www.elsevier.nlrlocateraqua-online
Alternative competitive strategies in juvenile
ž
/
Atlantic salmon Salmo salar : evidence from fin
damage
Amanda MacLean
a,), Neil B. Metcalfe
a, David Mitchell
ba
Fish Biology Group, DiÕision of EnÕironmental and EÕolutionary Biology, Institute of Biomedical and Life
Sciences, UniÕersity of Glasgow, Glasgow G12 8QQ, Scotland, UK
b
Marine HarÕest McConnell, Farms Office, Blar Mhor Industrial Estate, Fort William, InÕerness-shire,
Scotland, UK
Accepted 15 October 1999
Abstract
Dorsal fin damage in salmonid fishes is primarily caused by aggression. While undesirable in fish culture, it can nevertheless be a useful tool to study social interactions in large groups of fish where it is difficult to study the behaviour of known individuals directly. We used low
Ž .
temperature treatment to manipulate the growth rates of juvenile Atlantic salmon Salmo salar in the spring and followed the development of fin damage in tagged individuals. Fin damage did not develop until mid-summer, possibly because of a qualitative change in the nature of aggressive
Ž
attacks. The probability of having fin damage was strongly related to relative body size fork
.
length within each group of fish: the largest fish in a tank were up to six times more likely to have damaged fins than the smallest fish. While studies of small groups of salmonids have demonstrated that subordinates are the main recipients of fin damage, the results of this study indicate that the reverse is true in larger groups. We propose that this is because dominant fish compete aggressively amongst themselves and incur fin damage, while less aggressive individuals adopt alternative feeding strategies that result in lower levels of food intake and growth, but reduce the risk of injury. Similar studies could be used to assess the success of feeding regimes in reducing the level of aggression in cultured populations.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Atlantic salmon; Salmo salar; Fin rot; Aggression; Feeding; Competition
)Corresponding author. Tel.:q44-141-330-3534; fax:q44-141-330-5971.
Ž .
E-mail address: [email protected] A. MacLean .
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
1. Introduction
Ž .
In the wild, Atlantic salmon Salmo salar spend at least a year and usually more in fresh water, where they are territorial and engage in frequent bouts of agonistic
Ž .
behaviour Kalleberg, 1958; Keenleyside and Yamamoto, 1962 . In aquaculture, densi-ties are much higher than in the wild and thus, the physical damage caused by aggression is more frequent and severe: so much so that fin condition can be used to
Ž .
distinguish between farmed fish and wild stocks e.g., Bosakowski and Wagner, 1994 . Aggression has a negative impact on growth and welfare in farmed fish, as it can result in physical damage, which may lead to secondary infections with pathogens such as
Ž .
Aeromonas salmonicida Schneider and Nicholson, 1980; Turnbull et al., 1996 .
Inequal-ities in food intake resulting from social hierarchies maintained by aggression can lead
Ž
to growth depensation where initial small size differences within a group become more
. Ž .
pronounced as time goes on Jobling, 1985; Jobling and Wandsvik, 1983 . Social
subordination is also associated with chronic stress, which can have detrimental effects
Ž .
on health and growth Schreck et al., 1997; Wedemeyer, 1997 .
Ž .
In small groups of fish fewer than 20 , there are usually pronounced social
hierarchies dominated by one or two aggressive individuals that monopolise the food supply and reduce the feeding activity and thereby the growth of their social
subordi-Ž
nates Jobling and Wandsvik, 1983; Koebele, 1985; Huntingford et al., 1993; Adams et
.
al., 1998 . Dominant fish perform more aggressive acts than subordinates, and the subordinates usually receive more aggressive nips and exhibit more fin damage than
Ž
dominants Fenderson and Carpenter, 1971; Abbott and Dill, 1989; Gregory and
.
Griffith, 1996; Moutou et al., 1998 . However, the conclusions reached from such studies may not necessarily hold true for larger groups of fish. For instance, in paired
Ž .
encounters between juvenile Arctic charr SalÕelinus alpinus , the more aggressive fish of the two usually acquired more food, but in culture conditions the same individuals
Ž
were no more likely to grow well than their subordinate partners Adams and
Hunting-.
ford, 1996 . This may be because the social hierarchy is less stable in larger groups
ŽFenderson and Carpenter, 1971 . A difficulty in studying aggressive interactions in.
culture conditions is the large number of fish involved. This makes it practically impossible to observe the behaviour of known individuals. It is here that the damage caused by aggressive behaviour can be used as an indicator to shed light on the subject. The best-known physical damage caused by aggression is inflicted on the fins and is termed fin damage, fin erosion or fin rot. These terms cover a range of symptoms including splitting of the fin rays, tissue loss and pale nodular thickening of the distal
Ž .
portion of the fin Turnbull et al., 1996; Winfree et al., 1998 . While other factors can be
Ž .
involved see Winfree et al., 1998, for a summary , there is little doubt that the principal cause of fin erosion in farmed salmonids, especially when it occurs on the dorsal fin, is
Ž
aggressive behaviour. In paired encounters between steelhead trout Oncorhynchus
. Ž . Ž
mykiss Abbott and Dill, 1985 , and in small groups of Atlantic salmon Turnbull et al.,
.
ŽTurnbull, 1992 . Most importantly, scanning electron micrographs of damaged fins.
from fish-farms showed clear tooth marks and an absence of bacterial infection
ŽTurnbull et al., 1996 ..
Fin damage has been used as an indicator of the strength of the social hierarchy by
Ž . Ž .
Christiansen and Jobling 1990 and Moutou et al. 1998 , providing useful insights into the dynamics of aggression within larger groups of fish than can easily be studied otherwise. Fin splitting is the primary symptom of fin damage, and repeated splitting eventually leads to tissue loss and thickening of the remaining tissue. Splitting heals
Ž
rapidly, whereas re-growth and reduction in thickening take longer to occur Turnbull,
.
1992 . Therefore, splitting is likely to be the best indicator of current levels of aggression.
The aim of this study is to examine the effect of body size on the incidence of fin damage in large groups of fish kept under culture conditions. Using data from individu-ally marked fish previously subjected to different manipulations of growth rates, we are
Ž .
able to compare the effects of both relative to other group members and absolute body size on the timing and duration of fin damage. We demonstrate a strong and consistent effect of relative body size, which indicates the existence of alternative strategies of aggression and feeding within groups of fish.
2. Materials and methods
The experiments involved a population of farmed Atlantic salmon parr from pooled hatchery stock. The experiment started approximately 2 weeks after first-feeding, on 17
Ž .
April 1997, at the freshwater hatchery of Marine Harvest McConnell MHM at
Ž .
Invergarry, Scotland, where four tanks labelled A–D were stocked with 1550 "7%
fish per tank. In order to manipulate growth rates, group D, the control, remained at Invergarry throughout the experiment, while groups A, B and C successively spent 3
Ž . Ž
weeks in colder water mean of 8.3"0.028C in Glasgow University’s aquaria A from
.
17 April–8 May, B from 9–29 May and C from 30 May–19 June before being returned
to Invergarry. The water at Invergarry was heated to ca. 128C until mid-May, when
ambient temperatures reached that level. The fish were then kept at the ambient water temperature until the third week of October, when the water was heated to keep
Ž
temperatures at ca. 88C. The fish were initially kept in small, circular tanks diameter 0.6
.
m, water depth 0.25–0.3 m , but on 20th June all four groups, now permanently at
Ž .
Invergarry, were transferred to larger 2-m square tanks water depth 0.5 m , where they remained until the end of the experiment.
Throughout the experiment the tanks were lit by overhead fluorescent strip-lights; the
photoperiod regime was that used commercially to produce accelerated ‘‘S1r2’’ smolts,
Ž .
with long days 24L:0D until the end of May, and thereafter 22L:2D separated by a
Ž . Ž .
A random sample of 150 fish was measured on 18 April, the first day of the experiment. The fish were anaesthetised using Benzocaine and measurements were made
Ž .
of fork length to 1 mm . Random samples of 150 fish per group were then measured on 9 May, 29–30 May and 19–20 June. On 22–25 July, random samples of 100 fish per
Ž .
tank were measured and tagged with Passive Integrated Transponder PIT tags. The PIT
Ž .
tags length 17 mm, mass 0.1 g were inserted into the body cavity through an incision
made in the body wall. The incision was then dusted with a 50:50 mix of Cicatrine
Ž . Ž
antibiotic powder Wellcome, London, UK and Orahesivee Protective Powder ER
.
Squibb and Sons, Hounslow, UK to help prevent infection and close the wound. The tagged fish were re-measured on 1–3 September, 3–9 November and 9 December. From July onwards only the tagged fish were assessed for fin damage, and data are presented only for the tagged fish that survived to the end of the experiment and were assessed on
Ž .
all sampling dates ns314 . Thus, the changes in frequency should represent actual
healing or incurring of damage rather than sampling error.
On each of the measurement days, damage to the dorsal fin was assessed by
Ž
comparison with Fig. 1. Three separate measures were assessed: tissue loss judged by
. Ž .
fin size , splitting and thickening. Fin size was scored on a five-point scale Fig. 1 . As it was difficult to distinguish whether a fin was intact or only slightly reduced in size,
the 90%qcategory was taken to be undamaged and the lower categories were classed
Ž . Ž .
as damaged. Splitting and thickening were both classed as either absent 0 , mild 1 or
Ž .
severe 2 . Fin splitting categories 1 and 2 were combined for analysis as the distinction between the two was judged in retrospect to be unsatisfactory.
Specific Growth Rate in length between measurement periods was calculated as:
SGRs100= ln FL
Ž
t 2.
yln FLŽ
t1.
rŽ
t2yt1.
Ž .
where t1sfirst sampling day; t2ssecond sampling day and FLsfork length mm .
3. Results
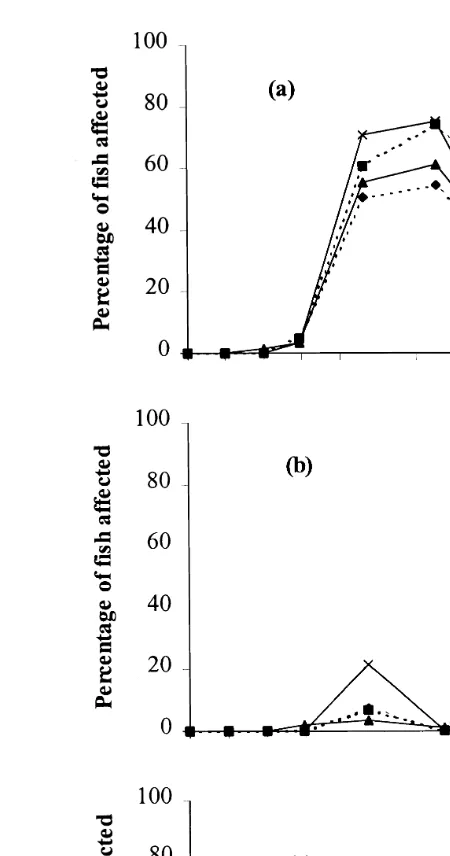
No fin damage of any kind was visible until the end of May, when it was evident in small numbers of fish. There was a rapid rise in the incidence of damage between June
Ž .
and July Fig. 2 . From July onwards, splitting was the most common type of fin
Ž .
damage Fig. 2a . It then declined during the photoperiod winter, but increased to previous levels by December.
Ž .
Thickening and tissue loss were always less prevalent than splitting Fig. 2b–c . Neither category of damage was particularly severe. Thickening category 2 was never recorded, while only two fish ever had less than 30% of the dorsal fin remaining, and
Ž .
most of those affected 130 out of 139 had 60% to 90% remaining. There were some
Ž .
differences between groups in the frequency of fin damage Table 1 , but all four groups
Ž .
showed similar patterns of development of fin damage Fig. 2 . Since fin splitting was the most common category of damage, and it is the best indicator of current levels of aggression, all subsequent analyses are based only on this measurement.
Ž .
By November, 2.2% 7 fish were very small parr that had failed to smolt, while
Ž .
4.1% 13 fish were sexually mature male parr. As the growth patterns of both of these
Ž .
categories of fish differed from the majority of the fish which were immature smolts they have been excluded from the following analysis, except where indicated.
Ž .
Table 2 gives the mean fork length "SE of fish with and without split fins in July, September and December when fin splitting was evident in large numbers of fish in all groups. Experimental group had a significant effect on fork length at all times due to the effect of the temperature manipulation, while fish that had split fins were, on average,
Ž
larger than fish without split fins in July and September, but not in December Two-way
ANOVA with group as a factor in: July, F3, 286s46.4, P-0.01; September, F3,
December, F1, 286s1.6, n.s. . In December, there was a significant interaction between group and fin condition, as the fish in group C that had split fins were still larger than those without split fins, but there was no longer any such relationship within the other
Ž
groups Interaction between group and fin condition in: July, F3, 286s2.5, n.s.;
.
September, F3, 286s1.1, n.s.; December, F3, 286s3.1, P-0.05 .
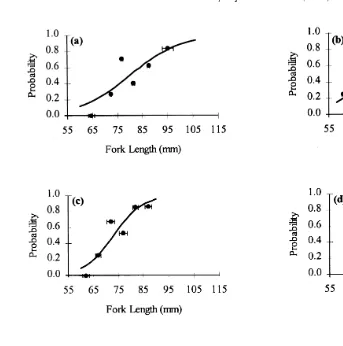
The relationship between fin damage and length within each group of fish in July was very strong, as shown by logistic regressions relating fin splitting to fish length for each
Ž .
group Fig. 3a–d . In all groups, there was a strong and significant positive relationship between the probability of having split fins and the size of the fish, with the probability
Fig. 2. Changes over time in the percentage of fish affected by three measures of dorsal fin damage in four
Ž . Ž . Ž .
groups of juvenile Atlantic salmon: a splitting, b thickening and c tissue loss.
Table 1
Comparisons byx2 test of frequencies of three categories of fin damage between groups of Atlantic salmon
of different mean length on five sampling dates. x2 values were regarded as invalid and are omitted if
cross-tabulation yielded expected frequencies of less than five in one or more cells. dfs3 in all cases.
2
Level of statistical significance: P-0.05.
UU
Level of statistical significance: P-0.01.
middle of the size range and would be considerably less likely to have split fins
Žprobabilities of 0.62 and 0.61, respectively ..
To adjust for the differences in size among the four groups, fish size is expressed as
Ž .
the deviation from the mean length of the group as a proportion of the mean Fig. 3e .
Table 2
Ž . Ž .
Mean fork length "SE of fish with and without split fins in four groups A to D of Atlantic salmon in July, September and December, 1997
Ž .
Month Group n Mean fork length mm"
Fig. 3. The effect of body size on the probability of having split fins in juvenile Atlantic salmon in July 1997
Ž . Ž . Ž . Ž . Ž .
in a Group A, b Group B, c Group C, d Group D, and e all four groups combined. The effect of
Ž .
deviation from mean fork length for fish in a group see text for explanation on the probability of having split
Ž . Ž .
fins is shown in e ; symbols squares are the actual proportion of fish that had split fins, within each 0.05
Ž .
range. The logistic regression lines have been calculated using only immature smolts ns294 , but symbols
Ž . Ž .
representing non-smolting parr ns7; open triangle and mature male parr ns13; open circle have been
Ž . Ž .
added to e for comparison. Lines are the logistic regression line given by the equation: Ysar1qa , where
wqz X Ž Ž . Ž .. Ž Ž ..
ase , and Xsfork length in a to d or deviation from mean fork length in e . The values of w
Ž . Ž . Ž .
and z for each graph are as follows: a wsy7.9442, zs0.0994, b wsy9.4217, zs0.1322, c
Ž . Ž . 2
wsy12.5787, zs0.1720, d wsy11.0913, zs0.1357, e ws0.4002, zs10.4572. The values of x
Ž . Ž . Ž . Ž . Ž .
were as follows: a 10.56, b 14.04, c 22.39, d 25.59, e 71.44, and P-0.025 in all cases.
Parr that would not smolt were as likely as other fish of their relative size to have fin
Ž 2 .
damage x s0.002, 1 df, ns29, n.s. , while mature male parr were somewhat more
Ž 2
likely to have fin damage than immature fish in the same relative size range x s4.42,
.
4. Discussion
When it first appeared, fin damage was strongly associated with size. The largest fish in a tank were up to six times more likely to have damaged fins than the smallest fish. This is the first time that such a relationship has been demonstrated, and indeed it appears to contradict some previous findings. Most previous studies have demonstrated
Ž
that social subordinates bear the brunt of the aggressive attacks of dominant fish Abbott
.
and Dill, 1989; Gregory and Griffith, 1996; Moutou et al., 1998 . If this was the case, it would imply that the smaller fish in this study were actually dominant. However, this is
Ž
unlikely, as fish that had delayed smolting and are usually socially subordinate,
.
Metcalfe et al., 1989 had the same low levels of fin damage that were observed in pre-smolts of the same relative size. Furthermore, although large body size in Atlantic
Ž
salmon is a consequence, rather than a cause, of social dominance Huntingford et al.,
.
1990 , the advantage that dominance confers in terms of increased feeding opportunity
Ž .
soon leads to increased growth rates and hence greater body size Metcalfe et al., 1992 . Thus, the larger fish in the current experiment were almost certainly aggressive fish that could compete effectively for food and therefore achieve rapid growth.
Why, then, was fin damage more prevalent in the larger fish? As the size of a group increases, it becomes increasingly difficult for a single fish to monopolise the food
Ž .
supply, and a group of dominant fish emerges Alanara and Brannas, 1996 . In small
¨ ¨
¨ ¨
groups of Atlantic salmon held at production densities, the fish with the highest food
Ž .
intake also received the largest number of aggressive attacks Adams et al., 1998 , while the dorsal fin was frequently nipped in reciprocal bouts of fighting in steelhead trout
ŽAbbott and Dill, 1985 . Therefore, in groups of hundreds of fish, it is likely that there.
are many large, aggressive and dominant fish that fight amongst themselves for food. What about the less aggressive fish? They may have adopted alternative feeding strategies that reduced the risk of injury. For instance, fish may avoid injury by sneaking
Ž .
in to feed while others fight Pettersson et al., 1996 , or by darting in and out of the
Ž .
feeding area as soon as food becomes available Adams et al., 1998 . Another possibility
Ž .
is a sit-and-wait strategy. Kadri et al. 1996 found that in a sea-cage of one sea-winter Atlantic salmon, the most successful fish, i.e., those that achieved the greatest food
Ž
intake, fed at the surface and contested many pellets although there was little overt
.
aggression . The less successful fish avoided contests by staying well below the surface and feeding on pellets that dropped down through the water column. Subordinate fish might also avoid competition by feeding at different times of day, thus avoiding
Ž .
interaction with dominants, as suggested by studies of post-smolts Kadri et al., 1997 . When food is available in excess these less aggressive strategies should still result in viable, if lower, rates of food acquisition and growth while reducing the risk of injury. However, when food is scarce, such strategies might not pay off and fin damage should be more evenly distributed between size classes, or even concentrated amongst the smaller fish, if they are forced to compete with dominant fish.
Ž
not increase between November and December, when fin damage and therefore
. Ž
aggression increased once again. For any given temperature, gut evacuation rate and
.
therefore the maximum potential food intake of juvenile Atlantic salmon is lower in the
Ž .
autumn than in the spring Higgins and Talbot, 1985 . Thus, the increase in day length
Ž .
after the photoperiod winter analogous to spring may have been a cue for a resurgence in appetite.
Overall, the fin damage observed in this experiment was less severe than is often
Ž
encountered on fish farms, where the dorsal fin can be almost entirely eroded Turnbull
.
et al., 1996 . The gross thickening of the fin tissue that is the classic symptom of ‘‘fin rot’’ was not observed in this experiment, although mild thickening was evident in some cases. Thickening is part of the healing process and involves the migration of epithelial
Ž .
cells to the damaged area Turnbull, 1992; Turnbull et al., 1998 . Thickening is more severe when damage occurs regularly, without time to heal, as it results in an accumulation of pathological changes. This is most noticeable in the cold, as the healing
Ž .
process is slowed Turnbull, 1992 . Thus the combination of warm water and the relatively low severity of damage may have prevented severe cases of ‘‘fin rot’’ from developing.
Since wild salmonids become aggressive within days of emergence from the redd
ŽKalleberg, 1958; Dill, 1977; Gustafson-Greenwood and Moring, 1990; Titus and
.
Mosegaard, 1991 , it is perhaps surprising that fin damage was uncommon before July.
Ž .
Kalleberg 1958 noticed that agonistic behaviour in Atlantic salmon parr went through a qualitative change when the fish were 60–70 mm in length. At smaller sizes, agonistic encounters mainly involved frontal attacks, with the dorsal fin lowered close to the back, while larger fish tended to use lateral displays with the dorsal fin erect. Clearly, the latter posture would expose the dorsal fin to a far greater chance of damage. In the present study, the rapid appearance of fin damage coincided with the majority of the fish passing the 60 mm threshold. Possibly, then, an increased tendency for lateral display behaviour exposed the dorsal fin to damage for the first time.
In conclusion, we have used fin damage as an indicator of aggressive interactions in large groups of juvenile Atlantic salmon in culture conditions. Fin damage was strongly related to relative body size, indicating that there are alternative feeding strategies within groups of fish. This study serves as a warning against uncritically extrapolating the findings of small-scale studies to culture conditions: social interactions may differ markedly according to group size. Fin damage can give valuable insights into the nature of aggressive interactions in large groups of fish, and could be useful in comparing the success of feeding regimes in reducing the level of aggression in cultured populations.
Acknowledgements
References
Ž .
Abbott, J.C., Dill, L.M., 1985. Patterns of aggressive attack in juvenile steelhead trout Salmo gairdneri . Can. J. Fish. Aquat. Sci. 42, 1702–1706.
Abbott, J.C., Dill, L.M., 1989. The relative growth of dominant and subordinate juvenile steelhead trout
ŽSalmo gairdneri fed equal rations. Behaviour 108, 104–113..
Adams, C.E., Huntingford, F.A., 1996. What is a successful fish? Determinants of competitive success in
Ž .
Arctic char SalÕelinus alpinus in different social contexts. Can. J. Fish. Aquat. Sci. 53, 2446–2450.
Adams, C.E., Huntingford, F.A., Turnbull, J.F., Beattie, C., 1998. Alternative competitive strategies and the
Ž .
cost of food acquisition in juvenile Atlantic salmon Salmo salar . Aquaculture 167, 17–26.
Alanara, A., Brannas, E., 1996. Dominance in demand feeding behaviour in Arctic charr and rainbow trout:¨ ¨ ¨ ¨ the effect of stocking density. J. Fish Biol. 48, 242–254.
Bosakowski, T., Wagner, E.J., 1994. Assessment of fin erosion by comparison of relative fin length in hatchery and wild trout in Utah. Can. J. Fish. Aquat. Sci. 51, 636–641.
Christiansen, J.S., Jobling, M., 1990. The behaviour and the relationship between food intake and growth of juvenile Arctic charr, SalÕelinus alpinus L., subjected to sustained exercise. Can. J. Zool. 68, 2185–2191.
Dill, P., 1977. Development of behaviour in alevins of Atlantic salmon. Anim. Behav. 25, 116–121. Fenderson, O.C., Carpenter, M.R., 1971. Effects of crowding on the behaviour of juvenile hatchery and wild
Ž .
landlocked Atlantic salmon Salmo salar L. . Anim. Behav. 19, 439–447.
Gregory, R.S., Griffith, J.S., 1996. Winter concealment by subyearling rainbow trout — space size selection and reduced concealment under surface ice and in turbid water conditions. Can. J. Zool. 74, 451–455. Gustafson-Greenwood, K.I., Moring, J.R., 1990. Territory size and distribution of newly emerged Atlantic
Ž .
salmon Salmo salar . Hydrobiologia 206, 125–131.
Higgins, P.J., Talbot, C., 1985. Growth and feeding in juvenile Atlantic salmon. In: Cowey, C.B., Mackie,
Ž .
A.M., Bell, J.G. Eds. , Nutrition and feeding in fish. Academic Press, London, pp. 243–263.
Huntingford, F.A., Metcalfe, N.B., Thorpe, J.E., Graham, W.D., Adams, C.E., 1990. Social dominance and body size in Atlantic salmon parr, Salmo salar L. J. Fish Biol. 36, 877–881.
Huntingford, F.A., Metcalfe, N.B., Thorpe, J.E., 1993. Social status and feeding in Atlantic salmon Salmo
salar parr — the effect of visual exposure to a dominant. Ethology 94, 201–206.
Jobling, M., 1985. Physiological and social constraints on growth of fish with special reference to Arctic charr,
SalÕelinus alpinus L. Aquaculture 44, 83–90.
Jobling, M., Wandsvik, A., 1983. Effect of social interactions on growth rates and conversion efficiency of Arctic charr, SalÕelinus alpinus L. J. Fish Biol. 22, 577–584.
Kadri, S., Huntingford, F.A., Metcalfe, N.B., Thorpe, J.E., 1996. Social interactions and the distribution of
Ž .
food among one-sea-winter Atlantic salmon Salmo salar in a sea-cage. Aquaculture 139, 1–10. Kadri, S., Metcalfe, N.B., Huntingford, F.A., Thorpe, J.E., 1997. Daily feeding rhythms in Atlantic salmon: II.
Size-related variation in feeding patterns of post-smolts under constant environmental conditions. J. Fish Biol. 50, 273–279.
Kalleberg, H., 1958. Observations in a stream tank of territoriality and competition in juvenile salmon and
Ž .
trout Salmo salar L. and S. trutta L. . Rep. Inst. Freshwat. Res., Drottningholm 39, 55–98.
Ž .
Keenleyside, H.A., Yamamoto, F.T., 1962. Territorial behaviour of juvenile Atlantic salmon Salmo salar L. . Behaviour 19, 139–169.
Koebele, B.P., 1985. Growth and the size hierarchy effect: an experimental assessment of three proposed mechanisms; activity differences, disproportional food acquisition, physiological stress. Environ. Biol. Fishes 12, 181–188.
Metcalfe, N.B., Huntingford, F.A., Graham, W.D., Thorpe, J.E., 1989. Early social status and the development of life-history strategies in Atlantic salmon. Proc. R. Soc. London, Ser. B 236, 7–19.
Metcalfe, N.B., Wright, P.J., Thorpe, J.E., 1992. Relationships between social status, otolith size at 1st feeding
Ž .
and subsequent growth in Atlantic salmon Salmo salar . J. Anim. Ecol. 61, 585–589.
Moutou, K.A., Mccarthy, I.D., Houlihan, D.F., 1998. The effect of ration level and social rank on the development of fin damage in juvenile rainbow trout. J. Fish Biol. 52, 756–770.
Schneider, R., Nicholson, B.L., 1980. Bacteria associated with fin rot disease in hatchery reared Atlantic
Ž .
salmon Salmo salar . Can. J. Fish. Aquat. Sci. 37, 1505–1513.
Schreck, C.B., Olla, B.L., Davis, M.W., 1997. Behavioural responses to stress. In: Iwama, G.K., Pickering,
Ž .
A.D., Sumpter, J.P., Schreck, C.B. Eds. , Fish Stress and Health in Aquaculture. Cambridge University Press, Cambridge, pp. 145–170.
Ž .
Titus, R.G., Mosegaard, H., 1991. Selection for growth potential among migratory brown trout Salmo trutta fry competing for territories: evidence from otoliths. Can. J. Fish. Aquat. Sci. 48, 19–27.
Turnbull, J.F., 1992. Studies on dorsal fin rot in farmed Atlantic salmon Salmo salar parr. PhD thesis, Stirling University, UK.
Turnbull, J.F., Richards, R.H., Robertson, D.A., 1996. Gross, histological and scanning electron-microscopic appearance of dorsal fin rot in farmed Atlantic salmon, Salmo salar L., parr. J. Fish Dis. 19, 415–427. Turnbull, J.F., Adams, C.E., Richards, R.H., Robertson, D.A., 1998. Attack site and resultant damage during
Ž .
aggressive encounters in Atlantic salmon Salmo salar L. parr. Aquaculture 159, 345–353.
Wedemeyer, G.A., 1997. Effects of rearing conditions on the health and physiological quality of fish in
Ž .
intensive culture. In: Iwama, G.K., Pickering, A.D., Sumpter, J.P., Schreck, C.B. Eds. , Fish Stress and Health in Aquaculture. Cambridge University Press, Cambridge, pp. 35–71.