Chapter 6.
Free Radical Polymerization
Table 6. 1 Commercially Important Vinyl Polymers
6.1. Introduction
6. 2 Free Radical Initiators
Four typesPeroxides and hydroperoxides
6.2.1. Peroxides and Hydroperoxides ( ROOR ) ( ROOH )
Thermal homolysis
C O O C O
Ph O
Ph
∆ C O
O Ph
2
Decomposition
C O
O
Ph Ph + CO2
Radical combination ∵ Cage effect
(confining effect of solvent molecules) Wastage reaction
Ph Ph Ph
2
C O O
Ph Ph C O
O
Ph Ph
+
Induced decomposition
C O O C
O
Ph O
Ph Ph C O O C
O
Ph O
Ph
Ph +
O C
O
Ph
C O
O
Ph Ph +
Radical stability
Ph C CH3
CH3
O CH3 C
CH3
CH3
O C O
O
Ph C O
O CH3
> > >
Unstable radical More wastage reaction Stable radical
Less wastage reaction
Half-lifes
BPO (Benzoyl peroxide)
CH3 C CH3
CH3
O O C CH3
CH3 CH3
C O O C
O
Ph O
Ph
1.4 h at 85oC
1.38 h at 145oC
Ph C CH3
CH3
O O H
C O O C O
CH3 O
CH3
Promoters
C O O C
O
Ph O
Ph
CH3 Ph N
CH3 +
+ CH3
Ph N
CH3
O C
O
Ph +
O O
Ph C
-CH3
Ph N
CH3
O C
O
Ph +
+
No initiation of polymerization
∵ No N in polymers
6.2.2. Azo Compounds
C(CH3)2 N N
CN CN
(CH3)2C
CN
(CH3)2C
2 + N2
AIBN (Azobisiso(butyronitrile))
Radical combination
CN
C(CH3)2 CN
(CH3)2C CN
(CH3)2C 2
C N C(CH3)2 CN
(CH3)2C
(CH3)2C
C N
(CH3)2C
6.2.3. Redox Initiators
Production of free radicals by one–electron transfer reactions. Useful in low–temp polymerization and emulsion polymerization
C(CH3)2 OOH
Ph Fe2+
O
Ph C(CH3)2 + OH + Fe3+
+
-HOOH Fe2+ + OH Fe
3+
+ HO - +
O
3SOOSO
3-
+
S
2O
32-+
SO
42-+
-
SO
-
-4
S
2O
36.2.4. Photoinitiators
RSSR
hν
2 RS
C O
CH Ph
OH
Ph
O
C Ph
OH
Ph CH
+ hν
C O
Ph C
O
Ph C
O
Ph hν
2
The major advantages of photoinitiation:
The reaction is essentially independent of temp
Polymerization may be conducted even at very low temperatures
Better control of the polymerization reaction
6.2.5. Thermal Polymerization
Ph H
Ph +
2
∆ CH
CH2
CH CH2
CH CH3
Diels-Alder dimer
Transfer of H atom to monomer Molecule-induced homolysis
6.2.6. Electrochemical Polymerization
RCH
CH
2+
e
-RCH
CH
2_
RCH
CH
2RCH
CH
2+
e
-+
Radical ions : initiate free radical or ionic polymerization or both
6. 3 Techniques of Free Radical Polymerization Techniques
Method Advantages Disadvantages
Bulk Simple Rxn exotherm difficult to control No contaminants added High viscosity
Suspension Heat readily dispersed Washing and drying required Low viscosity Agglomeration may occur Obtained in granular form Contamination by stabilizer
Solution Heat readily dispersed Added cost of solvent
Low viscosity Solvent difficult to remove
Used directly as solution Possible chain transfer with solvent Possible environmental pollution
Emulsion Heat readily dispersed Contamination by emulsifier
Low viscosity Chain transfer agents often needed High MW obtainable Washing and drying necessary Used directly as emulsion.
6. 4 Kinetics and Mechanism of Polymerization
Formation of the initiator radicalAddition of the initiator radical to monomer Initiation
Initiator R
R CH2 CH
Y
+ R CH2 CH
Y
Propagation
CH CH2
Y
CH CH2
Y
+ CH2 CH
Y
CH CH2
Y
Termination CH CH2
Y
HC H2C
Y +
Coupling or combination
Disproportionation
CH CH2
Y
HC H2C Y
CH CH
Y
H2C H2C Y
Coupling almost exclusively at low temp Polystyrene
CH
CH2 + HC H2C CH2 CH HC H2C
C CH2
C
+ O OCH3 CH3
C H2C C
O
OCH3 CH3
C CH
C
+ O OCH3 CH3
HC H2C C
O
OCH3 CH3 Disproportionation
PMMA
∵
Steric repulsionElectrostatic repulsion
Initiator
k
dR
R
+ M
k
iM
1 InitiationPropagation
+ M
kp
M2 M1
+ M
kp
M3 M2
+ M
kp
M(x+1) Mx
Termination
+ My
ktc
M(x+y)
Mx Coupling
+ My
ktd
Initiation rate (Ri)
[ ]
[ ]
I
fk
2
dt
M
d
R
i=
•
=
dwhere
[M•] = total conc of chain radicals
[I] = molar conc of initiator
f = initiator efficiency = 0.3 ~ 0.8
Termination rate (Rt)
[ ]
[ ]
2 tt
2
k
M
dt
M
d
R
=
−
•
=
•
Steady-state assumption Ri = Rt
[ ]
[ ]
2t
d
I
2
k
M
fk
2
=
•
[ ]
[ ]
t d
k
I
fk
M
•
=
[ ]
[ ][ ]
[ ]
[ ]
t d p
p p
k
I
fk
M
k
M
M
k
dt
M
d
R
=
−
=
•
=
Propagation rate (Rp)
Average kinetic chain length ( )
ν
= Average # of monomer units polymerized per chain initiated
[ ][ ]
[ ]
[ ]
[ ]
(
[ ]
[ ]
)
21 dt p
t p 2
t p
t p
i p
I
k
fk
2
M
k
M
k
2
M
k
M
k
2
M
M
k
R
R
R
R
=
•
=
•
•
=
=
=
ν
ν
=
DP
ν
2
Coupling•
Gel effect, Trommsdorff effect, Norris-Smith effectOccurs in bulk or concentrated solution polymerizations
η↑ Chain mobility↓ R [M•] ↑
t↓
Rp↑ Release of more heat R
i↑ Rp↑
Polymerization of MMA in benzene 50°C benzoylperoxide
•
Chain transfer reactionsTransfer of reactivity from the growing polymer chain
to another species
Chain-end radical abstract a H atom from a chain
Chain branching
2) Backbiting : intramolecular chain transfer
3) C.T. to Initiator or Monomer
Important with monomers containing allylic hydrogen, such as propylene
Reactive radical
Resonance-stabilized radical
∴ High-mol-wt polypropylene cannot be prepared
CH2 C CH3
COOCH3
CH2 C CH3
COOCH3
+ allylic hydrogen
C.T. to monomer
Propagation
CH2 CH CH3
COOCH3
CH2 C CH2
COOCH3 +
CH2 C CH2
COOCH3
Resonance-stabilized radical CH2 C
CH3
COOCH3
CH2 C
CH3
C
O
OCH3
CH2 C CH3
COOCH3
CH2 C
CH3
C
O
OCH3
Resonance-stabilized radical
4) C.T. to Solvent or Chain transfer agents
CCl4
Transfer reaction rate
[ ][ ]
M
T
k
R
tr=
tr•
where T = transfer agent Kinetic chain length
[ ]
[ ]
• = ν M k 2 M k t p[ ][ ]
[ ]
• +∑
[ ][ ]
• =[ ]
• +[ ]
∑
[ ]
• = + = ν T k M k 2 M k T M k M k 2 M M k R R R tr t p tr 2 t p tr t p trw/o transfer agent
with transfer agent
[ ]
[ ]
[ ]
[ ]
[ ]
[ ]
M[ ]
T C 1 M k T k 1 M k T k M k 2 1 T p tr p tr t tr
∑
∑
∑
+ ν = + ν = + • = ν T p trC
k
k
=
Chain transfer constant[ ]
[ ]
M
Table 6.5 CTfor St and MMA
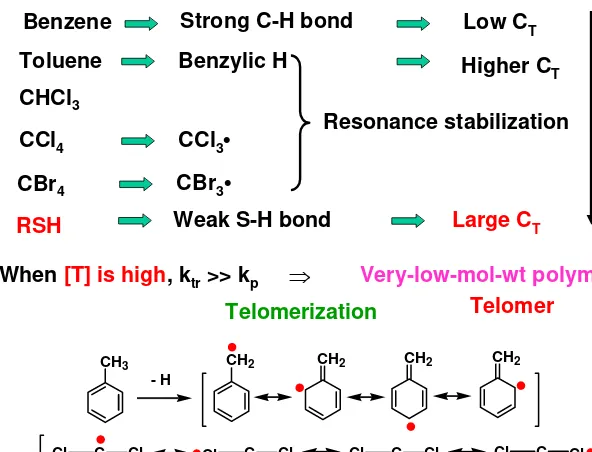
Strong C-H bond
Benzene Low CT
Toluene Benzylic H Higher C
T
Weak S-H bond Large CT CCl3• Resonance stabilization
CHCl3
CCl4 CT↑
CBr3•
CBr4 RSH
Very-low-mol-wt polymer
Telomerization
When [T] is high, ktr >> kp ⇒
Telomer
CH3 CH2 CH2 CH2 CH2
- H
C Cl Cl
Cl C Cl
Cl
Cl C Cl
Cl
Cl C Cl
ο Inhibitors
Added to monomers to prevent polymerization
during shipment or storage
Must be removed by distillation of monomer or extraction
Alkylated Phenol
R' . +
OH R R
R
R'H + R R
R
R R
R O
R R
R
R
R R
R
. O O
.
R .
R' . OR' R R
R
R R
R
R R
R O
R
R R
R
O
+ 'R +
% Polymerization
Time 0
No inhibitor
Inhibitor
More inhibitor
Induction period
Induction periods are common even with purified monomer
o Atom Transfer Radical Polymerization (ATRP)
ο Living free radical Polymerization
No chain termination or chain transfer
CH3CHCl
Ph
+ Cu(I)(bpy) CH3CH Ph
. + Cu(II)(bpy)Cl
H2C CH Ph
CH3CHCH2CHCl
Ph Ph
+ Cu(I)(bpy) CH3CHCH2CH Ph Ph
.
+ Cu(II)(bpy)Cl
bpy = bipyridyl =
N N
Polymerization of styrene
Transfer of Halogen atom
propagation
Preventing radical termination
o Addition of a Stable Free Radical
CH2CH Ph
. +
N
H3C CH3
H3C
CH3 O
.
CH2CH Ph
N
H3C CH3
H3C
CH3 O
TEMPO (2,2,6,6-tetramethylpiperidinyl-1-oxy) Too stale to initiate polymerization
Promotes decomposition of BPO to benzoyloxy radicals
Propagation Prevent termination
[ ]
[ ]
o o I M DP =All the chains are initiated at about the same time. No chain termination or transfer reactions.
ο Emulsion Polymerization
Smith-Ewart kinetics
[ ]
⎟
⎠
⎞
⎜
⎝
⎛
=
2
N
M
k
R
p pwhere [M] = concentration of monomer in the polymer particle
Rate of radical entry into the particle
[ ]
[ ]
[ ]
[ ]
I
fk
2
N
M
k
N
R
M
k
M
k
d p i pp
=
=
ρ
=
ν
Average kinetic chain length
ν
=
DP
N Ri = ρ[ ]
(
)
0.6s 4 . 0
i a E
R k N ⎟ ⎠ ⎞ ⎜ ⎝ ⎛ µ =
k = constant (0.37~0.53)
µ = rate of increase in the volume of a polymer particle [E] = concentration of micellar emulsifier
as = interfacial area occupied by an emulsifier molecule in the micelles
where
[ ] [ ]
0.4 0.6E
,
I
6.5. Stereochemistry of Polymerization
Steric and electrostatic interactions are responsible for stereochemistry of free radical polymerization
1) Interaction between Terminal unit & Monomer
Mirror approach
CH2C CH2C Y
X Y X .
C C Y X H
H
CH2C CH2C Y X
CH2 Y X
C Y
X .
etc
Isotactic polymer
Nonmirror approach
CH2C CH2C X
Y Y X .
C C Y X H
H
CH2C CH2C Y X
CH2 X Y
C Y
X .
etc
2) Interaction between Terminal unit & Penultimate unit
C
C
P CH3
CO2CH3 H
H
C
CO2CH3 CH3 CH2 C
CH3
CO2CH3
C
C
P CH3
CO2CH3 H
H
C
CO2CH3 CH3 CH2 C
CH3
CO2CH3
P: bulkiest group
If terminal carbon has sp2 planar structure,
Interaction between terminal unit & monomer is dominant factor
If terminal carbon has sp3 hybridization,
Interaction between penultimate unit & terminal unit is dominant factor
Stereoregularity ↑ as T
↓
Free radical polymerization of MMA at T < 0 oC
Crystalline polymer
Syndiotactic
(Expect) As size of substituent groups ↑, stereoregularity ↑
(Experiment) bulkier substituent
Less syndiotactic than PMMA under the same conditions
CH2 C CH3
6.6 Polymerization of Dienes
6.6.1. Isolated DienesCrosslinked polymer
Cooperative addition of one double bond to the other of the monomer
Independent reaction
Cyclic polymer
5- or 6-membered ring
Cyclopolymerization:
Cyclopolymerization of divinylformal
.
R +
O O O O
R
O O R
O O R
. .
O O R
O O R
.
head-to-tail mode six-membered ring
head-to-tail mode five-membered ring
Head-to-tail mode
Head-to-head mode
Six-membered ring
Highly crosslinked polymers Diallyl monomer
C
C O
O O
O
Used for manufacturing
electrical or electronics items (circuit boards, insulators,
television components, etc)
preimpregnating glass cloth or fiber for
fiber-reinforced plastics
Diallyl phthalate
O O
O O
2
Used for applications requiring good optical clarity
(eyeware lenses, camera filters,
panel covers, and the like)
6.6.2. Conjugated Dienes
H2C CH HC CH2 RH2C CH2
H
C CH2 .
RH2C HC CH CH2 .
.
R
CH2CH CH CH2
CH2
C C
CH2
H H
CH2
C C H
H CH2
Pendant vinyl groups unsaturation in the chain
cis trans
1,3-butadiene
1,4-addition 1,2-addition
CH2 C CH CH3
CH2
CH2CH CH CH2
CH3
CH2CH C CH2
CH3
CH3
CH2
C C
CH2
H H
CH2
C C H
H CH2
cis trans
1,2-addition 3,4-addition cis-1,4-addition trans-1,4-addition Isoprene (2-methyl-1,3-butadiene)
Natural rubber
(Hevea) Predominant
10%
trans-1,4 s-trans
s-cis cis-1,4 As T↑ , cis-1,4 ↑
CH2 C CH CH2
Sn(n-C4H10)3
cis-1,4 s-cis
Table 6.6 Structures of Free Radical-Initiated Diene Polymers
percent
Monomer
Polymerization
Temperature(℃) cis-1,4 trans-1,4 1,2 3,4
kp:
6.7 Monomer Reactivity
-C6H5 < -CN < -COOCH3 < -Cl < -OCOCH3 o Three factors in monomer reactivity
1) Resonance stabilization of free radicals: kp↓
-C6H5 > -CN > -COOCH3 > -Cl > -OCOCH3
Reactive monomer Stable monomer
Reactive radical Stable radical
Inverse relationship between monomer reactivity and kp Radical reactivity is the major factor.
R +
R R R R
2) Polarization of double bond by substituents: kp↓
Extreme polarization prevents free radical polymerization
e.g.
CH2 C
CN
CN CH2 CH
OCH3 No radical polymerization
C C C C
C C
Homolytic fission Heterolytic fission
Anionic Cationic polymerization
3) Steric hindrance: kp↓
CH2 CH
X
CH2 C
Y
X CH CH CH CH
Y X
kp: MMA < MA
CH2 C
COOCH3
C
H H
H CH2 C
COOCH3 H
MA MMA
∵ Resonance stabilization of the radical by hyperconjugation and steric hindrance
o Free energy of polymerization
∆Gp = ∆Hp - T∆Sp
∆H < 0 : exothermic, π-bond in monomer → σ-bond in polymer ∆S < 0 : monomer → covalently bonded chain structure
∴ Degree of freedom (randomness) ↓
favorable
unfavorable
(1) The higher the resonance stabilization of monomer, l∆Hl ↓ (2) Steric strain in polymer, l∆Hl ↓
(3) Decrease in H-bonding or dipole interaction on polymerization, l∆Hl ↓
Monomer Polymer
energy
π-bond σ-bond
Resonance Stabilization
of monomer ∆H Steric strain
Decrease in
Table 6.7 ∆H and ∆S of Polymerization
Monomer
-△H (kJ/mol)
-△S
(J/mol)
Acylonitrile 77 109
1,3-Butadiene 78 89
Ethylene 109 155
Isoprene 75 101
Methyl methacrylate 65 117
Propylene 84 116
Styrene 70 104
Tetrafluoroethylene 163
-Vinyl acetate 90
-o P-olymerizati-on-dep-olymerizati-on Equilibrium
Mx + M M(x+1) kp
kdp
where
kdp = depropagation rate constant
Tc T
kdp
kp [M]
kp [M] - kdp
At Tc
[ ][ ]
M• M = k[ ]
M•kp dp
Equilibrium constant
[ ]
[ ][ ]
e[ ]
e kdpp k M1 M
M M
K = =
• • =
where [M]e = equilibrium monomer concentration
∆G = ∆Go + RT ln K
where ∆Go= ∆G in standard state
(pure monomer or 1-M solution monomer,
pure polymer or 1-M repeating units of polymer) At equilibrium
∆G = 0
∆Go = ∆Ho - T
c∆So = - RTc ln K = RTc ln [M]e
[ ]
e oo c
M ln R S
H T
+ ∆
∆
=
[ ]
R S RT
H M
ln
o
c o
e
∆ − ∆
=
R
S
o
∆
−
[
]
R
S
RT
H
M
ln
o
c
o
e
∆
−
∆
=
R
H
o
∆
[
]
eM
ln
1
c
T
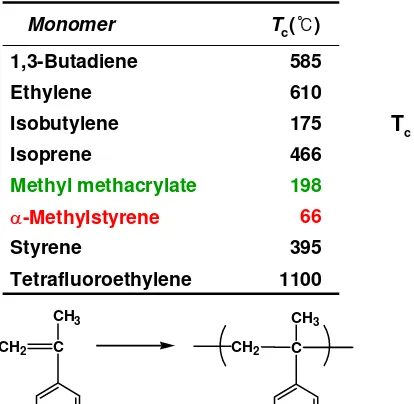
Table 6.8 Tc of Pure Liquid Monomers Monomer Tc(℃)
1,3-Butadiene 585
Ethylene 610
Isobutylene 175
Isoprene 466
Methyl methacrylate 198
α-Methylstyrene 66
Styrene 395
Tetrafluoroethylene 1100
[ ]
e oo c
M ln R S
H T
+ ∆
∆ =
CH2 C
CH3
CH2 C
CH3
-△H = 35 kJ/mol
Tc = 66 oC
6.8 Copolymerization
Reaction Rate
k11
M1
•
+ M1 M1•
k11 [M1•] [M1] k12M1
•
+ M2 M2•
k12 [M1•] [M2] k21M2
•
M1
•
+ M k
21 [M2•] [M1] 1
k22
M2
•
+ M2 M2•
k22 [M2•] [M2]o Steady-state assumption for [M1•] and [M2•] k12 [M1•] [M2] = k21 [M2•] [M1]
o Rate of consumption of M1 and M2
[ ]
[
][ ]
[
][ ]
1 2 21 1 1 11 1 M M k M M k dt M d • + • = −[ ]
[
][ ]
[
][ ]
2 2 22 2 1 12 2 M M k M M k dt Md = • + •
−
[ ]
[ ]
o Instant composition of copolymer
[ ]
[ ]
[
[
]
]
[
[
]
]
⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ • + • • + • = 2 22 1 12 2 21 1 11 2 1 2 1 M k M k M k M k M M M d M d[
]
[ ][
[ ]
]
2 12 2 1 21 1 M k M M kM • = •
o Monomer reactivity ratio
1 12 11 r k k = 2 21 22 r k k =
o Copolymer (composition) equation
o Instantaneous composition of feed and polymer
[ ]
[ ] [ ]
1 21 2 1 M M M f 1 f + = − =
f1 = mole fraction of M1 in the feed f2 = mole fraction of M2 in the feed
[ ]
[ ] [ ]
1 21 2 1 M d M d M d F 1 F + = − =
F1 = mole fraction of M1 in the copolymer F2 = mole fraction of M2 in the copolymer
[ ]
[ ]
11 2 1 F 1 F M d M d − =
[ ]
[ ]
2 1 2 1 f f M M =[ ]
[ ]
[ ]
[ ]
[ ] [ ]
[ ] [ ]
⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ + + = 2 2 1 2 1 1 2 1 2 1 M r M M M r M M M d M d ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ + + = − = − 2 1 1 2 2 1 1 2 1 1 1 f f r f r f f f 1 F 1 F F 1 ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ + + =− 1 2 2
2 1 1 2 1 1 1 f r f f f r f f F 1 F 2 1 2 1 1 2 2 2 2 1 2 1 1 2 1 1 2 2 1 1 2
1 r f f f
[ ]
[ ]
212 1 M d M d F F
F = =
[ ]
[ ]
2 1 2 1 M M f f f = =
[ ]
[ ]
[ ]
[ ]
[ ] [ ]
[ ] [ ]
⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ + + = 2 2 1 2 1 1 2 1 2 1 M r M M M r M M M d M d 2 2 1 2 1 r f f f r r f 1 f r f F + + = ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ + + =f
f
r
F
r
fF
+
2=
1 2+
(
)
1 2 2 r F f r F F 1 f ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ − = −
Finemann and Rose Equation
(
)
F
F
1
f
−
1 - r1r2
2 2 2 2
1 2
1 1
2 1 2
1 1 1
f
r
f
f
2
f
r
f
f
f
r
F
+
+
+
=
1) r1 = r2 = 1
No preference for homopolymerization or copolymerization Random copolymer
F1 = f1 e.g. M
1 = ethylene M2 = vinyl acetate r1 = 0.97 r2 = 1.02
2) r1 = r2 = 0
Alternating copolymer
F1 = 0.5 e.g. M
1 = styrene r1 = 0.041
3) 0 < r1, r2 < 1
More common e.g. M
1 = styrene r1 = 0.52
M2 = methyl methacrylate r2 = 0.46
Azeotropic copolymerization
[ ]
[ ]
[ ]
[ ]
2 1 2 1 M M M d Md =
[ ]
[ ]
[ ]
[ ]
[ ] [ ]
[ ] [ ]
⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ + + = 2 2 1 2 1 1 2 1 2 1 M r M M M r M M M d M d[ ] [ ]
[ ] [ ]
M r M 1M M r 2 2 1 2 1 1 = + +
[ ]
[ ]
14) 1 << r1 and r2 << 1
e.g. M
1 = styrene M2 = vinyl acetate r1 = 55 r2 = 0.01
M1 = styrene M2 = vinyl chloride r1 = 17 r2 = 0.02
Essentially homopolymer
5) r1
•
r2 = 1e.g. M
1 = MMA
r1 = 10 r2 = 0.1
M2 = vinyl chloride Ideal copolymerization
r1
=
r2 = 1 Real random copolymerization The more r1 and r2 diverge, the less random2 2 2 2 1 2 1 1 2 1 2 1 1 1 f r f f 2 f r f f f r F + + + =
(
)
(
)
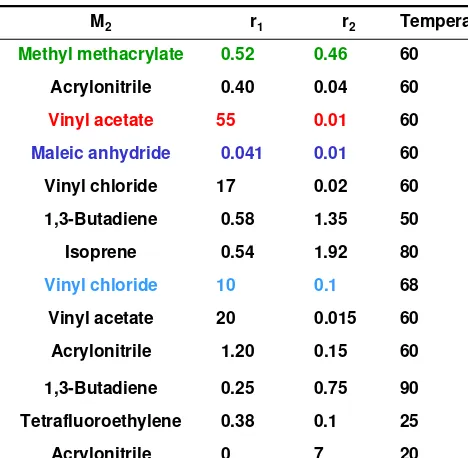
1 1 2Table 6.9 Reactivity Ratios
M1 M2 r1 r2 Temperature(℃)
Styrene Methyl methacrylate 0.52 0.46 60
Styrene Acrylonitrile 0.40 0.04 60
Styrene Vinyl acetate 55 0.01 60
Styrene Maleic anhydride 0.041 0.01 60
Styrene Vinyl chloride 17 0.02 60
Styrene 1,3-Butadiene 0.58 1.35 50
Styrene Isoprene 0.54 1.92 80
Methyl methacylate Vinyl chloride 10 0.1 68
Methyl methacylate Vinyl acetate 20 0.015 60
Methyl methacylate Acrylonitrile 1.20 0.15 60
Methyl methacylate 1,3-Butadiene 0.25 0.75 90
Ethylene Tetrafluoroethylene 0.38 0.1 25
Ethylene Acrylonitrile 0 7 20
r1 = r2 = 1 r1 = r2 = 0
0 < r1, r2 < 1 1 << r1 and r2 << 1
r1
•
r2 = 1 r1 =10, r2 = 0.1Azeotropic composition
2 1
2 1
r r 2
r 1 f
− −
Q-e Scheme (Alfrey-Price Treatment)
k12 = P1Q2 exp (- e1e2)
P1 = reactivity of radical M1
•
Q2 = reactivity of monomer M2 e1 = polarity of monomer M1 e2 = polarity of monomer M2 where(
)
(
)
[
1(
1 2)
]
2 1 2 1 2 1 1 1 1 1 12 11
1 exp e e e
Q Q e e exp Q P e e exp Q P k k
r ⎟⎟ − −
⎠ ⎞ ⎜⎜ ⎝ ⎛ = − − = =
(
)
[
2 2 1]
1 2
2 exp e e e
Q Q
r ⎟⎟ − −
⎠ ⎞ ⎜⎜ ⎝ ⎛ =
Styrene: standard
Q = 1.00 e = - 0.80
Resonance factor ( + steric factor)
Polarity factor
+ : electron-withdrawing group
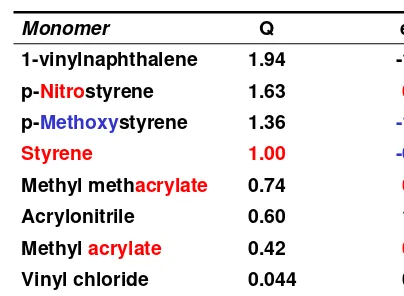
Table 6.10 Reactivity (Q) and Polarity (e) of Monomer
Monomer Q e
1-vinylnaphthalene 1.94 -1.12
p-Nitrostyrene 1.63 0.39
p-Methoxystyrene 1.36 -1.11
Styrene 1.00 -0.80
Methyl methacrylate 0.74 0.40
Acrylonitrile 0.60 1.20
Methyl acrylate 0.42 0.60
Vinyl chloride 0.044 0.20
CH2 CH + CH2 CH
O C CH3 O
CH2 CH
O C CH3 O
Little tendency
No resonance-stabilization
Resonance-stabilization
M1 = Styrene M2 = Vinyl acetate r1 = 55 r2 = 0.01
Styrene Q = 1.00 e = - 0.80 Vinyl acetate Q = 0.026 e = - 0.22
M1 = Styrene M2 = MMA
Styrene Q = 1.00 e = - 0.80
r1 = 0.52 r2 = 0.46
MMA Q = 0.74 e = 0.40
H3
CH2 C C CH3
O
OCH3 + CH2 C
CH3
C O
OC CH2 CH
Each radical has about twice as much tendency to react with its opposite monomer
Copolymerization of styrene and maleic anhydride
Strong tendency toward alternation (r1 and r2 = ~ 0) 1) Polar effects in the transition state
Electron transfer
Electron transfer
2) Formation of Charge-transfer complexes between comonomers Homopolymerization of charge-transfer complex
~ (DA)n D+..A- + D+..A- ~ (DA)
n+1 D+..A -charge-transfer complex
<Evidence>
(1) Rp maximum when monomer composition ratio = 1:1 maximum conc. of donor-acceptor complex (2) Alternation is independent of monomer feed ratios,
and other reactive monomers included with the feed fail to react while alternating copolymer is forming
(3) Rp is enhanced by addition of Lewis acids,
which increase the acceptor properties of one of the monomers (4) Chain transfer agents have little effect on the mol. wt.
Other compounds that undergo free radical-initiated copolymerization with vinyl monomers are CO and SO2
CH2 CH R CH2 CH
R
CO
SO2
CH2 CH R
C O
CH2 CH R
SO2 polyketones