Review article
Sucrose and the integration of metabolism in vascular plants
John Farrar
a, Chris Pollock
b,*, Joe Gallagher
baSchool of Biological Sciences,Uni
6ersity of Wales,Bangor LL57 2UW,Wales,UK bInstitute of Grassland and En
6ironmental Research,Aberystwyth SY23 3EB,Wales,UK
Received 29 August 1999; received in revised form 29 November 1999; accepted 29 November 1999
Abstract
We consider the hypothesis that sucrose is a signal as well as a substrate. We suggest that the significance of sugar sensing in plants is the integration of whole-plant carbon flux so that the capacity of sources to produce sucrose matches the capacity of sinks to consume it. We pay particular attention to difficulties with this hypothesis and the areas where further or better evidence is needed. We conclude that there is strong correlative evidence for a link between sucrose metabolism and the level of expression of key genes, but that a number of different mechanisms may be involved. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Sucrose; Sucrose metabolism; Vascular plants
www.elsevier.com/locate/plantsci
1. Introduction
The vast majority of higher plants exploit their environment through the interception of light en-ergy, together with the acquisition of a range of compounds (principally water, carbon dioxide and inorganic nutrients). Considerable specialisation at both the tissue and organ level is associated with the capture of different resources. This specialisa-tion generates a requirement to transport re-sources between and integrate the function of different organs.
Sucrose is one of the major forms in which material is transported around higher plants. Commonly, it is a major product of photosynthe-sis, the main form of translocated carbon and the main substrate for sink metabolism. We propose that sucrose has also got a significant regulatory
and integrative function. In particular, we suggest that excess sucrose can feed forward to stimulate sink processes and feed back to down-regulate photosynthesis [1 – 3]. Sucrose metabolism is inti-mately linked to the metabolism of inorganic and organic N, and thus may be part of a wider mechanism for balancing resource acquisition and allocation within and between organs [4].
In this review, we will consider mainly the longer-term, integrative effects of sucrose on metabolism and development. Such effects are be-lieved to be mediated via modulation of gene expression or protein turnover. We consider de-tailed examples of where sucrose is thought to modulate metabolic flux and discuss how such modulation might help to integrate spatially sepa-rate processes. We also consider how changes in sucrose content may be transduced into changes in gene expression. We pay particular attention to the quality of, and gaps in, the evidence support-ing the general hypothesis that sucrose is a signal molecule.
* Corresponding author.
1.1. Why sucrose?
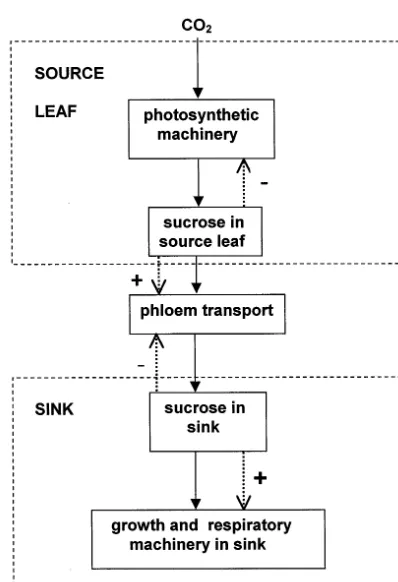
The formal statement of the mechanism we propose is indicated in Fig. 1, and demonstrates the pivotal position of sucrose. There are three components of any regulatory system: a sensing mechanism, a transmission mechanism and a transduction mechanism. If sucrose is important as a regulator, it must be involved in one or more of these mechanisms in a way that is beyond any direct role as a substrate. The sucrose content of a plant or plant part represents a relatively long-term integral of the balance between supply (pho-tosynthesis) and demand (growth, nutrient assimilation, storage etc). A simple calculation illustrates the potential for changes in pool size. A
barley leaf produces sucrose photosynthetically at about 2 g m−2 h−1; since its sucrose content is
typically 6 g m−2, the size of the sucrose pool
would increase 5-fold during a single photoperiod, if export was prevented. The sucrose pool clearly has the capacity to sense both internal and envi-ronmental change; what is the evidence that it does so?
There is no doubt that changes in assimilation by leaves and in metabolism by sinks are associ-ated with changes in the overall sugar pool. Two examples will suffice. Increased irradiance leads to accumulation of both sucrose and starch in barley leaves within 8 h of treatment [5], and chilling roots and stem apices of Lolium temulentum
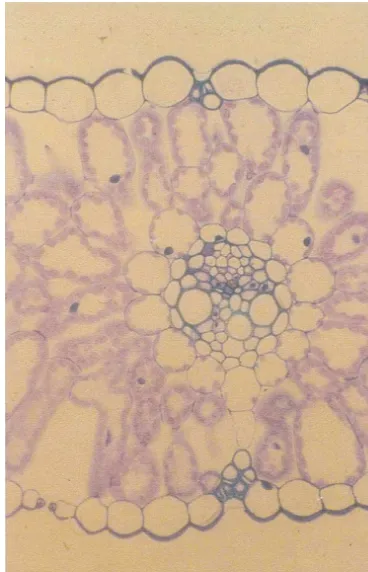
(whilst maintaining leaves at 20°C) led to similar effects, together with the accumulation of fructose polymers. Reversal of this treatment was accom-panied by mobilisation of reserves and a resump-tion of root and shoot growth [6]. Similar effects have been described for sink tissues although the changes are less marked, since the sucrose pool appears to be buffered (see Ref. [2] and Refs. therein). Bulk changes in sucrose content ignore the effects of cell or tissue compartmentation, and concentrations of sucrose and fructans can vary greatly between epidermis (nearly sugar free), mes-ophyll and parenchymatous bundle sheath in bar-ley leaves ([7,8]; Fig. 2). Clearly, attempts to correlate sugars with the features they are thought to control should be focused at the level of the single cell, not a heterogeneous tissue. There is interchange between the various inter- and intra-cellular pools, giving a series of potential re-sponses with different sensitivity, based upon the different size and turnover rates of the various components.
There is also abundant evidence that the rapid movement of sucrose through plants via the phloem is sensitive to the sugar status of both source and sink. The exact relationship between pressure-driven mass flow in the phloem and the overall sucrose abundance in source and sink is not clear, particularly given tissue-specific differ-ences in distribution. It has been suggested that export rates are related to bulk sucrose concentra-tion [9] although other evidence suggests that the rate correlates more closely with the nonvacuolar sucrose contents of mesophyll and vasculature ([10], B.E. Collis, J.F. Farrar and C.J. Pollock, unpublished observations). There is no doubt,
Fig. 2. A barley leaf in transverse section, stained with periodic acid-Schiffs reagent and toluidine blue. A main vein, mesophyll cells and epidermis are visible, together with the prominent parenchymatous bundle sheath. The picture em-phaises the complexity of plant tissues and the potential for localisation of function [7,8]. In this case, there is spatial separation of the biosynthesis and export of sucrose between the mesophyll and the phloem, with the possibility of distinct sugar pools being involved in the regulation of photosynthesis [11] and phloem loading [40]. Micrograph courtesy of O. Koroleva.
than the balancing of supply and demand [11]. There are other examples of longer-term regula-tion involving sugars. Whilst genes of respiratory (cytochrome c; [12]) and 1-C [13] metabolism are sugar-regulated, naturally most attention has fo-cused on photosynthesis. Sheen [14] showed that promoter – reporter fusions for genes coding for photosynthetic proteins were sensitive to sucrose, acetate and hexose but not to mannitol when measured in a maize propoplast transient assay system. More directly, increased concentration of assimilates were generated in tobacco leaves by over-expressing a yeast invertase in the cell wall and thus blocking phloem loading [15]. The trans-genic plants expressing invertase showed reduced growth and photosynthesis and a decline in the activity of certain photosynthetic enzymes [16]. Exogenous supply of sugars to intact spinach leaves or to photosynthetic cell cultures of Chenopodium rubrum and endogenous accumula-tion induced by cold-girdling all gave similar ef-fects [17,18].
Unfortunately, down-regulation of photosyn-thesis is not a universal response to assimilate accumulation in leaves. In temperate gramineae, rates of dry weight gain [19] or of carbohydrate accumulation and photosynthetic oxygen evolu-tion [20] were linear over time, even though the tissue concentration of soluble sugars increased about fiftyfold during the experiment. One of the problems with comparing the results from this type of experiment is that bulk measurements of sugar accumulation may mask significant differ-ences between different cell types [8]. It is possible, therefore, that ‘nonresponsive’ plants merely se-quester the accumulated sugars at a site where they do not cause photosynthetic down-regulation. It is also easy to confuse direct effects on photo-synthesis with indirect effects mediated through altered leaf ontogeny [2].
The effects of elevated CO2 should be
pre-dictable if our hypothesis is correct: in general,
growth at elevated CO2 results in both higher
concentrations of non-structural carbohydrates and a down-regulation of photosynthesis in source leaves, the former being held to cause the latter [21]. Some data are consistent, with correlations between carbohydrate content and photosynthetic rate [22,23]. However, there are exceptions. For example, just 24 h after switching barley from 350
to 700 ppm CO2, the carbohydrate content of the
therefore, that changes in sucrose concentration can accompany changes in source or sink perfor-mance and can communicate these imbalances be-tween different organs.
2. Do changes in sucrose content produce long-term changes in metabolism?
2.1. Sources
There is convincing evidence for the
involve-ment of sugars in the regulation of leaf
second leaf is substantially increased but photo-synthesis is not down-regulated (B.E. Collis, J.F. Farrar and C.J Pollock unpublished observations; [24]), possibly because 24 h is too soon to see evidence of depressed rates of photosynthesis and emphasising the importance of a time-course in such experiments. Further the rate of photosynthe-sis depends on N as well as carbohydrate status [22,25,26], confounding experiments where simple correlations between carbohydrate and photosyn-thetic downregulation are sought.
Down-regula-tion of photosynthetic rate can occur without a reduction in the amount of rubisco protein [27]. Clearly, we need direct evidence of causality be-tween carbohydrates and photosynthetic gene ex-pression at elevated CO2. We need experiments
where carbohydrate and nitrogen partitioning are measured along with photosynthetic rates, amounts of rubisco protein and transcript levels, over a time course following a change in CO2
concentration.
Nevertheless, the balance of probability is that accumulation of sucrose or a closely related metabolise can feed back to reduce the expression of key photosynthetic genes; the most persuasive case is made by Moore et al. [28]. Sucrose accumu-lation may be a necessary, but not a sufficient, condition. There are, of course, a considerable number of signalling systems in plants that act by modifying gene expression and/or protein turnover and it seems improbable that these all act in isolation. The abundance and activity of individ-ual proteins and the resultant flux through specific pathways will usually reflect the balance between a range of regulatory mechanisms, some of which are responsive to assimilate abundance.
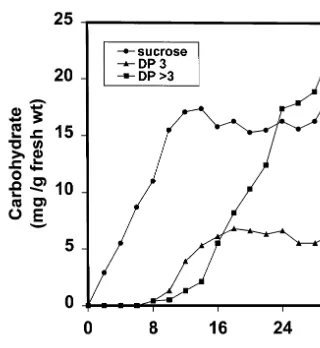
One of the clearest demonstrations of a link between elevated sucrose contents and the induc-tion of changes in the patterns of gene expression is shown during the induction of fructan accumu-lation in leaves of C3 grasses and cereals. The kinetics of the process is shown in Fig. 3. The imposition of sucrose accumulation is followed by the progressive accumulation of short- and long-chain fructose polymers. Administration of in-hibitors of gene expression (Table 1) has no effect on the photosynthetic accumulation of sucrose, but blocks the subsequent conversion of these to fructans. The enzymology of this pathway in gramineae is not completely understood [29]. However, we have shown that, under conditions of sucrose accumulation, there is progressive up-reg-ulation of a number of mRNA species [13]. One of these up-regulated mRNA species is closely related to a fructosyl transferase cloned from barley [30] and is a member of the acid invertase gene family (J. Gallagher, C. Pollock, unpublished observa-tions). In this case, accumulation of sucrose ap-pears to be closely linked to the up-regulation of enzymes concerned in its subsequent metabolism. There are a number of other examples where genes associated with photosynthesis, resource allocation
Fig. 3. Progressive accumulation of sucrose, oligosaccharides and high molecular weight fructan following blockage of sucrose export in leaves ofLolium temulentum. Redrawn with permission from reference [65].
Table 1
Specific effects of inhibitors of transcription and translation on the conversion of sucrose to fructan in excised, illuminated leaves ofLolium temulentuma
Inhibitor Total water-soluble carbo- Percentage as hydrate accumulated in 24 fructan h (ma g−1fresh wt)
43.3 58.4
Water control
2.5 Cycloheximide 40.5
(100mM)
44.9
Cordycepin 2.8
(1 mM)
a-Amanitin 48.6 4.2
(1 mM)
53.0 44.5
L-MDMP
(10mM)b
D-MDMP 40.8 1.0
(10mM)b
aData from reference [66].
bMDMP (2-(4-methyl-2,6-dinitroanilino)-N-methyl
propi-onamide) is an inhibitor of cytoplasmic translation. Only the
and carbon metabolism in leaves appear to be sensitive to assimilate abundance [4,31], and it is tempting to regard this sensitivity as part of a generic regulatory framework selected to ensure that carbohydrate synthesis, storage and export are maintained in balance. However, we suggest that too much of the evidence for sugar-regulated gene expression is correlative and that there is a pressing need for more direct evidence.
2.2. Sinks
Once again, there is good evidence that a range of metabolic processes in sink tissues are sensitive to the supply of assimilate, but here the effects are stimulatory: the greater the sugar status, the greater the capacity for sinks to grow. Because of their sensitivity to experimental manipulations, their relatively low internal storage capacity and their broadly indeterminate habit, much of the work has been done on roots, but similar evidence exists for other types of sink. The total growth of all sinks on the plant cannot exceed the ability of source organs to supply them, and there is ample evidence that the size and respiration rate of the root system is regulated relative to that of the source leaves [32]. Reduction of sugar supply by darkening, defoliation or excision leads to a pro-gressive decline in root growth and respiration, accompanied by declines in the amounts of en-zymes involved in sugar metabolism and respira-tion but increases in proteolytic enzymes [2,33,34]. Significantly, many of these processes can be sus-tained in starved roots by the administration of exogenous sugars [2]; for example the activity of fumarase and acid invertase and the amount of cytochrome c oxidase increase, and the activity of endoprotease and the transcript level of polyubiq-uitin decrease, on supplying metabolised sugars to excised barley root tips (B.E. Collis, J.F. Farrar, C.J. Pollock, unpublished observations), and there is evidence that cell division, which is arrested in G1 and G2 stages during starvation, can be restarted by the supply of sucrose or glucose to root tips [35].
The study of assimilate-responsiveness in genes coding for enzymes of sucrose metabolism has, however, identified an additional layer of complex-ity [31]. For sucrose synthase (Susy) and invertase (I6r), there is evidence both for sugar modulation
and for the reciprocal sensitivity of the products of
different genes coding for the same activity. This has led to the idea of ‘feast’ and ‘famine’ genes. Koch suggests that up-regulation of ‘feast’ genes for Susy and Ivr in the presence of abundant imported sucrose could balance import and metabolism relative to supply. By contrast, the equivalent ‘famine’ genes appear to be localised in the epidermis and vascular strands and may have a role in maintenance of function during starva-tion by altering import priority towards essential tissues. This hypothesis is supported by the obser-vation that cortical cells in roots are often sac-rificed during stress [31] and emphasises our poor knowledge of how phloem transport relates to sugar metabolism within adjacent tissues. We would predict that the proteolytic responses to low sugar would, at least initially, be confined to the cortex whilst sugar pools and respiratory activity are retained in the vasculature.
There is, therefore, a significant body of evi-dence that is consistent with sink processes being directly responsive to assimilate supply and with that responsiveness being mediated via changes in gene expression. Furthermore there are examples of where these responses are themselves part of the mechanisms that regulate both carbohydrate metabolism and sink activity. Taken in conjunc-tion with the observaconjunc-tions from leaves, it is possi-ble to postulate a pivotal role for sucrose in the sensing of source – sink balance and in the regula-tion of growth and metabolism that is required to maintain such a balance.
However, our information for sinks is not as good as that for source leaves. We also need equivalent information for shoot apices. Critically, we need information on whether the rate-con-trolling processes in sinks are sugar-regulated. This is vital since respiration rate mirrors demand [32] and we do not know which genes and gene products in sinks are central in determining that demand. If our hypothesis is correct, it is these genes that should be sugar-sensitive.
2.3. The transport pathway
relative balance between supply of and demand for fixed carbon throughout the plant, facilitating the integration of the source and sink processes de-scribed above. Although the transport pathway itself can contribute to buffering change by virtue of the considerable amount of sucrose contained within it, there is no doubt that information on source – sink balance can be transmitted rapidly over significant distances. Chilling the growing points of grasses produces an immediate reduction in extension growth [36]. Within 30 min of this reduction, sucrose begins to accumulate at an enhanced rate in mature source leaves [37]. Simi-larly, when root temperature is reduced or in-creased, import of carbon into the root alters with minutes [38]. There is, therefore, no reason a priori to suppose that changes in sucrose concentration cannot contribute to the integration of plant metabolism over large distances. The possible reg-ulation of phloem transport by the sucrose trans-porters involved in phloem loading [39] may add complexity, since whatever regulates the expres-sion of these transporters will control sucrose flux partly independently of its abundance in source leaves and consumption in sinks. Transporter ac-tivity in isolated plasma membrane vesicles has indeed been shown to be sensitive to sucrose abun-dance in the tissues prior to extraction, as has expression of the appropriate mRNA species [40].
3. Cross-talk between regulatory systems: the case of C – N interactions
As indicated above, it is inappropriate to con-sider sucrose-regulation of plant processes in isola-tion. For example, the effects of changes in assimilate abundance on plant metabolism can be modified significantly by edaphic or biotic stress [4]. The importance of considering interacting reg-ulatory mechanisms is particularly obvious in the case of the links between primary carbon and nitrogen metabolism.
At one level, physiologists have suggested that the basis of shoot – root allometry is the ‘func-tional equilibrium’ between carbon and nitrogen assimilation [41]. Under this model, carbon is ac-quired by the shoot, with provision to the root controlling the rate of root growth. In turn, nitro-gen is acquired by the root; supply to the shoot determines the investment in growth and
photo-synthetic machinery and in turn controls the rate of carbon supply. Changes in the allometric con-stant under low light or low nutrient conditions are consistent with this model [42], but Farrar and Gunn [43] argue that shoot:root (S:R) ratio is regulated for resource acquisition, but none of the partial processes which determine it are themselves regulated in a simple way so that it is far from obvious how control of S:R is achieved. Further, we cannot answer the question of whether there is co-ordinated regulation of the amount of an organ and the density of resource-acquiring machinery within it — for example, is it the amount of shoot, or the amount of rubisco per unit leaf area, that is regulated?
At the biochemical level, nitrate reduction, su-crose synthesis and organic acid synthesis are all ‘sinks’ for the immediate products of photosynthe-sis. There is now compelling evidence that the relative demands of these processes for fixed car-bon, ATP and reducing power are balanced via the coordinated regulation of sucrose phosphate synthase, nitrate reductase and PEP carboxylase activities [44]. This regulation occurs by specific and reversible phosphorylation that causes changes in existing enzyme activity.
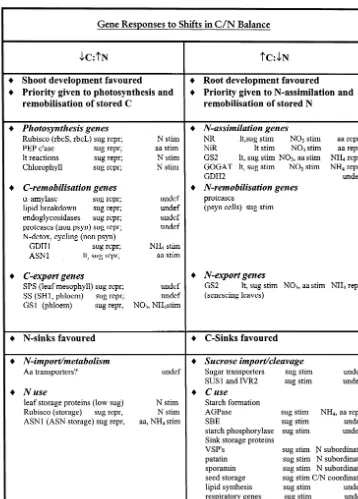
Fig. 4. Gene responses to shifts in C/N balance. Redrawn with permission from Koch, 1997. Abbreviations are as follows: stim, stimulated; repr, repressed; undef, undefined; sug, sugar; lt, light; aa, amino acid.
that both together are needed for metabolism to respond.
4. Possible mechanisms for the regulation of gene expression by sucrose
Although induction or suppression of metabolic pathways by sugars is well-known in microbes, there are few mechanistic models for how sucrose
might act in this way. We are aware of two: the induction of levansucrase activity in Bacillus sub
-tilis [46] and the hexose repression of invertase activity in yeast [47]. The former involves a specific role for sucrose, the latter does not. In B. subtilis, sucrose is phosphorylated in the cell membrane during uptake. This is associated with the dephos-phorylation of the product of the sac X gene. In this state, the product no longer inhibits the sac Y
anti-terminator and facilitates transcription of the structural gene for levan sucrase [48].
In yeast, it appears that a specific hexokinase functions in a regulatory as well as a catalytic role and thus senses flux rather than abundance. The hexokinase, possibly as a substrate – enzyme – effec-tor complex, initiates a protein kinase cascade that acts as a regulator of transcription [4,49]. At least one of the kineses has a homolog in higher plants, suggesting that a similar system may operate there [50 – 52]. In plants, sucrose signalling would be initiated by the cleavage of sucrose molecules ei-ther by invertase or by sucrose synthase. This would generate substrates for the hexokinase, and the extent of flux through this enzyme would determine the extent of up-regulation of genes that responded via the protein kinase cascade [49]. Jang and Sheen [49] provide experimental evidence, us-ing a maize transient expression assay, that phos-phorylation of hexoses is a key part of this transduction pathway. Importantly, transgenics over- or under-expressing hexokinase are hyper-and hypo-sensitive to sugar, respectively [53]. In-terestingly enough, the possession by plants of alternative pathways for sucrose cleavage could affect the level of response. Inversion of sucrose liberates two potential substrate molecules for hex-okinase, whereas cleavage by Susy generates only one. Additionally, invertase isoforms with differ-ent catalytic properties are presdiffer-ent in the vacuole, the apoplast and the cytosol, and this may also affect substrate availability for the hexokinase step. Hexokinase-based regulation would appear to sense the hexose/hexose phosphate pool, whereas regulation of transporter activity, and of fructan accumulation appear to be more specifi-cally associated with changes in sucrose content [40,54,55] These remain as provisional models for sucrose-sensing and transduction pathways in plants, but the frequency of occurrence of sugar-responsive pathways in microbes suggests a degree of conservation and the probability of a restricted number of common mechanisms.
There are some deep problems remaining. For example, how is sucrose-mediated gene regulation related to sugar concentration: is it a linear func-tion of concentrafunc-tion, is there a threshold, or is the regulation sensitive to flux rather than to concen-tration? There are other issues to be resolved. How is the signal time-integrated? Since sucrose concen-tration in leaves can alter 5 – 10-fold in a day
[8,56], is there continuous modulation of gene activity with the amount of individual mRNAs and proteins providing the integration? Simply understanding the signal transduction pathway will not enable us to understand how sugar sig-nalling fits fully into the daily life of the plant. Finally, Trewavas [57] has suggested that the sen-sitivity of tissues to plant growth substances can vary greatly during ontogeny and may be the dominant factor determining the effects of growth substances: could the same be true of sugar regula-tion? The question is especially pertinent since there may be an interaction of sugar levels and hormonal regulation [58,59].
5. Higher-order integration: sucrose as a morphogen
The mechanisms discussed above principally in-volve sucrose as a substrate, either directly or via its potential to modulate the activities of enzymes involved in its synthesis, cleavage or subsequent metabolism. There are also a few reports of su-crose acting to alter developmental pathways in groups of cells. Perhaps the clearest indication of this is the demonstration that sucrose in combina-tion with auxin can substitute for an excised bud in inducing the differentiation of vascular tissue within a block of undifferentiated callus tissue. At constant auxin concentration, increasing concen-trations of sucrose increased the proportion of phloem vessels in the vascular tissue [60] Jeffs and Northcote [61] showed that this effect could be obtained only with a-glucosyl disaccharides (e.g. sucrose, trehalose and maltose).
There is some evidence for a similar effect dur-ing the floral transition and fruit abortion (dis-cussed in reference [2]) but no mechanistic explanations exist for these observations. It re-mains possible, however, that the overall effects of sucrose include morphogenetic as well as sub-strate-level and regulatory interactions.
6. Concluding remarks
two ‘rate limiting’ enzymes [62]. Above this, we have a much clearer idea of how the amount of specific enzyme protein is regulated via changes in gene expression, post-translational modification and protein turnover. We are also using the preci-sion and high resolution of molecular methodolo-gies to gain an understanding of how processes may be regulated and integrated in time and space. This broader approach has produced a consider-able body of evidence that is highly suggestive (if rarely conclusive) of a key role for sucrose in the integration of plant growth and function. The increased availability of defined transgenic and mutant plants will permit many of the hypotheses outlined in this review to be tested more rigor-ously, but it is important to remember the extent of plant diversity, even in primary carbon metabolism. There are, for example, species that translocate a range of sugars other than sucrose, that store an even wider range of carbohydrates [63] and that have very different allocation strate-gies to those most frequently studied [64]. We should not neglect this diversity as a valuable tool in the assessment of the various roles for sucrose in higher plant metabolism.
Acknowledgements
We acknowledge financial assistance from the Biotechnology and Biological Sciences Research Council and from the Natural Environment Re-search Council.
References
[1] T.F. Neales, L.D. Incoll, The control of leaf photosyn-thesis rate by the level of assimilate concentration in the leaf a review of the hypothesis, Bot. Rev. 34 (1968) 107 – 124.
[2] C.J. Pollock, J.F. Farrar, Source – sink relations: the role of sucrose, in: N.R. Baker (Ed.), Photosynthesis and the Environment, Kluwer, Netherlands, 1996, pp. 261 – 279. [3] T. Roitsch, Source – sink regulation by sugars and stress,
Curr. Opin. Plant Biol. 2 (1999) 198 – 206.
[4] K.E. Koch, Molecular crosstalk and the regulation of C-and N-responsive genes, in: C.H. Foyer, W.P. Quick (Eds.), A Molecular Approach to Primary Metabolism in Higher Plants, Taylor and Francis, London, 1997, pp. 105 – 124.
[5] S.C. Farrar, J.F. Farrar, Effects of photon fluence rate on carbon partitioning in barley source leaves, Plant Physiol. Biochem. 25 (1987) 541 – 548.
[6] R.J. Simpson, R.P. Walker, C.J. Pollock, Fructan exohy-drolase in leaves ofLolium temulentum L, New Phytol. 119 (1991) 499 – 507.
[7] O.A. Koroleva, J.F. Farrar, A.D. Tomos, C.J. Pollock, Patterns of solute in individual mesophyll, bundle sheath and epidermal cells of barley leaves induced to accumu-late carbohydrate, New Phytol. 136 (1997) 97 – 104. [8] O.A. Koroleva, J.F. Farrar, A.D. Tomos, C.J. Pollock,
Carbohydrates in individual cells of epidermis, mesophyll and bundle-sheath in barley leaves with changed export or photosynthetic rate, Plant Physiol. 118 (1998) 1525 – 1532.
[9] L.C. Ho, J.H.M. Thornley, Energy requirements for assimilate translocation from mature tomato leaves, Ann. Bot. 42 (1978) 481 – 483.
[10] S.C. Farrar, J.F. Farrar, Fluxes of carbon compounds in leaves and roots of barley plants, in: B. Jeffcoat, A.F. Hawkins, A.D. Stead (Eds.), Regulation of Sources and Sinks in Crop Plants, British Plant Growth Regulator Group, Bristol, 1985, pp. 67 – 84.
[11] M. Stitt, Metabolic regulation of photosynthesis, in: N.R. Baker (Ed.), Photosynthesis and the Environment, Kluwer, Dordrecht, 1996, pp. 151 – 190.
[12] S.A. Felitti, D.H. Gonsales, Carbohydrates modulate the expression of the sunflower cytochrome c gene at the mRNA level, Planta 206 (1998) 410 – 415.
[13] A.L. Winters, J. Gallagher, C. Pollock, J.F. Farrar, Isolation of a gene expressed during sucrose accumula-tion in leaves ofLolium temulentum L, J. Exp. Bot. 46 (1995) 1345 – 1350.
[14] J. Sheen, Metabolic repression of transcription in higher plants, Plant Cell 2 (1990) 1027 – 1038.
[15] A. von Schwaewen, M. Stitt, U. Sonnerwald, L. Willmitzer, Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photo-synthesis and strongly influences growth and phenotype of transgenic tobacco plants, EMBO J. 9 (1990) 3033 – 3044.
[16] M. Stitt, A. von Schwaewen, L. Willmitzer, Sink regula-tion of photosynthetic metabolism in transgenic tobacco plants expressing yeast invertase in the cell wall involves a down-regulation of the Calvin cycle and an up-regula-tion of glycolysis, Planta 183 (1990) 40 – 50.
[17] C. Schafer, H. Simper, B. Hofman, Glucose feeding results in co-ordinated changes of chlorophyll content, ribulose-1,5-bisphosphate carboxylase-oxygenase activity and photosynthetic potential in photoautotrophic sus-pension cultured cells ofChenopodium rubrum, Plant Cell Environ. 15 (1992) 343 – 350.
[18] A. Krapp, B. Hofman, C. Schafer, M. Stitt, Regulation of the expression ofrbcsand other photosynthetic genes by carbohydrates: a mechanism for the ‘sink regulation’ of photosynthesis?, Plant J. 3 (1993) 817 – 828.
[19] L. Natr, Time-course for photosynthesis and maximum figures for the accumulation of assimilates in barley leaf segments, Photosynthetica 1 (1967) 29 – 36.
[20] T.L. Housley, C.J. Pollock, Photosynthesis and carbohy-drate metabolism in detached leaves ofLolium temulen
[21] J.F. Farrar, S. Gunn, Effects of temperature and atmo-spheric carbon dioxide on source-sink relations in the context of climate change, in: E. Zamski, A.A. Schaffer (Eds.), Assimilate Distribution in Plants and Crops, Dekker, New York, 1996, pp. 389 – 406.
[22] R. Baxter, S.A. Bell, T.H. Sparks, T.W. Ashenden, J.F. Farrar, Effects of elevated CO2concentrations on three
montane plant species. III. Source leaf metabolism and whole-plant carbon partitioning, J. Exp. Bot. 46 (1995) 917 – 929.
[23] S. Plum, J.F. Farrar, Carbon partitioning in barley fol-lowing manipulaiton of source and sink, Aspects Appl. Biol. 45 (1996) 177 – 180.
[24] J.F. Farrar, Carbohydrate: where does it come from, where does it go?, in: J.A. Bryant, M.M. Burrell, N.J. Kruger (Eds.), Plant Carbohydrate Biochemistry, BIOS Scientific Publishers, Oxford, 1999, pp. 29 – 46.
[25] H. Harmens, C.M. Stirling, C. Marshall, J.F. Farrar, Elevated CO2and N: interactive effects on
photosynthe-sis ofDactylis glomerata, in: Y. De Kok, I. Stulen (Eds.), Responses of Plant Metabolism to Air Pollution and Global Change, Backhuys, Leiden, 1998, pp. 319 – 322. [26] H. Harmens, C.H. Stirling, C. Marshall, J.F. Farrar,
Does down-regulation of photosynthetic capacity by ele-vated CO2 depend on N supply in Dactylis glomerata?
Phys. Plant. (1999) in press.
[27] J.M. Hibberd, P. Richardson, R. Whitbread, J.F. Farrar, Effects of leaf age, basal meristem and infection with powdery mildew on photosynthesis in barley grown in 700 umol mol−1CO
2, New Phytol. 134 (1996) 317 – 325.
[28] B.D. Moore, S.H. Cheng, D. Sims, J.R. Seeman, The biochemical and molecular basis for photosynthetic accli-maiton to elevated atmospheric CO2, Plant Cell Environ.
22 (1999) 567 – 582.
[29] A.J. Cairns, R. Nash, M.-A. Machado de Carvalho, I.M. Sims, Characterisation of the enzymatic polymerisation of 2,6-linked fructan by leaf extracts from timothy grass (Phleum pratense), New Phytol. 142 (1999) 79 – 91. [30] N. Sprenger, K. Bortlik, A. Brandt, T. Boller, A.
Wlernken, Purification, cloning and functional expres-sion of sucrose fructan 6-transferase, a key enzyme of fructan synthesis in barley, Proc. Natl. Acad. Sci. USA 92 (1995) 11652 – 11656.
[31] K.E. Koch, Carbohydrate-modulated gene-expression in plants, Ann. Rev. Plant Physiol. Plant Mol. Biol. 47 (1996) 509 – 540.
[32] J.F. Farrar, The whole plant: carbon partitioning during development, in: C.J. Pollock, J.F. Farrar, A.J. Gordon (Eds.), Carbon Partitioning Within and Between Organ-isms, BIOS Scientific Publishers, Oxford, 1992, pp. 163 – 179.
[33] F. James, R. Brouquisse, A. Pradet, P. Raymond, Changes in proteolytic activity in glucose-starved maize root tips-regulation by sugars, Mant. Physiol. Biochem. 31 (1993) 845 – 856.
[34] M. Dieuaide-Noubhani, P. Canioni, P. Raymond, Sugar-starvation induced changesof carbon metabolism in ex-cised root tips of maize, Plant Physiol. 115 (1997) 1505 – 1513.
[35] J. Van’t Hof, Control points within the cell cycle, in: J.A. Bryant, D. Francis (Eds.), The Cell Division Cycle in
Plants, Cambridge University Press, Cambridge, 1985, pp. 1 – 13.
[36] J.L. Stoddart, H. Thomas, E.J. Lloyd, C.J. Pollock, The use of a temperature-profiled position transducer for the study of low-temperature growth in Gramineae, Planta 167 (1986) 359 – 363.
[37] C.J. Pollock, Sucrose accumulation and the initiation of fructanbiosynthesis inLolium temulentumL, New Phytol. 96 (1988) 527 – 534.
[38] P.E.H. Minchin, J.F. Farrar, M.R. Thorpe, Partitioning in split root systems of barley: effect of temperature of the root, J. Exp. Bot. 45 (1994) 1103 – 1109.
[39] D. Rentsch, W.B. Frommer, Molecular approaches to-wards an understanding of loading and unloading of assimilates in higher plants, J. Exp. Bot. 47 (1996) 1199 – 1204.
[40] T.-J. Chiou, D.R. Bush, Sucrose is a signalling molecule in assimilate partitioning, Proc. Natl. Acad. Sci. USA 95 (1988) 4784 – 4788.
[41] H. Brouwer, Functional equilibrium sense or nonsense?, Neth. J. Ag. Sci. 31 (1983) 335 – 348.
[42] T.W. Rufty, Probing the carbon and nitrogen interac-tion: a whole-plant perspective, in: C.H. Foyer, W.P. Quick (Eds.), A Molecular Approach to Primary Metabolism in Higher Plants, Taylor and Francis, Lon-don, 1997, pp. 255 – 273.
[43] J.F. Farrar, S. Gunn, Allocation: allometry, acclimation-and alchemy?, in: H. Larnbers, H. Poorter, M.M.I. Van Vuuren (Eds.), Inherent Variation in Plant Growth, Backhuys, Leiden, 1998, pp. 183 – 198.
[44] C. Macintosh, Regulation of C/N metabolism by re-versible protein phosphorylation, in: C.H. Foyer, W.P. Quick (Eds.), A Molecular Approach to Primary Metabolism in Higher Plants, Taylor and Francis, Lon-don, 1997, pp. 179 – 201.
[45] M. Stitt, A. Krapp, The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background, Plant Cell Environ. 22 (1999) 583 – 621.
[46] G.L. Cote, J.A. Ahlgren, Metabolism in microorganisms. Part 1. Levan and levansucrase, in: M. Suzuki, N.J. Chatterton (Eds.), Science and Technology of Fructans, CRC Press, Boca Raton, 1993, pp. 142 – 168.
[47] M. Johnston, Feasting, fasting and fermenting: glucose sensing in yeast and other cells, Trends Genetics 15 (1999) 29 – 33.
[48] A.M. Crutz, M. Steinmetz, S. Aymerich, R.R. Richter, D. Le Coq, Induction of levansucrase inBacillus subtilis; an antitermination mechanism negatively controlled by the phosphotransferase system, J. Bacteriol. 172 (1990) 1043 – 1050.
[49] J.C. Jang, J. Sheen, Sugar sensing in higher plants, Plant Cell 6 (1994) 1665 – 1679.
[50] A. Alderson, P.A. SabeDi, J.R. Dickinson, D. Cole, M. Richardson, M. Kreis, P.R. Shewry, N.G. Halford, Complementation of snf 1, a mutation affecting global regulation of carbon metabolism in yeast, by a plant protein kinase cDNA, Proc. Natl. Acad. Sci. USA 88 (1991) 8602 – 8605.
sen-sors of the eukaryotic cell?, Annu. Rev. Biochem. 67 (1998) 821 – 855.
[52] S. Smeekens, Sugar regulation of gene expression in plants, Curr. Opin. Plant Biol. 1 (1998) 230 – 234. [53] J.C. Jang, P. Leon, L. Zhou, J. Sheen, Hexokinase as a
sugar sensor in higher plants, Plant Cell 9 (1997) 5 – 19. [54] S. Lalonde, E. Boles, H. Hellmann, L. Barker, J.W. Patrick, W.B. Frommer, J.M. Ward, The dual function of sugar carriers: transport and sugar sensing, Plant Cell 4 (1999) 59 – 69.
[55] W. Wagner, A. Wiemken, P. Matile, Regulation of fruc-tan metabolism in leaves of barley (Hordeum6ulgareL.
cv Gerbel), Plant Physiol. 81 (1986) 444 – 447.
[56] J.F. Farrar, Fluxes and turnover of sucrose and fructans in healthy and diseased plants, J. Plant Physiol. 134 (1989) 137 – 140.
[57] A. Trewavas, How do plant growth substances work? 2. Opinion, Plant Cell Environ. 14 (1991) 1 – 12.
[58] P.B. Kaufman, N.S. Ghosheh, D. LaCroix, S.L. Soni, H. Ikoma, Regulation of invertase levels in A6ena stem
segments by GA, sucrose, glucose and fructose, Plant Physiol. 52 (1973) 221 – 228.
[59] P. Perata, C. Matsukura, P. Vernieri, J. Yamaguchi, Sugar repression of a gibberellin-dependent signalling pathway in barley embryos, Plant Cell 9 (1997) 2197 – 2208.
[60] R.H. Wetmore, J.P. Rier, Experimental induction of vascular tissue in callus of angiosperms, Am. J. Bot. 50 (1963) 418 – 430.
[61] R.A. Jeffs, D. Northcote, The influence of indol-3-yl acetic acid and sugar on the pattern of induced differentiation in plant tissue culture, J. Cell. Sci. 2 (1967) 77 – 88. [62] D. Fell, Understanding the Control of Metabolism,
Port-land Press, PortPort-land, 1997.
[63] C.J. Pollock, A.J. Cairns, J. Gallagher, M.-A. Machado deCarvalho, A. Kochhar, The metabolism of sugars based upon sucrose, in: J.A. Bryant, M.M. Burrell, N.J. Kruger (Eds.), Plant Carbohydrate Biochemistry, BIOS Scientific Publishers, Oxford, 1999, pp. 47 – 60.
[64] C.J. Pollock, Resource use efficiency and crop perfor-mance: what is the link?, in: C.H. Foyer, W.P. Ouick (Eds.), A Molecular Approach to Primary Metabolism in Higher Plants, Taylor and Francis, London, 1997, pp. 327 – 336.
[65] A.J. Cairns, C.J. Pollock, Fructan biosynthesis in excised leaves ofLolium tenulentumL. I Chromatographic cgarac-terisation of oligofructans and their labelling patterns following14CO
2feeding, New Phytol. 109 (1988) 399 – 405.
[66] A.J. Cairns, C.J. Pollock, Fructan biosynthesis in excised leaves of Lolium tenulentum L. II Changes in fructosyl transferase activity following excision and application of inhibitors of gene expression, New Phytol. 109 (1988) 407 – 413.