold higher than wounded con-trol levels. At 48 h after treatment, peroxidase activity increased by approximately two-fold above that observed in wounded control fruit. An increase in peroxidase activity with time was de-tected also in wounded and non-wounded control fruit and the level of increase was slightly higher in wounded fruit, especially 48 h after treatment.
4. Discussion
The present data show that A. pullulans when applied as a wound treatment was effective in controlling postharvest decay of apple fruit caused by B. cinerea and P. expansum. It also induced the accumulation of chitinase, b-1,3-glu-canase, and peroxidase in apple fruit over and above the stimulation of these enzymes in wounded, non-inoculated fruit. Comparable levels of decay control have been reported with other microbial antagonists (Roberts, 1990; Wilson and Wisniewski, 1994; Bull et al., 1997; Chand-Goyal and Spotts, 1997; Droby et al., 1998; El Ghaouth et al., 1998; Janisiewicz, 1998; Nigro et al., 1999) and attributed to a complex mechanism that may involve nutrient competition, site exclusion, direct parasitism, production of lytic enzymes, and
sibly induced resistance (Droby and Chalutz, 1994). Supporting evidence for a putative in-volvement of nutrient and space competition and direct parasitism in the mode of action of antagonistic yeasts and yeast-like fungi comes from: (1) observations that biocontrol activity of microbial antagonists is concentration-dependent and can be reversed by the addition of exoge-nous nutrients (Droby et al., 1989), and (2) the fact that they are capable of attaching tena-ciously to fungal cell walls and causing severe cell-wall alterations (Wisniewski et al., 1991; El Ghaouth et al., 1998).
The possibility that antagonists may also in-duce host defense mechanisms has been inferred but not substantiated. Antagonistic yeasts were shown to induce the accumulation of scoparone in lemon fruit (Rodov et al., 1994) and the for-mation of structural barriers in apple (El Ghaouth et al., 1998). Similarly, in the present study A. pullulans caused a transient increase in chitinase, b-1,3-glucanase, and peroxidase activ-ity in apple fruit. It is not clear, however, whether or not the total chitinase and b-1,3-glu-canase activity detected in yeast-treated wounds is mainly of host origin, especially since A. pul -lulans like other antagonists is capable of pro-ducing b-1,3-glucanase (Castoria et al., 1997) and chitinase. Preliminary studies, however, indi-cated that none of the chitinase and b-1,3-glu-canase isoforms detected in yeast-treated tissue were of yeast origin (data not shown). Detailed analysis of chitinase and b-1,3-glucanase iso-forms using polyacrylamide gel electrophoresis (Trudel and Asselin 1989) will provide further insight as to their regulation and origin.
Antifungal glucanohydrolases and peroxidases are considered potentially important in host re-sistance mechanisms. Peroxidase is involved in lignin formation (Hammerschmidt et al., 1982; Kuc, 1990), while chitinase and b-1,3-glucanase are capable of hydrolyzing fungal cells, and in combination they have been shown to inhibit the growth of several pathogenic fungi in vitro (Schlumbaum et al., 1986; Sela-Buurlage et al., 1993). These observations, together with the fact that the induction and accumulation of glu-canohydrolases and peroxidases is often
corre-lated with the onset of induced resistance, suggest an active role for these enzymes in de-fense against pathogenic fungi (Kuc, 1990; Sticher et al., 1997; Van Loon et al., 1998). It is not possible, however, to determine the extent of the role played by host defense responses in the observed protection because of the antago-nistic activity of A. pullulans at the wound site. The accumulation of chitinase, b-1,3-glucanase, and peroxidase can be expected to retard fungal growth. Considering that the antagonist, A. pul -lulans, also possesses b-1,3-glucan and chitin in its cell wall (Bartnicki-Garcia, 1968), this raises the question of its sensitivity to chitinases and b-1,3-glucanases produced by the fruit.
The results from the analysis of the wound site suggest that growth of A. pullulans in wound cavities was not impaired by the accu-mulation of chitinase and b-1,3-glucanase. It is quite possible that the lytic enzymes of host origin may act differently on antagonistic yeast confined to wound cavities than on necrophitic pathogens. In ectomycorrhizal interactions, plant secreted chitinases and b-1,3-glucanases in-hibitory toward pathogenic fungi showed no ef-fect on ectomycorrhizal fungi, thus allowing the establishment of a symbiotic interactions (Peter et al., 1997). Also, filamentous fungi, despite having similar cell wall constituents (Bartnicki-Garcia, 1968), are known to display differential sensitivity to chitinases and b-1,3-glucanases (Schlumbaum et al., 1986).
Acknowledgements
The stay of Dr A. Ippolito in the laboratory of C.L. Wilson was partially supported by a FUL-BRIGHT fellowship.
References
Abeles, F.B., Forrence, L.E., 1979. Temporal and hormonal controlb-1,3-glucanase inPhaseolus6ulgarisL. Plant Phys-iol. 45, 395 – 400.
Bartnicki-Garcia, S., 1968. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Ann. Rev. Microbiol. 22, 87 – 108. Bull, C.T., Stack, J.P., Smilanick, J.L., 1997. Pseudomonas syringaestrains ESC-10 and ESC-11 survive in wounds on citrus and control green and blue molds of citrus. Biol. Control 8, 81 – 88.
Castoria, R., De Curtis, F., Lima, G., Pacifico, S., De Cicco, V., 1997. Extracellular fungal wall depolymerases and an-tagonism of an isolate ofAureobasidiumsp. against posthar-vest pathogen. In: Bertolini, P., Sijmons, P.C., Guerzoni, M.E, Serra, F. (Eds.), Non Conventional Methods for the Control of Post-harvest Disease and Microbiological Spoilage. Workshop Proceedings COST 914 – COST 915, Bologna, Italy, pp. 105 – 110.
Chand-Goyal, T., Spotts, R.A., 1997. Biological control of postharvest diseases of apple and pear under semi-commer-cial and commersemi-commer-cial conditions using three saprophytic yeasts. Biol. Control 10, 199 – 206.
Droby, S., Chalutz, E., Wilson, C.L., Wisniewski, M.E., 1989. Characterization of the biocontrol activity ofDebaryomyces hansenii in the control ofPenicillium digitatumon grape-fruit. Can. J. Microbiol. 35, 794 – 800.
Droby, S., Chalutz, E., 1994. Mode of action of biocontrol agents for postharvest diseases. In: Wilson, C.L., Wis-niewski, M.E. (Eds.), Biological Control of Postharvest Diseases of Fruits and Vegetables — Theory and Practice. CRC Press, Boca Raton, FL, pp. 63 – 75.
Droby, S., Cohen, A., Weiss, B., et al., 1998. Commercial testing of Aspire: a yeast preparation for the biological control of postharvest decay of citrus. Biol. Control 12, 97 – 100.
El Ghaouth, A., Wilson, C., Wisniewski, M., 1998. Ultrastruc-tural and cytochemical aspect of the biocontrol activity of Candida saitoana in apple fruit. Phytopathology 88, 282 – 291.
Eckert, J.W., Sievert, J.R., Ratnayake, M., 1994. Reduction of imazalil effectiveness against citrus green mold in California packinghouses by resistant biotypes of Penicillium digi -tatum. Plant. Dis. 78, 791 – 794.
Fokkema, N.J., Lorbeer, J.W., 1974. Interactions between Alternaria porri and the saprophytic mycoflora of onion leaves. Phytopathology 64, 1128 – 1133.
Hammerschmidt, R., Nuckles, E.M., Kuc, J., 1982. Association of enhanced peroxidase activity with induced systemic
resistance of cucumber toColletotrichum lagenarium. Phys-iol. Plant Pathol. 20, 73 – 82.
Ippolito, A., Nigro, F., 2000. Impact of preharvest application of biocontrol agents on postharvest rots of fresh fruits and vegetables. Crop Prot. (in press).
Ippolito, A., Nigro, F., Romanazzi, G., Campanella, V., 1997. Field application ofAureobasidium pullulansagainstBotry -tisstorage rot of strawberry. In: Bertolini, P., Sijmons, P.C., Guerzoni, M.E, Serra, F. (Eds.), Non Conventional Meth-ods for the Control of Post-Harvest Disease and Microbio-logical Spoilage. Workshop Proceedings COST 914 – COST 915, Bologna, Italy, pp. 127 – 133.
Janisiewicz, W.J., 1998. Biocontrol of postharvest diseases of temperate fruits: challenges and opportunities. In: Boland, G.J., Kuykendall, L.D. (Eds.), Plant-Microbe Interactions and Biological Control. Marcel Dekker, Inc, New York, pp. 171 – 198.
Kohl, J.R., Belanger, R., Fokkema, N.J., 1997. Interaction of four antagonistic fungi withBotrytis aclatain dead onion leaves: a comparative microscopic and ultrastructural study. Phytopathology 87, 634 – 642.
Kuc, J., 1990. Immunization for the control of plant disease. In: Hornby, D. (Ed.), Biological Control of Soil-borne Plant Pathogens. CAB International, pp. 355 – 366.
Lima, G., Ippolito, A., Nigro, F., Salerno, M., 1997. Effective-ness ofAureobasidium pullulansandCandida oleophilaon postharvest strawberry rots. Postharvest Biol. Technol. 10, 169 – 178.
McCormack, P.J., Wildman, H.G., Jeffries, P., 1994. Produc-tion of antibacterial compounds by phylloplane-inhabiting yeasts and yeast-like fungi. Appl. Environ. Microbiol. 60, 927 – 931.
Nigro, F., Ippolito, A., Romanazzi, G., Salerno, M., 1997. Prove di integrazione tra UV-C, curing e Aureobasidium pullulansnella lotta contro la muffa grigia dell’actinidia in postraccolta. Primi risultati. Proceedings V Convegno An-nuale S.I.Pa.V., Agripolis, Legnaro, (PD), Italy, 3 (Abstract).
Nigro, F., Finetti Sialer, M.M., Gallitelli, D., 1999. Transfor-mation ofMetschnikowia pulcherrima320, biocontrol agent of storage rot, with the green fluorescent protein gene. J. Plant Pathol. 81, 205 – 208.
Peter, S., Hubner, B., Sirrenberg, A., Hager, A., 1997. Differen-tial effect of purified spruce chitinases andb-1,3-glucanases on the activity of elicitors from ectomycorrhizal fungi. Plant Physiol. 114, 957 – 968.
Roberts, R.G., 1990. Postharvest biological control of gray mold of apple byCryptococcus laurentii. Phytopathology 80, 526 – 530.
Rodov, V., Ben-Yehoshua, S., Albaglis, R., Fang, D., 1994. Accumulation of phytoalexins scoparone and scopoletin in citrus fruits subjected to various postharvest treatments. Proceedings of International Scientific Symposium on Nat-ural Phenols in Plant Resistance, Freising-Weihenstephan, Germany, September 1993, Acta Hortic. 381, 517 – 523 Schena, L., Ippolito, A., Zahavi, T., Cohen, L., Nigro, F.,
Schlumbaum, A., Mauch, F., Vogeli, U., Boller, T., 1986. Plant chitinases are potent inhibitors of fungal growth. Nature 324, 365 – 367.
Sela-Buurlage, M.B., Ponstein, A.S., Bres-Vloemans, B., Melchers, L.O., Van den Elzen, P., Cornelissen, B.J.C., 1993. Only specific tobacco chitinases andb-1,3-glucanases exhibit antifungal activity. Plant Physiol. 101, 857 – 863. Sticher, L., Mauch-Mani, B., Metraux, J.P., 1997. Systemic
acquired resistance. Ann. Rev. Phytopathol. 35, 235 – 270. Trudel, J., Asselin, A., 1989. Detection of chitinase activity after polyacrilamide gel electrophoresis. Anal. Biochem. 178, 362 – 366.
Van Loon, L.C., Bakker, P.A.H.M., Pieterse, M.J., 1998.
Systemic resistance induced by rhizosphere bacteria. Ann. Rev. Phytopathol. 36, 453 – 483.
Wirth, S.A., Wolf, G.A., 1990. Dye-labelled substrates for the assay and detection of chitinase and lysozyme activity. J. Microbiol. Meth. 11, 197 – 205.
Wisniewski, M., Biles, C.L., Droby, S., McLaughlin, R., Wilson, C.L., Chalutz, E., 1991. Mode of action of postharvest biocontrol yeast,Pichia guilliermondii. I: char-acterization of attachment to Botrytis cinerea. Physiol. Mol. Plant Pathol. 39, 245 – 258.
Wilson, C.L., Wisniewski, M.E., 1994. Biological Control of Postharvest Diseases of Fruits and Vegetables — Theory and Practice. CRC Press, Boca Raton, FL.