Purification and properties of a major isoform of
b
-1,3-glucanase
from pearl millet seedlings
K. Ramachandra Kini
a, N.S. Vasanthi
a, S. Umesh-Kumar
b, H. Shekar Shetty
a,*
aDepartment of Studies in Applied Botany and Biotechnology,Downy Mildew Research Laboratory,Uni6ersity of Mysore,Manasagangotri, Mysore570 006,India
bDepartment of Microbiology and Bioengineering,Molecular Biology Unit,Central Food Technological Research Institute,Mysore570 013,India
Received 6 June 1999; received in revised form 30 August 1999; accepted 30 August 1999
Abstract
A major isoform of b-1,3-glucanase from pearl millet seedlings was purified following ammonium sulfate precipitation, ion-exchange chromatography and gel filtration techniques. The enzyme had a molecular weight of 20.5 kDa on SDS – PAGE and was highly basic with a pIof 9.6. It was thermostable with a broad temperature optima for activity ranging from 37 to 70°C and had an optimum pH of 5.2. Mercuric chloride and para-chloromercuric benzoate inhibited completely the enzyme while manganese chloride activated it. Antibodies raised against the purifiedb-1,3-glucanase identified another protein with an apparent molecular weight of 30 kDa in western reactions. Significance of this enzyme in pearl millet – downy mildew host – pathogen interaction is discussed. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:b-1,3-Glucanase; Pearl millet; Downy mildew; Host – pathogen interaction
www.elsevier.com/locate/plantsci
1. Introduction
Downy mildew of pearl millet (Pennisetum glau -cum (L.) R. Br.) caused by an oomycetous biotrophic fungus Sclerospora graminicola (Sacc.) Schroet, is a devastating disease of this important cereal crop. Even though the disease has been known for a long time and resistant cultivars have been used for its control, the resistance is not durable and often there is breakdown of resistance in cultivars [1]. The knowledge on the biochemical basis of resistance of pearl millet to downy mildew disease is also far from complete. In order to develop breeding strategies for pearl millet culti-vars with durable host resistance to downy mildew, it is important to generate information about the components involved in pearl millet resistance against downy mildew.
b-1,3-Glucanases found in most of the plant species, studied initially for their role in the regula-tion of the developmental activities of plants, have recently gained importance as important enzymes governing resistance of the plants to diseases [2 – 4]. Along with chitinases, b-1,3-glucanases have been shown to possess antifungal properties [5,6] and have been implicated in the defense reactions of a number of plants [7 – 9]. b-1,3-Glucanases have been purified and the genes encoding them isolated and sequenced in several higher plants [2]. However, the information on properties of b -1,3-glucanases from graminaceous plants is still lim-ited [10 – 12]. Although the exact role of
b-1,3-glucanase in pearl millet – downy mildew in-teractions is still unknown, preliminary studies have indicated involvement of this enzyme in resis-tance of pearl millet against downy mildew [13]. Better understanding of the role ofb-1,3-glucanase in this host – pathogen interaction may require iso-lation and analysis of properties of b -1,3-glu-canases from pearl millet seedlings. In this paper,
* Corresponding author. Tel.: +91-821-515126; fax: + 91-821-411467.
E-mail address:[email protected] (H. Shekar Shetty)
we report the purification and properties of a major isoform ofb-1,3-glucanase from pearl millet seedlings inoculated with S. graminicola and its probable function in the pearl millet – downy mildew interaction.
2. Materials and methods
2.1. Seed samples
Seeds of pearl millet cultivars highly resistant (IP18296) and highly susceptible (HB3) to downy mildew (S. graminicola) obtained from Interna-tional Crop Research Institute for Semi-Arid Tropics (ICRISAT), Hyderabad, India and All India Coordinated Pearl Millet Improvement Pro-gram (AICPMIP), Pune, India, respectively, were used.
2.2. Pathogen
S. graminicola pathotype 1 maintained on its
susceptible host HB3 cultivar of pearl millet under greenhouse conditions was used.
2.3. Inoculation of the plant material
Seeds of pearl millet cultivars IP18296 and HB3 surface sterilized in 0.1% (v/v) sodium hypochlor-ite solution for 15 min were washed thoroughly with sterile distilled water and germinated on moist filter paper under aseptic conditions at 259 1°C in the dark for 3 days. The 3-day-old seedlings were root-dip inoculated with 4×104 zoospores
ml−1 suspension of S. graminicola following the
standardized method of Safeeulla [14]. Seedlings dipped in sterile distilled water served as control. The seedlings (0.5 g) were harvested 24 h after inoculation and used for enzyme extraction.
2.4. Enzyme extraction
The seedlings were homogenized using acid-washed glass beads and 0.05 M sodium acetate buffer [pH 5.2; 1 ml (gram fresh weight)−1] in a
pre-chilled pestle and mortar at 4°C. The ho-mogenate was centrifuged at 10 000×gfor 20 min at 4°C (Himac Centrifuge, Hitachi) and the super-natant was used as crude extract.
2.5. b-1,3-Glucanase assay
b-1,3-Glucanase was assayed following the method of Isaac and Gokhale [15] with glucose standard. Laminarin (Sigma Chemicals Co., St. Louis, MO; 0.1%) in 0.05 M sodium acetate buffer (pH 5.2) was used as substrate. Protein content in extracts was estimated by the dye binding method [16] using bovine serum albumin (Sigma) as stan-dard. The enzyme activity was expressed in terms of mmol min−1 mg−1 protein.
2.6. Purification of b-1,3-glucanase
Seedlings of the highly resistant cultivar IP18296 harvested 24 h after inoculation were used for enzyme extraction. For enzyme purifica-tion crude extract was prepared from 500 g of these seedlings in 0.05 M sodium acetate buffer (pH 5.2). Following ammonium sulphate fraction-ation (50% saturfraction-ation) at 4°C, the proteins ob-tained after centrifugation were dissolved in a minimum amount of 0.05 M sodium acetate buffer (pH 5.2) and dialysed against the same buffer.
2.6.1. Ion-exchange chromatography
The dialysed proteins were subjected to ion-ex-change chromatography on DEAE – Sepharose (Sigma) column (bed: 10×2.5 cm) and the flow through obtained with sodium acetate buffer (0.05 M, pH 5.2) containing b-1,3-glucanase was collected. This was concentrated by lyophilization and used for gel filtration.
2.6.2. Gel filtration
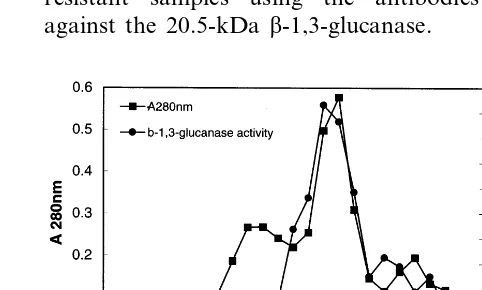
The proteins were separated on Sephadex G-100 column (60×1 cm) equilibrated with sodium ac-etate buffer (0.05 M, pH 5.2) and eluted with the same buffer. Two-mililitre fractions were collected and assayed for b-1,3-glucanase. The enzyme con-taining fractions were concentrated by lyophiliza-tion separately and used for electrophoresis.
2.6.3. Electrophoresis and electrofocusing
performed cathodically by the method of Reis-feld et al. [18] in 14% separating and 5% stack-ing gel. Methylene blue was used as trackstack-ing dye. Isoelectric focusing (IEF) was performed on a 1.5 mm 7.5% polyacrylamide gel containing 2% ampholyte (pH 3 – 10, Sigma) using a Multiphor II (LKB) system according to the manufacturer’s protocol. The pI markers (Sigma), ranging from pI 3.6 to 9.3 were co-electrophoresed to estimate the pI of the proteins. Purified enzyme (5 mg) was loaded at the center of the horizontal gel maintained at 2°C. The voltage was increased stepwise: 200 V for 30 min, 400 V for 30 min, 600 V for 30 min, 800 V for 30 min and 1000 V for 1 h. The gels during the run were maintained at 2°C.
After native PAGE and IEF the proteins were stained for b-1,3-glucanase activity following the method described by Pan et al. [19].
2.7. Enzyme characterization
The effect of pH on the activity of purified
b-1,3-glucanase was determined at 37°C over the pH range 3.0 – 9.0 in 0.05 M buffers (citrate/ Na2HPO4, pH 3.0 – 3.5; sodium acetate, pH 4.0 –
5.5; sodium phosphate, pH 6.0 – 7.0; Tris – HCl, pH 7.5 – 9.0). The effect of temperature was de-termined for the temperature range 4 – 98°C in 0.05 M sodium acetate buffer (pH 5.2). The ther-mal stability of the enzyme was determined by measuring the activity remaining after incubation of the enzyme at various temperatures (28 – 98°C) for 60 min in 0.05 M sodium acetate buffer (pH 5.2).
The effect of various metal ions and enzyme inhibitors on the enzyme activity was measured by pre-incubation of the enzyme with 10 mM of the compounds at 37°C for 15 min and checking the residual activity under standard assay condi-tions.
2.8. Assay for antifungal acti6ity
Antifungal activity of the purified b -1,3-glu-canase was tested on some common fungi like
Trichoderma harzianum, Fusarium oxysporum and
Aspergillus fla6us following the method of Mauch
et al [5]. Fungal spores were harvested from well sporulating colonies and suspended in sterile dis-tilled water. The concentration of the spore
sus-pensions were determined in a hemocytometer and adjusted to 2×106 spores ml−1. The freshly
prepared suspensions (0.5 ml) were plated out on petridishes containing potato dextrose agar medium for maintenance of the test fungus. To allow for spore germination and initial vegetative growth, the plates were incubated for 20 to 24 h at room temperature. At this time, sterile filter paper discs (8 mm diameter) were laid on the agar surface. Twenty-five micrograms of protein equivalent to the crude extract and 10 mg of purified b-1,3-glucanase were applied to separate discs. Sodium acetate buffer (0.05 M, pH 5.2) and sterile distilled water were also loaded on separate discs to serve as controls. The plates were further incubated at room temperature and observed for inhibition zones.
2.9. Immunological methods
Antiserum was raised against the purified b -1,3-glucanase from pearl millet in New Zealand white rabbits. For immunization, 500 mg of protein was used. Injections containing the protein in phosphate buffered saline (PBS; 0.145 M NaCl, 0.01 M NaH2PO4, 0.01 M Na2HPO4,
pH 7.2) mixed with equal volume of Freund’s complete adjuvant were administered through in-tramuscular routes on days 0, 14, 28 and 42. The antiserum was separated from the blood 2 days after the last injection and was stored at −20°C.
2.10. Western blot analysis
Table 1
Summary ofb-1,3-glucanase purification from S.graminicolainoculated seedlings of pearl millet
Total protein
Step Total activity Specific activity Purification Yield (mmol min−1mg−1 protein) (fold) (%)
(U) (mg)
545.6 4.33
Crude extract 126 1 100
28 186.5 6.66 1.5
Ammonium sulphate pre- 34.2
cipitation
73 16.98 3.9
DEAE–Sepharose 4.3 13.4
33 41.33 9.5 6.05
0.8 Sephadex G-100
3. Results
3.1. Purification of b-1,3-glucanase
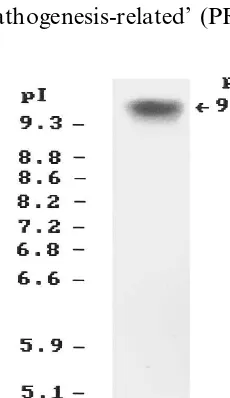
The recovery of the enzyme after various steps of purification is presented in Table 1. Ammonium sulphate fractionation removed contaminating proteins and b-1,3-glucanase enzyme was precipi-tated at 50% saturation. On DEAE – Sepharose column chromatography, the enzyme was eluted as unbound protein. Sephadex G-100 column chromatography eluted the proteins in the frac-tions 14 – 17 (Fig. 1). When electrophoresed by SDS – PAGE, a single major protein was detected from the fraction 16 of the G-100 column (Fig. 2). The apparent molecular mass of this protein was found to be 20.5 kDa. Native PAGE showed a single protein in that fraction also. Detection of the enzyme with the substrate after native PAGE confirmed this protein to be b-1,3-glucanase. Isoelectric focusing showed that the purified en-zyme had a pI of 9.6 (Fig. 3).
3.2. Physico-chemical properties
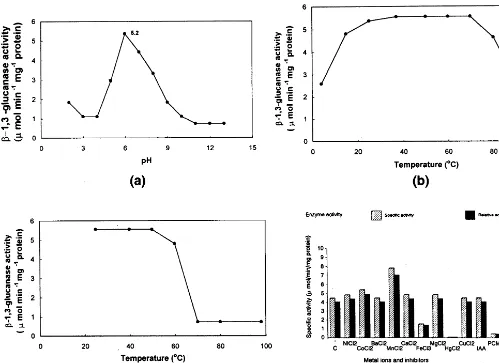
The properties of the purified b-1,3-glucanase are summarised in Fig. 4. The purified enzyme was found to have optimum activity at pH 5.2 (Fig. 4a). The temperature profile showed that the opti-mum temperature of the enzyme ranged from 37 to 70°C (Fig. 4b). The enzyme was thermostable as more than 80% enzyme activity could be recov-ered after incubation at 60°C for over 1 h (Fig. 4c).
Studies on the influence of metal ions and en-zyme inhibitors revealed HgCl2and p-chloro
mer-curic benzoate to completely inhibit the enzyme activity. While FeCl3inhibited the enzyme by 67%,
MnCl2 activated it by 75% (Fig. 4d). The
inhibi-tion by HgCl2 was overcome when cystein (100
mM) was included in the reaction mixture.
3.3. Antifungal acti6ity
The purified b-1,3-glucanase enzyme was tested for antifungal activity. Sodium acetate buffer (0.05 M, pH 5.2) and sterile distilled water were used as controls. On the T. harzianum plate, faint inhibi-tion zone was observed surrounding the disc treated with purified b-1,3-glucanase (data not shown). No inhibition in growth was observed in the other two fungi F. oxysporum and A. fla6us.
3.4. Western blot analysis
The 20.5-kDa protein corresponding to the purified b-1,3-glucanase was observed in the crude extracts of inoculated and control seedlings of both resistant and susceptible pearl millet cultivars (Fig. 5). In addition, another protein with appar-ent molecular mass of 30 kDa was detected only in the resistant samples. This protein was detected more strongly than the 20.5-kDa protein in the resistant samples using the antibodies raised against the 20.5-kDa b-1,3-glucanase.
Fig. 2. SDS – PAGE of purified b-1,3-glucanase. Lane 1, molecular weight markers; lane 2, purified enzyme (5mg); lane 3, crude extract (50mg).
includeb-1,3-glucanases, have been identified. It is generally known that extracellular forms are acidic and intracellular are basic [2,22]. The vacuolar enzymes might act as a last line of defense, whereas the extracellular b-1,3-glucanase could be involved in recognition and the release of elicitors. However, in potato [23] and sunflower [24], basic protein, in particular basic b-1,3-glucanase corre-sponding to the accepted definition of PR proteins have been found to be extracellular also. The basic isozymes were also identified as major stress-in-duced b-1,3-glucanases in bean leaves [25] and were induced in germinating wheat seeds infected
withF. culmorum[9]. In pea seedlings, basicb
-1,3-glucanases are strongly induced in response to pathogen attack and in combination with basic chitinases, are efficient in inhibiting fungal growth in vitro [5]. Only basic isozymes of chitinase and
b-1,3-glucanase from tomato were inhibitory to
Alternaria solani whereas, no inhibitory activity
was observed with acidic isozymes [26]. A basic
b-1,3-glucanase (34 kDa, pI 9.3) isolated from pepper stems had inhibitory activity against chitin-negative fungus Phytophthora capsici but not against some chitin-containing fungi [6]. It is re-ported also that the specific enzyme activity of basic b-1,3-glucanases is much higher than that of the acidic isoforms [27,28]. In the light of the above reports, identification of basic isoform of
b-1,3-glucanase in pearl millet suggested an impor-tant role for the enzyme in active defense of pearl millet against S. graminicola. The purified basic
b-1,3-glucanase isoform showed limited antifungal activity only on T. harzianum, and not against other test fungi like F. oxysporum and A. fla6us.
This may be due to insensitivity of these test fungi towards the purified b-1,3-glucanase or due to the inability of b-1,3-glucanase alone to act as power-ful antifungal agent. Mauch et al. [5] have re-ported that purified pea b-1,3-glucanase or chitinase alone were not effective in inhibiting the fungal growth while a combination of both these enzymes inhibited significantly the growth of sev-eral fungi. Chitin containing fungi A. mali, Col
-letotrichum gloeosporioides, F, oxysporum f.sp.
cucumerinum and Magnaporthe grisea were found
also to be insensitive to b-1,3-glucanase purified from pepper stems [6].
The immunoblot analysis using antiserum raised against the purified b-1,3-glucanase identified two proteins from the total proteins of resistant and
4. Discussion
Our preliminary studies have shown that in seedlings of resistant pearl millet cultivars, the activity ofb-1,3-glucanase increased upon inocula-tion with the downy mildew pathogen S. gramini -cola [13]. In the present study, the major isoform of b-1,3-glucanase purified from pearl millet seedlings inoculated withS. graminicola, showed a pI of 9.6 and molecular weight of 20.5 kDa on SDS – PAGE. This apparently supports the reports that molecular weight of plant b-1,3-glucanases involved in defense range from 20 to 35 kDa and have extreme pI values [2,21]. In many plants, both intracellular (vacuolar) and extracellular forms of ‘pathogenesis-related’ (PR) proteins, that
Fig. 4. Properties of purified b-1,3-glucanase from pearl millet. (a) Effect of pH on purified b-1,3-glucanase. (b) Effect of temperature on b-1,3-glucanase. (c) Thermal stability of b-1,3-glucanase. (d) Effect of metal ions and enzyme inhibitors on
b-1,3-glucanase.
susceptible cultivars of pearl millet seedlings. Even though the antiserum was raised against the 20.5-kDa isoform ofb-1,3-glucanase, it reacted strongly with another protein of 30 kDa, only in the resis-tant samples. The cross-reactivity of the antiserum with the 30-kDa protein suggested a high degree of homology of this protein from resistant cultivar with the 20.5-kDa isoform of b-1,3-glcanase. En-zyme zymograms of resistant plant extracts after isoelectric focusing and western reactions showed no relation of the 30-kDa protein to any of the
b-1,3-glucanase isoforms (data not shown) but its prominent reaction with the antibody (Fig. 5) suggested a programmed rearrangement of b -1,3-glucanase precursor which offers resistance in in-compatible interaction. In the light of the above, further studies are underway to study the
develop-mental regulation of b-1,3-glucanase in relation to its role in resistance of pearl millet against downy mildew.
Acknowledgements
The authors are grateful to the Indian Council of Agricultural Research, New Delhi, for funds. KRK thank CSIR, New Delhi, for financial assistance.
References
[1] S.L. Ball, Pathogenic variability of downy mildew (Scle
-rospora graminicola) in pearl millet. I. Host cultivar reactions to infection by different pathogen isolates, Ann. Appl. Biol. 102 (1983) 257 – 264.
[2] C.R. Simmons, The physiology and molecular biology of plant 1,3-b-D-glucanases and 1,3;1,4-b-D-glucanases,
Crit. Rev. Plant Sci. 13 (1994) 325 – 387.
[3] S. Roulin, P. Xu, A.H.D. Brown, G.B. Fincher, Expres-sion of specific (13)-b-glucanase genes in leaves of near-isogenic resistant and susceptible barley infected with the leaf scald fungus (Rynchosporium secalis), Phys-iol. Mol. Plant Pathol. 50 (1997) 245 – 261.
[4] V.V. Lozovaya, A. Waranyuwat, M. Widholmj, b -1,3-Glucanase and resistance toAspergillus fla6usinfection in
maize, Crop Sci. 38 (1998) 1255 – 1260.
[5] F. Mauch, B. Mauch-Mani, T. Boller, Antifungal hydro-lases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and b-1,3-glucanase, Plant Physiol. 88 (1988) 936 – 942.
[6] Y.J. Kim, B.K. Hwang, Isolation of a basic 34 kDa
b-1-3-glucanase with inhibitory activity againstPhytoph
-thora capsici from pepper stems, Physiol. Mol. Plant Pathol. 50 (1997) 103 – 115.
[7] Y.J. Kim, B.K. Hwang, Differential accumulation of
b-1,3-glucanase and chitinase isoforms in pepper stems infected by compatible and incompatible isolates ofPhy
-tophthora capsici, Physiol. Mol. Plant Pathol. 45 (1994) 195 – 209.
[8] J.M. Cachinero, F. Cabello, J. Jorrin, M. Tena, Induc-tion of different chitinase and b-1,3-glucanase isoen-zymes in sunflower (Helianthus annus L.) seedlings in response to infection by Plasmopara halstedii, Eur. J. Plant Pathol. 102 (1996) 401 – 405.
[9] C. Caruso, G. Chilosi, C. Caporale, L. Leonardi, L. Bertini, P. Magro, V. Buonocore, Induction of pathogen-esis-related proteins in germinating wheat seeds infected with Fusarium culmorum, Plant Sci. 140 (1999) 107. [10] J. Wang, P. Xu, G.B. Fincher, Purification,
characteriza-tion and gene structure of (13)-b-glucanase isoenzyme GIII from barley (Hordeum 6ulgare), Eur. J. Biochem.
209 (1992) 103 – 109.
[11] M. Hrmova, G.B. Fincher, Purification and properties of three (13)-b-glucanase isoenzymes from young leaves of barley (Hordeum 6ulgare), Biochem. J. 289 (1993)
453 – 461.
[12] T. Akiyama, H. Kaku, N. Shibuya, Purification and properties of a basic endo-1,3-b-glucanase from rice (Oryza sati6aL.), Plant Cell Physiol. 37 (1996) 702 – 705.
[13] K.R. Kini, Biochemical and molecular analysis of vari-ability inSclerospora graminicola and resistance mecha-nism in pearl millet, PhD thesis, University of Mysore, Mysore, India, 1998, p. 177.
[14] K.M. Safeeulla, Biology and Control of the Downy Mildew of Pearl Millet, Sorghum and Finger Millet, Wesley Press, Mysore, India, 1976.
[15] S. Isaac, A.V. Gokhale, Autolysis: a tool for protoplast production fromAspergillus nidulans, Trans. Br. Mycol. Soc. 78 (1982) 389 – 394.
[16] M.M. Bradford, A rapid and sensitive method for the quantification of microgram quantities of protein utiliz-ing the principle of protein – dye bindutiliz-ing, Anal. Biochem. 72 (1976) 248 – 254.
[17] U.K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature 227 (1970) 680 – 685.
[18] R.A. Reisfeld, U.J. Lewis, D.E. Williams, Disk elec-trophoresis of basic proteins and peptides on polyacry-lamide gels, Nature 195 (1962) 281 – 283.
[19] S.Q. Pan, X.S. Ye, J. Kuc, Direct detection ofb -1,3-glu-canase isozymes on polyacrylamide electrophoresis and isoelectrofocusing, Anal. Biochem. 182 (1989) 136 – 140. [20] S. Winston, S. Fuller, J. Huller, Western blotting, in:
E.M. Ausubel (Ed.), Current Protocols in Molecular Biology, Wiley, New York, 1987, pp. 10.8.1 – 10.8.6. [21] J.F. Bol, H.J.M. Linthorst, B.J.C. Cornelissen, Plant
pathogenesis-related proteins induced by virus infection, Ann. Rev. Phytopathol. 28 (1990) 113 – 138.
[22] H.J.M. Linthorst, Pathogenesis-related proteins of plants, Crit. Rev. Plant Sci. 10 (1991) 123 – 150. [23] M. Schroder, K. Hahlbrock, E. Kombrink, Temporal
and spatial patterns of 1,3-b-glucanase and chitinase in potato leaves infected byPhytophthora infestans, Plant J. 2 (1992) 161 – 172.
[24] J.L. Jung, B. Fritig, G. Hahne, Sunflower pathogenesis-related proteins, Plant Physiol. 101 (1993) 873 – 880. [25] F. Mauch, L.A. Staehlin, Functional implications of the
subcellular localization of ethylene-induced chitinase and
b-1,3-glucanase in bean leaves, Plant Cell 1 (1989) 447 – 457.
[26] C.B. Lawrence, M.H.A.J. Joosten, S. Tuzan, Differential induction of pathogenesis-related proteins in tomato by
Alternaria solaniand the association of a basic chitinase isozyme with resistance, Physiol. Mol. Plant Pathol. 48 (1996) 361 – 377.
[27] S. Kauffmann, M. Legrand, P. Geoffroy, B. Fritig, Bio-logical function of pathogenesis-related proteins: four PR proteins of tobacco have 1,3-b-glucanase activity, EMBO J. 6 (1987) 3209 – 3212.
[28] M. Legrand, S. Kauffmann, P. Geoffroy, B. Fritig, Bio-logical function of pathogenesis-related proteins: four tobacco pathogenesis-related proteins are chitinases, Proc. Natl. Acad. Sci. USA 84 (1987) 6750 – 6754.