Development of the arbuscular mycorrhizal symbiosis
Maria J Harrison
The arbuscular mycorrhizal (AM) symbiosis formed between plant roots and fungi is one of the most widespread symbiotic associations found in plants, yet our understanding of events underlying its development are limited. The recent integration of biochemical, molecular and genetic approaches into analyses of the symbiosis is providing new insights into various aspects of its development. In the past year there have been advances in our understanding of the signals required for the formation of appressoria, the molecular changes in the root in response to colonisation, and components of the signal transduction pathways common to both the AM andRhizobiumsymbioses.
Addresses
The Samuel Roberts Noble Foundation, 2510 Sam Noble Parkway, Ardmore, Oklahoma, 73402 USA;e-mail: [email protected]
Current Opinion in Plant Biology1998,1:360–365 http://biomednet.com/elecref/1369526600100360 Current Biology Ltd ISSN 1369-5266
Abbreviations
AM arbuscular mycorrhizal Mt4 Medicago truncatula 4
Introduction
Over 80% of vascular flowering plants are capable of entering into symbiotic associations with arbuscular mycorrhizal (AM) fungi, and in natural environments the roots of many plants are really symbiotic organs termed mycorrhizae. The fungi that form these associations are members of the zygomycetes and the current classification places them all into one Order, the Glomales [1]. The AM association is a relatively non-specific, highly compatible, long lasting mutualism from which both partners derive benefit. The plant supplies the fungus with carbon, on which it is entirely dependent. The fungal contribution is more complex — it is clear that the fungus assists the plant with the acquisition of phosphate and other mineral nutrients from the soil, and it is also apparent that it may influence the plant’s resistance to invading pathogens [2]. In addition to its ecological significance, the association may also have applications in agriculture, particularly in sustainable systems [3], where the intimate link between the soil and the plant created by the mycorrhiza, and its impact on nutrient movement, plant and soil nutrition and soil conservation, may be fully exploited.
Development of the symbiosis is associated with sig-nificant alterations in the interior morphology of the root cortex and in the physiology of the plant. These aspects have been reviewed elsewhere in detail [4–7]. In brief, the interaction begins when fungal hyphae, arising
from spores or from adjacent colonised roots, contact the root surface. Here they differentiate to form appressoria via which they penetrate the root (Figure 1). Once inside the root, the fungus may grow both inter- and intra-cellularly throughout the cortex but does not in-vade the vasculature or the meristematic regions. The types of internal structures that develop depend on the plant/fungal combination and may include intracellular, differentiated hyphae called arbuscules and/or intracellular
coils [8•]. Although the fungal hypha penetrates the
cortical cell wall to form the arbuscule within the cell, it does not penetrate the plant plasma membrane and this extends to surround the arbuscule (Figure 1). In addition to internal growth within the root, the fungus also maintains external mycelia which ramify out into the soil. These external hyphae access phosphate which is then transported to the internal structures and eventually released to the root. The interface between the fungal arbuscule and the cortical cell is probably important for nutrient transfer between the symbionts, but this has not yet been demonstrated directly [9]. In comparison to other plant/microbe interactions, we still know relatively little about the molecular events underlying development of the AM symbiosis. The obligate, biotrophic nature of the fungi has contributed to the difficulties with molecular and genetic analyses of these associations and it is only recently that such approaches have been applied. This review surveys the most recent molecular genetic and biochemical studies of the association and their contributions to basic knowledge of the AM symbiosis.
Signals for the development of the AM
symbiosis
The formation of the symbiosis requires the co-ordinate development of both the fungus and the plant and is assumed to require an interchange of signals. As AM fungal spores are capable only of germination and limited hyphal growth in the absence of the plant, it seems likely that plant signals are essential for the initial stages of the symbiosis. Root exudates have been shown to stimulate growth and branching of the AM fungal hyphae and low concentrations of certain flavonoid/isoflavonoid compounds, which are major components of some plant root exudates, are also capable of promoting hyphal growth [10,11]. Recent data suggest that AM fungi have a receptor for the flavonoid/isoflavonoid compounds. Flavonoids, particularly isoflavonoids are known to have estrogenic
activity and the estrogen 17β-estradiol was shown to
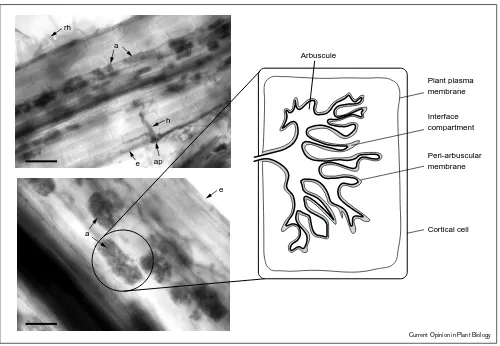
Figure 1
Plant plasma membrane Arbuscule
Peri-arbuscular membrane ap
e h a
rh
e
a Cortical cell
Interface compartment
Current Opinion in Plant Biology
Roots colonised with a mycorrhizal fungus and a diagrammatic representation of a cell containing an arbuscule. The upper left panel shows a
Medicago truncatularoot colonised with an arbuscular mycorrhizal fungus,Glomus versiforme. An appressorium (ap) and hypha (h) penetrating the root are visible. Arbuscules (a), slightly out of the focal plane are visible in the inner cortex. Root hairs are also shown (rh) and the epidermis is labelled (e). The lower panel shows arbuscules in the inner cortical cells of aM. truncatularoot. The right hand side panel is a diagram showing the features of an arbuscule within a cortical cell. The peri-arbuscule membrane is continuous with the plant plasma membrane and surrounds the arbuscule. The space between the membrane and the arbuscule is referred to as the interface compartment. The roots were stained with Cholorazol Black E to allow visualisation of the fungus. The magnification of the upper panel is x300 and the lower panel x600.
are likely to be important features for recognition of the molecule ([12]; Figure 2). Despite the evidence that flavonoid/isoflavonoids promote growth of these fungi it seems unlikely that they are essential for development of the association, as maize mutants lacking flavonoids are still able to form the symbiosis [13]. In addition, isoflavonoids are unlikely to be a universal signal, as they are found only in a subset of the plants capable of forming the symbiosis. Instead, such compounds probably signal the presence of a root and the fungus can respond by increasing hyphal growth and branching to enhance the possibility of contacting the root.
On contact with the root, the fungus differentiates to form appressoria on the surface of the epidermal cells. These structures have only been observed on plant roots and they do not form on synthetic surfaces even in the presence of growth-stimulating exudates [10,14]. Recently it was demonstrated that the mycorrhizal fungus,
Gigaspora margarita was able to form appressoria on
purified carrot epidermal cell wall fragments [15•]. These
Following the formation of appressoria, the fungus pen-etrates the root and proceeds to colonise the cortex and differentiate to form arbuscules. Plant mutants on which the fungus is able to form appressoria but unable to penetrate the root have been identified in a number of legumes [16–19]. Some of these mutants may be lacking a signal for entry and the cloning of the mutated genes has the potential to provide insight into signalling at this stage of the symbiosis. The nature of the signals involved in the later stages of the association is entirely unknown; however, it is conceivable that the more common secondary metabolites are involved. Flavonoids increase in mycorrhizal legume roots and sesquiterpenoid cyclohexenone derivatives were shown recently to accumulate during mycorrhizal development in
many members of the Poaceae [20,21,22•]. Other possible
candidates for later signals include hormones. Induction of cytokinins in mycorrhizae has been reported previously and this was confirmed recently in alfalfa, where significant induction of ZR (trans-zeatin riboside) type cytokinins was
observed in mycorrhizal roots [23,24•]. At the moment,
signals derived from the fungus are entirely unknown.
Figure 2
Current Opinion in Plant Biology
Chemical structure of biochanin A showing the numbering system used to identify carbon atoms and rings.
Molecular events associated with the
development of the mycorrhiza
The identification of plant mutants unable to form a complete symbiosis indicates that development of the association is controlled, at least in part, by the plant. The nature of this control, however, is currently unknown [16]. There has been recent progress in the identification of genes showing differential expression in mycorrhizal roots and although it will require extensive analyses to determine whether these genes have a significant role in the symbiosis, they currently provide information on the molecular events occurring during formation of the associ-ation. In addition they may serve as molecular markers for different developmental stages in the symbiosis. A
mycor-rhizal inducibleβ-tubulin gene was identified in maize and
expression of promoter–reporter gene fusions in tobacco indicated that this gene is induced in the cells in which arbuscules are developing [25]. This is consistent with alterations in the cytoskeleton which occur as the internal structure of the cell is reorganising to accommodate the arbuscule and to develop the peri-arbuscular membrane
(Figure 1). The cell wall of the arbuscule and the peri-arbuscular membrane that surrounds it are separated by a narrow interface compartment that is continuous with the plant cell wall although it differs in structure ([26]; Figure 1). Antibody probes and stains have revealed that the compartment is a complex mixture of molecules
including xyloglucans, arabinogalactans,β-D-1→4 glucans
and hydroxy rich glycoproteins [27–28]. The significance of this molecular composition is currently unknown but it has been speculated that some of the components may act as signals [5]. Potential candidates might include small oligosaccharides or arabinogalactan proteins, both of which are known to act as signaling molecules in other systems. The importance of cell wall signals in the early stages
of the AM symbiosis [15•] indicates that these types of
signals should not be overlooked.
One of the major alterations in the cortical cells in which the arbuscules form is the massive extension of the plasma membrane to form the peri-arbuscular membrane ([29]; Figure 1). Given that the exchange of nutrients is assumed to occur at this interface, it might be expected that membrane transport mechanisms will be induced. Consistent with this suggestion, a cDNA representing a mycorrhizal inducible plasma membrane ATPase was identified recently in barley [30] and a novel gene, predicted to encode a membrane anchored protein with structural similarities to phospholamban, a vertebrate
protein that regulates the activity of a Ca2+-ATPase,
is induced in mycorrhizal pea [31]. The induction of an ATPase gene is consistent with biochemical data indicating increases in plasma membrane ATPase activity in mycorrhizal roots of some species [32] and with earlier studies that demonstrated high levels of ATPase activity on the peri-arbuscular membrane [33]. The putative function of ATPases on this membrane is to provide energy for nutrient transfer processes; however, transport proteins involved in nutrient movement at the arbuscular interface are currently unknown. A mycorrhizal
inducible sugar transporter has been identified inMedicago
truncatula [34] and a gene encoding a member of the membrane intrinsic protein gene family is induced in parsley mycorrhizae. Members of this family have been shown to facilitate the movement of water and other small molecules [35].
Phosphate regulates the symbiosis between AM fungi and plants and the extent of colonisation in the roots is inversely correlated with the phosphate status of the plant [36,37]. Since the symbiosis usually results in increased levels of phosphate in the plant some of the plant genes showing differential expression in mycorrhizal roots may be regulated in response to phosphate, rather than signals from the fungus. There is, however, evidence for independent regulation of expression of one gene,
Mt4, via both of these stimuli. Mt4 is a novel cDNA
representing a root specific gene fromM. truncatula[38•].
starvation and down-regulated by phosphate or in response to colonisation by a mycorrhizal fungus. It was initially assumed that down regulation in the symbiosis was
a consequence of increased phosphate; however, Mt4
expression is also down regulated in a symbiotic plant mutant in which colonisation is limited to growth on the surface of the root and in which phosphate transfer
does not occur. This suggests that the Mt4 gene is
regulated independently by two signals, phosphate and an unknown component(s) of the mycorrhizal fungus. Two phosphate transporters and an acid phosphatase show similar patterns of expression in response to phosphate starvation and colonisation by mycorrhizal fungi, but it is unknown whether they also show independent regulation in response to the two signals [39]. Together, these data indicate that, in general, phosphate starvation inducible genes are down regulated in the early stages of the symbiosis, ahead of significant levels of phosphate entering the root. Has the symbiosis co-evolved to the extent that the plant ‘anticipates’ an increased supply of phosphate before it is delivered? Certainly the symbionts have co-existed for the extensive periods of time that might be necessary for this to occur. Fossil evidence indicates that arbuscular mycorrhizae were present in Devonian land plants and thus plants and AM fungi have been associated for over 400 million years [40].
In comparison with the plant, even less is known about the molecular changes in the fungal symbiont as the association develops. The recent identification of genes expressed in the spores [41] coupled with the continuing identification of fungal genes expressed during the symbiotic phase of the life cycle [42,43] will be essential in unravelling aspects of AM fungal development.
Conserved signalling pathways in the
Rhizobium-legume and mycorrhizal
symbioses
It has been speculated that the Rhizobium–legume (bacterial–plant) symbiosis evolved from the much older mycorrhizal (fungal–plant) symbiosis and emerging sim-ilarities in the molecular events occurring during their development lend support to this idea [44]. The ob-servation that a number of nodulation mutants are also mycorrhizal mutants indicates the presence of genes essential for both symbioses; however, the identity of the genes responsible is unknown [16–19]. Recent studies indicate that a number of the nodulin genes induced during nodule development are also induced in the mycorrhizal symbiosis, although their function in the latter
is unclear. In Vicia faba, one of the four leghaemoglobin
genes induced in nodules is also induced in mycorrhizal
roots [45] and three other nodulin genes, PsENOD12,
MsENOD2andMsENOD40are induced in mycorrhizal pea and alfalfa roots, respectively [5,24•]. In mycorrhizal alfalfa
roots,MsENOD40transcripts are localised to the pericycle,
epidermis and cortex as seen in roots inoculated with
Rhizobia, and co-localise with cells containing immature
arbuscules. The MsENOD2 transcripts are present in
cells containing mature arbuscules, whereas in nodules these transcripts are localised in the nodule parenchyma.
Induction of MsENOD2 and 40 occurs in response to
cytokinin, which is elevated in roots during nodulation and has been shown recently to increase in mycorrhizal alfalfa roots [24•]. On the basis of these data the authors suggest that the downstream signalling events are conserved between the two symbioses; however, it is equally possible that the similarity lies only in the increase in cytokinin and the pathways prior to cytokinin induction are distinct.
Further support for conserved signalling pathways is
provided by studies of PsENOD5 and 12A expression in
mycorrhizal and nodulated pea and pea mutants [46•].
As in nodule development, PsENOD5 and PsENOD12A
are induced in the early stages of the pea mycorrhizal
symbiosis. Further experiments using the pea sym8
mutant, which is blocked in early stages of both nodulation and mycorrhiza formation, demonstrated that a functional Sym8 was necessary for expression of the downstream
PsENOD5andPsENOD12Agenes in both symbioses. Sym 8, therefore, is a common step in the signal transduction pathways for both of these symbioses. It seems unlikely that the initial signals for the two symbioses will be the same; however, at some point — probably shortly after the initial signal — the signal pathways converge and a number of downstream events are conserved.
Conclusions
As the application of molecular approaches to analyses of this symbiosis is relatively recent, it can be anticipated that the first few years will include groundwork to support future advances. In the past year molecular approaches have contributed to our understanding of some aspects of the symbiosis and generated molecular tools essential for future analyses. Thus, the end of the lag phase is approaching and the next few years should see a rapid increase in our understanding of the events underlying the AM symbiosis. The identification of mycorrhizal mutants is accelerating and the continued cloning of genes induced during the symbiosis will contribute to a molecular picture of the events accompanying development of the association. These genes will also serve as tools; both for the analysis of altered developmental processes in the mutants, and as a starting point for the analysis of signal transduction pathways operating in the symbiosis. The symbiosis is a complex system and it will be important to use integrated approaches to obtain a complete understanding of the events underlying its development and functioning.
Acknowledgements
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest •• of outstanding interest
1. Morton JB, Benny GL:Revised classification of arbuscular mycorrhizal fungi (zygomycetes): a new order, glomales, two new suborders, glomineae and gigasporineae, and two new families, acaulosporaceae and gigasporaceae, with an amendation of glomaceae.Mycotaxon1990,XXXVII:471-491. 2. Newsham KK, Fitter AH, Watterson AR:Arbuscular mycorrhiza
protect an annual grass from root pathogenic fungi in the field. J Ecol1995,83:991-1000.
3. Schreiner RP, Bethlenfalvay GJ:Mycorrhizal interactions in sustainable agriculture.Crit Rev Biotechnol1995,15:271-287. 4. Smith SE, Gianinazzi-Pearson V:Physiological interactions
between symbionts in vesicular–arbuscular mycorrhizal plants. Annu Rev Plant Physiol Plant Mol Biol1988,39:221-244. 5. Gianinazzi-Pearson V:Plant cell responses to arbuscular
mycorrhiza fungi: getting to the roots of the symbiosis.Plant Cell1996,8:1871-1883.
6. Bonfante-Fasolo P, Perotto S:Plant and endomycorrhizal fungi: the cellular and molecular basis of their interaction.In
Molecular Signals in Plant–Microbe Communications.Edited by Verma DPS. Boca Raton: CRC Press; 1992:445-470. 7. Harrison MJ:The arbuscular mycorrhizal symbiosis: an
underground association.Trends Plant Sci1997,2:54-56. •
8. Smith FA, Smith SE:Structural diversity in (vesicular)-arbuscular mycorrhizal symbioses.New Phytol1997,137 :373-388.
A detailed and informative review of the diversity of structures present in arbuscular mycorrhizae with discussion of their significance with respect to plant/fungal interfaces involved in nutrient transport. This is particularly im-portant; the presence of different types of structures tends to be overlooked because the majority of experimental work has involved plants forming the
Arum-type mycorrhiza.
9. Smith SE:Transport at the mycorrhizal interface.Mycorrhiza
1993,5:1-3.
10. Giovannetti M, Avio L, Sbrana C, Citernesi AS:Factors affecting appressorium development in the vesicular-arbuscular mycorrhizal fungusGlomus mosseae(Nicol. & Gerd.) Gerd. & Trappe.New Phytol1993,123:115-122.
11. Nair MG, Safir GR, Siqueira JO:Isolation and identification of vesicular-arbuscular mycorrhiza stimulatory compounds from clover (Trifolium repens) roots.Appl Environ Microbiol1991,
57:434-439.
12. Poulin M-J, Simard J, Catford J-G, Labrie F, Pich ´e Y:Response of symbiotic endomycorrhizal fungi to estrogens and antiestrogens.Mol Plant–Microbe Interact1997,10:481-487. 13. B ´ecard G, Taylor LP, Douds Jr DD, Pfeffer PE, Doner LW:
Flavonoids are not necessary plant signals in arbuscular mycorrhizal symbiosis.Mol Plant–Microbe Interact1995,8 :252-258.
14. Giovannetti M, Sbrana C, Avio L, Citernesi AS, Logi C:Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during pre-infection stages.New Phytol1993,125:587-593. •
15. Nagahashi G, Douds DD Jr:Appressorium formation by AM fungi on isolated cell walls of carrot roots.New Phytol1997,
136:299-304.
Elegant experiments demonstrating that the plant signal that induces ap-pressoria formation by AM fungi resides in the epidermal cell wall of host plants. Isolated epidermal cell walls were a suitable substrate for appressoria formation, however, penetration hyphae did not develop, suggesting that subsequent stages require the presence of an intact cell.
16. Duc G, Trouvelot A, Gianinazzi-Pearson V, Gianinazzi S:First report of non-mycorrhizal mutants (Myc-) obtained in pea (Pisum sativumL.) and Fababean (Vicia fabaL.).Plant Sci
1989,60:215-222.
17. Gianinazzi-Pearson V, Gianinazzi S, Guillemin JP, Trouvelot A, Duc G:Genetic and cellular analysis of resistance to vesicular arbuscular (VA) mycorrhizal fungi in pea mutants.InAdvances in Molecular Genetics of Plant–Microbe Interactions, 1.Edited
by Hennecke H, Verma DPS. Netherlands: Kluwer Academic Publishers; 1991:336-342.
18. Bradbury SM, Peterson RL, Bowley SR:Interaction between three alfalfa nodulation genotypes and twoGlomusspecies. New Phytol1991,119:115-120.
19. Sagan M, Morandi D, Tarenghi E, Duc G:Selection of nodulation and mycorrhizal mutants in the model plantMedicago truncatula(Gaertn.) afterγ-ray mutagenesis.Plant Sci1995,
111:63-71.
20. Harrison MJ, Dixon RA:Isoflavonoid accumulation and expression of defense gene transcripts during the
establishment of vesicular-arbuscular mycorrhizal associations in roots ofMedicago truncatula.Mol Plant–Microbe Interact
1993,6:643-654.
21. Peipp H, Maier W, Schmidt J, Wray V, Strack D:Arbuscular mycorrhizal fungus-induced changes in the accumulation of secondary compounds in barley roots.Phytochemistry1997,
44:581-587. •
22. Maier W, Hammer K, Dammann U, Schulz B, Strack D:
Accumulation of sesquiterpenoid cyclohexenone derivatives induced by an arbuscular mycorrhizal fungus in members of the Poaceae.Planta1997,202:36-42.
An extensive survey of secondary metabolite expression in 61 members of the Poaceae reveals that the accumulation of sesquiterpenoid cyclo-hexenone derivatives in response to colonisation by AM fungi is widespread. 23. Beyrle H:The role of phytohormones in the function and
biology of mycorrhizas.InMycorrhiza Structure, Function, Molecular Biology and Biotechnology. Edited by Varma A, Hock B: Springer-Verlag; 1995:365-391.
•
24. van Rhijn P, Fang Y, Galili S, Shaul O, Atzmon N, Wininger S, Eshed Y, Lum M, Li Y, To V, Fujishige N, Kapulnik Y, Hirsch AM:
Expression of early nodulin genes in alfalfa mycorrhizae indicates that signal transduction pathways used in forming arbuscular mycorrhizae andRhizobium-induced nodules may be conserved.Proc Natl Acad Sci USA1997,94:5467-5472. Elevated levels of cytokinin and the induction ofMsENOD2andMsENOD40
were observed in alfalfa roots following colonisation by mycorrhizal fungi. The data are consistent with the conservation of signal transduction pathways in the nodulation and mycorrhizal symbioses.
25. Bonfante P, Bergero R, Uribe X, Romera C, Rigau J,
Puigdomenech P:Transcriptional activation of a maize a-tubulin gene in mycorrhizal maize and transgenic tobacco plants. Plant J1996,9:737-743.
26. Bonfante P, Perotto S:Strategies of arbuscular mycorrhizal fungi when infecting host plants.New Phytol1995,130:3-21. 27. Balestrini R, Romera C, Puigdomenech P, Bonfante P:Location of a cell-wall hydroxyproline-rich glycoprotein, cellulose and
β-1,3-glucans in apical and differentiated regions of maize mycorrhizal roots.Planta1994,195:201-209.
28. Balestrini R, Jos ´e-Estanyol M, Puigdom ´enech P, Bonfante P:
Hydroxyproline-rich glycoproteins mRNA accumulation in maize root cells colonized by an arbuscular mycorrhizal fungus as revealed byin situhybridization.Protoplasma1997,
198:36-42.
29. Alexander T, Meier R, Toth R, Weber HC:Dynamics of arbuscule development and degeneration in mycorrhizas ofTriticum aestivumL. andAvena sativaL. with reference toZea maysL. New Phytol1988,110:363-370.
30. Murphy PJ, Langridge P, Smith SE:Cloning plant genes differentially expressed during colonization of roots of
Hordeum vulgareby the vesicular-arbuscular mycorrhizal fungusGlomus intraradices.New Phytol1997,135:291-301. 31. Martin-Laurent F, van Tuinen D, Dumas-Gaudot E,
Gianinazzi-Pearson V, Gianinazzi S, Franken P:Differential display analysis of RNA accumulation in arbuscular mycorrhiza of pea and isolation of a novel symbiosis-regulated plant gene.Mol Gen Genet1997,256:37-44.
32. Bago B, Donaire JP, Azc ´on-Aguilar C:ATPase activities of root microsomes from mycorrhizal sunflower (Helianthus annuus) and onion (Allium cepa) plants.New Phytol1997,176:305-311. 33. Gianinazzi-Pearson V, Smith SE, Gianinazzi S, Smith FA:
Enzymatic studies on the metabolism of vesicular-arbuscular mycorrhizas.New Phytol1991,117:61-74.
35. Roussel H, Bruns S, Gianinazzi-Pearson V, Hahlbrock K, Franken P:Induction of a membrane intrinsic protein-encoding mRNA in arbuscular mycorrhiza and elicitor-stimulated cell suspension cultures of parsley.Plant Sci1997,126:203-210. 36. Schwab SM, Menge JA, Leonard RT:Comparison of stages
of vesicular-arbuscular mycorrhiza formation in sudangrass grown at two levels of phosphorus nutrition.Amer J Bot1983,
70:1225-1232.
37. Thomson BD, Robson AD, Abbott LK:Effects of phosphorus on the formation of mycorrhizas byGigaspora calosporaand
Glomus fasciculatumin relation to root carbohydrates.New Phytol1986,103:751-765.
•
38. Burleigh SH, Harrison MJ:A novel gene whose expression inMedicago truncatularoots is suppressed in response to colonization by vesicular-arbuscular mycorrhizal (VAM) fungi and to phosphate nutrition.Plant Mol Biol1997,34:199-208. Reports a novel gene,Mt4, that is regulated in response to phosphate and to colonisation by mycorrhizal fungi. Studies ofMt4expression in a mycorrhizal mutant suggest that regulation by phosphate and mycorrhizal fungi occurs via two, initially independent pathways.
39. Liu H, Trieu AT, Blaylock LA, Harrison MJ:Cloning and
characterization of two phosphate transporters fromMedicago truncatularoots: Regulation in response to phosphate and to colonization by arbuscular mycorrhizal (AM) fungi.Mol Plant–Microbe Interact1998,11:14-22.
40. Remy W, Taylor TN, Hass H, Kerp H:Four hundred-million-year-old vesicular arbuscular mycorrhizae.Proc Natl Acad Sci USA
1994,91:11841-11843.
41. Franken P, Lapopin L, Meyer-Gauen G, Gianinazzi-Pearson V:
RNA accumulation and genes expressed in spores of the
arbuscular mycorrhizal fungus,Gigaspora rosea.Mycologia
1997,89:293-297.
42. Harrison MJ, van Buuren ML:A phosphate transporter from the mycorrhizal fungusGlomus versiforme.Nature1995,378 :626-629.
43. Burleigh SH, Harrison MJ:A cDNA from the arbuscular mycorrhizal fungusGlomus versiformewith homology to a cruciform DNA-binding protein fromUstilago maydis. Mycorrhiza1998,7:in press.
44. LaRue TA, Weeden NF:The symbiosis genes of the host.In
Proceedings of the 1st European Nitrogen Fixation Conference.
Edited by Kiss GB, Endre G. Szeged, Hungary: Officiana Press; 1994:147-151.
45. Fr ¨uhling M, Roussel H, Gianinazzi-Pearson V, P ¨uhler A, Perlick AM:
TheVicia fabaleghemoglobin gene VfLb29 is induced in root nodules and in roots colonized by the arbuscular mycorrhizal fungusGlomus fasciculatum.Mol Plant-Microbe Interact1997,
10:124-131. •
46. Albrecht C, Geurts R, Lapeyrie F, Bisseling T:Endomycorrhizae and rhizobial nod factors activate signal transduction pathways inducingPsENOD5andPsENOD12expression in which Sym8 is a common step.Plant J1998, in press.