www.elsevier.com / locate / bres
Short communication
Stimulation of prolactin secretion by chronic, but not acute,
administration of leptin in the rat
a ,
*
b a¨
Hajime Watanobe
, Helgi B. Schioth , Toshihiro Suda
a
Third Department of Internal Medicine, Hirosaki University School of Medicine, 5 Zaifu-cho, Hirosaki, Aomori 036-8562, Japan
b
Department of Neuroscience, Uppsala University, BMC, Box 593, SE 751 24 Uppsala, and Melacure Therapeutics, Uppsala, Sweden
Accepted 12 September 2000
Abstract
Leptin, the product of obese (ob) gene, has been reported to affect the secretion of all six anterior pituitary hormones, but data are especially scarce regarding the interplay between leptin and prolactin (PRL). Thus, in this study we examined and compared in vivo the effects of acute and chronic administrations of recombinant mouse leptin on PRL secretion in male rats. Normally-fed and 3-day-fasted rats received an intraperitoneal bolus injection of leptin [1.0 mg / kg body weight (BW)] or vehicle only. The leptin treatment was without effect on plasma PRL levels up to 5 h postadministration. Food deprivation for 3 days significantly decreased both PRL and leptin levels. This decrease in plasma PRL was prevented by a 3-day constant infusion of 75mg / kg BW/ day of leptin, which maintained plasma leptin levels similar to those of normally-fed rats. The administration of three times the higher dose of leptin (225mg / kg BW/ day) to fasted rats led to further increases in both PRL and leptin in the plasma. Thus, a dose-dependent stimulatory effect of chronic leptin treatment on PRL secretion was indicated. This study demonstrates that chronic, but not acute, administration of leptin stimulates PRL secretion in the rat. 2000 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Neuroendocrine regulation: other
Keywords: Leptin; Prolactin; Acute hyperleptinemia; Chronic hyperleptinemia
The obese gene product, leptin, is a hormone produced which does not produce leptin. There exist a few studies by adipocytes which plays a key role in the regulation of which reported a direct stimulatory effect of leptin or its food intake and energy expenditure [4,13,23,40]. Besides fragment of PRL secretion in vivo [10] and in vitro [39], its well known role in body weight homeostasis, leptin has but conflicting in vivo [14] and in vitro [33] data have also recently been shown to play a significant role in the been reported. We have also found that an intracerebroven-regulation of neuroendocrine function. Increasing evidence tricular (icv) administration of anti-leptin antisera sig-suggests that leptin may affect the secretion of all six nificantly delayed the onset of preovulatory PRL surge, anterior pituitary hormones. Of these, gonadotropins and and icv and systemic treatments of leptin to fasted rats growth hormone have thus far been studied in more detail resumed the hormonal surge to normality [20,35,37]. than the other pituitary hormones from the viewpoint of In spite of these preexisting studies, it still remains to be their interaction with leptin (for review, see Ref. [5]). elucidated whether leptin can acutely affect PRL secretion On the other hand, regarding the interplay between in vivo, and also whether acute and chronic administra-leptin and prolactin (PRL), relevant data are especially tions of leptin exert differential effects on PRL release. scarce. A stimulatory effect of leptin on PRL secretion Thus, in the present study we examined and compared the may be indirectly suggested from the evidence that leptin effects of acute and chronic administrations of leptin to partially restores lactation in the ob / ob mouse [6], a strain freely-moving male rats in order to better understand a possible physiological role of the adipocyte-derived hor-mone in regulating PRL secretion in the rat.
*Corresponding author. Fax:181-172-32-6846.
E-mail address: [email protected] (H. Watanobe). Animals: Adult male rats of the Wistar Strain [body
weight (BW), 240–250 g] were used. They were housed at chosen in order to achieve a supraphysiological concen-constant room temperature and humidity under a 12-h tration of plasma leptin. For the purpose of determining the light–dark cycle (light on at 08:00 h and off at 20:00 h). temporal change in plasma leptin levels in the four groups Food and water were available ad libitum, unless otherwise after implanting the minipump, tail blood (300 ml) was indicated. collected for leptin assay immediately before and 12, 24,
Acute leptin treatment: For this experiment, two groups and 48 h after the implantation. Seventy-two hours after were prepared, i.e. normally-fed and 3-day-fasted rats. The the minipump implantation, animals were sacrificed by latter group had started fasting 72 h before the injection of rapid decapitation, and trunk blood was collected to leptin as described below. Two days prior to the experi- measure both leptin and PRL. All these blood samples ment, all animals were implanted with a jugular vein were collected into tubes containing EDTA-2Na (2.5 mg / catheter filled with heparin solution under light ether ml blood), and their subsequent treatment was done in the anesthesia. At about 08:00 h on the day of the experiment, same manner as described above.
an extension tube for blood sampling was connected to the Assays: PRL levels in plasma were measured by RIA
jugular vein catheter. After leaving the animals undis- using the reagents kindly donated by Dr. A.F. Parlow turbed for about 4 h, the experiment was started at (NIDDK). Rat PRL-RP-3 was used as the standard, and approximately 12:00 h. Throughout the experimental the sensitivity of the assay was 0.8 ng / ml. Plasma leptin period, they were kept under a conscious, freely-moving levels were determined by the rat leptin RIA kit produced condition without apparent stress. Recombinant mouse by Linco Research (St. Louis, MO). In this kit, rm leptin (rm) leptin (1.0 mg / kg BW) dissolved in vehicle [0.01 M crossreacts with the rat counterpart at 100%. It was phosphate-buffered saline (PBS)], or the vehicle only, was reported by the manufacturer that the sensitivity of the rat administered intraperitoneally (ip) as a bolus injection. leptin RIA system is 0.5 ng / ml. However, we found in our Two separate groups from both normally-fed and 3-day- preliminary study that the use as the standard of the fasted rats were given rm leptin or vehicle, respectively. recombinant rat leptin produced by R&D Systems, Inc. Blood samples (400 ml) were collected 30 min before, (Minneapolis, MN, USA) yields a better sensitivity of the immediately before, and thereafter at 30-min intervals up assay. Thus, in our leptin assay we used as the standard the to 300 min after the injection. To prevent anemia and also R&D Systems’ rat leptin in place of a standard preparation the loss of circulating plasma volume, red blood cells were supplied in the kit. Sensitivity of leptin in our modified resuspended in 0.9% NaCl and returned to the animals assay system was 0.3 ng / ml. For both PRL and leptin, after each blood collection. The blood was immediately samples from individual rats were analyzed within the placed in EDTA-2Na (2.5 mg / ml blood)-containing tubes, same assay. Both the intra- and interassay coefficients of centrifuged, and the plasma was kept frozen at2208C until variation were less than 10% in the two assays.
assayed for PRL and leptin. Statistical analyses: Results were expressed as the Chronic leptin treatment: For this experiment, four mean6S.E.M. One-way or two-way ANOVA followed by groups were prepared, i.e. normally-fed, fasted (3 days), Scheffe’s post-hoc test was used to analyze the data, as fasted1rm leptin (75 mg / kg BW/ day), and fasted1rm appropriate. Differences were considered significant if P leptin (225mg / kg BW/ day) rats. The administration of rm was smaller than 0.05.
leptin was performed utilizing Alzet osmotic minipumps Fig. 1 shows the effects of ip administration of rm leptin (model 2001) which can deliver 24ml / day for up to 7 days (1.0 mg / kg BW) or vehicle only on plasma leptin and (Alzet, Palo Alto, CA). The minipumps were filled with PRL levels in normally-fed and 3-day-fasted rats. Vehicle 0.01 M PBS (vehicle) containing rm leptin which was alone was without effect on plasma leptin concentrations in adjusted for a daily delivery of 75 or 225 mg / kg BW. both normally-fed and fasted groups during the entire Normally-fed and fasted groups were treated with the same period of observation (normally-fed group, 1.360.2|
type of minipump which was loaded with the vehicle only. 1.560.3 ng / ml; fasted group, 0.360.1|0.460.1 ng / ml).
The minipump, one for each animal, was implanted As expected, ip administration of rm leptin produced a subcutaneously in the back under light ether anesthesia 72 marked elevation of plasma leptin in both normally-fed h before sacrifice. The dosage of 75 mg / kg BW/ day of rm and fasted groups. In both groups, plasma leptin reached leptin was chosen based on our previous report that 100 its peak 30 min postadministration (normally-fed group,
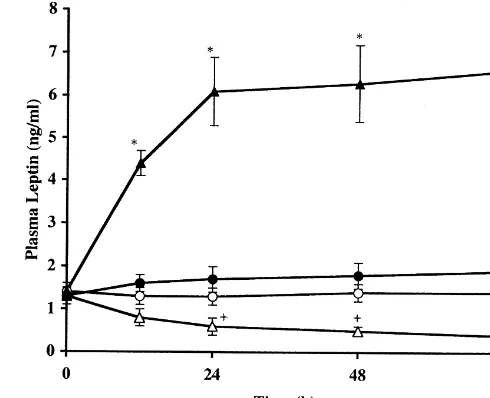
Fig. 2. Temporal profile of plasma leptin in normally-fed, fasted, and leptin-supplemented fasted male rats up to 72 h after commencement of the respective treatments. The number of rats examined was 7–9 per group.s—s, normally fed1PBS;n—n, fasted1PBS;d—d, fasted1 leptin (75mg / kg BW/ day);m—m, fasted1leptin (225mg / kg BW/ day).
w, P,0.01 vs. the remaining three groups. 1, P,0.05 vs. normally fed1PBS and fasted1leptin (75 mg / kg BW/ day) groups. For further details, see text.
normally fed1PBS group at the timepoints of 24, 48, and 72 h. The supplementation of 75 mg / kg BW/ day of rm leptin to fasted rats restored plasma leptin to such levels which were slightly higher than but statistically indis-tinguishable from those in normally-fed1PBS group. The administration of three times the higher dose of rm leptin (225 mg / kg BW/ day) to fasted rats produced about three times (P,0.01) the normal level of plasma leptin as early as 12 h postadministration and a consistently higher level (about 4.5 times the normal, P,0.01) from 24 h onward.
Fig. 1. Temporal profiles of plasma leptin and PRL after ip
administra-Fig. 3 shows plasma levels of leptin and PRL in
tion of rm leptin (1.0 mg / kg BW) or vehicle only in normally-fed and
3-day-fasted male rats. The number of rats examined was 7–8 per group. normally-fed, fasted, and leptin-supplemented fasted
s—s, normally fed (vehicle); d—d, normally-fed (leptin); n—n,
groups determined 72 h after commencing the respective
fasted (vehicle);m—m, fasted (leptin). In this and the following figures,
treatments. Both leptin and PRL levels in the plasma were
where standard errors are not shown, they were smaller than the symbols.
significantly (P,0.05) lower in fasted1PBS group than in
For further details, see text.
normally fed1PBS group, but these fasting-induced changes were completely reverted to normal by supple-leptin. Plasma PRL levels in both the vehicle- and leptin- menting 75mg / kg BW/ day of rm leptin. The administra-treated normally-fed groups were significantly (P,0.05) tion of three times the higher dose of rm leptin (225mg / kg higher than those in the two fasted groups between 230 BW/ day) to fasted rats led to further increases in both and 300 min. leptin and PRL concentrations, which exceeded the levels Fig. 2 shows the temporal profile of plasma leptin levels of normally fed1PBS group by 4.3 and 2.3 times (P,0.05 in normally fed, fasted, and leptin-supplemented fasted for both), respectively.
27
Tena-Sempere et al. [33] reported that 10 M of leptin did not modulate PRL release from incubated anterior pituitaries of fasted male rats within 2 h. It was recently reported by Gonzalez et al. [10] that an icv administration of mouse leptin-(116–130), an active fragment of the native molecule [11], caused a significant rise in serum PRL levels in food-deprived male rats. However, we feel that this study of Gonzalez et al. [10] contains two problems which may preclude us from accepting it as the first undisputed demonstration for leptin’s PRL-releasing activity in vivo. Firstly, the authors used a fragment of mouse leptin instead of the mature protein. Although leptin-(116–130) has been shown to act in a similar manner to the native protein on both food intake and body weight in ob / ob mice [11], it was also reported that the expression of the normal biological activity of leptin requires amino acid residues proximal to position 106 and also sites between residues 106–140 [24]. Moreover, it was recently reported that the effect of leptin-(116–130) on body weight homeostasis is not mediated by its binding to the long form of the leptin receptor, the receptor isoform which is predominantly expressed in the hypothalamus [12]. Thus, the data derived from using the leptin fragment may not necessarily represent all the biological actions of the mature leptin molecule. Secondly, as the authors themselves also stated, the icv dose (15 mg) of leptin-(116–130) which they administered appears to be fairly large, even pharmacological. In our previous studies in which the native leptin molecule was given into the rat cerebroventricle, the dose we chose was 0.15–0.3 nmol on a molar basis [20,35]. With this dose, we observed normalization or a fairly good recovery of prevulatory luteinizing hormone and PRL surges in fasted female rats [20,35]. In the study of Gonzalez et al. [10], they adminis-tered icv 9.61 nmol of the leptin fragment, which, on a
Fig. 3. Plasma levels of leptin and PRL in normally-fed, fasted, and
molar basis, is 32–64 times larger than the dose (0.15|0.3 leptin-supplemented fasted male rats determined 72 h after
commence-nmol) which we have previously given via the same route
ment of the respective treatments. The number of rats examined was 7–9
[20,35]. Thus, the data obtained from using this
pharmaco-per group. w, P,0.05 vs. the remaining three groups.1, P,0.05 vs.
normally fed (NF)1PBS and fasted1leptin (75mg / kg BW/ day) groups. logical dose of leptin fragment must await further evalua-For further details, see text. tion for their physiological meaning. At any rate, our own
data in the present study do not favor a PRL-releasing results were that the acute administration of leptin was action of acute hyperleptinemia.
without effect on PRL secretion in both normally-fed and In the second part of this study, we investigated whether fasted rats, despite the fact that plasma leptin levels chronic leptin treatment affects PRL secretion. The ob-attained 30–60 min after injecting the rm leptin (1.0 servation that fasting for 3 days significantly lowered mg / kg BW) were about 1,000 times higher than the plasma PRL levels, is in agreement with the preexisting concentration in normally-fed male rats. These results literature [2,3,7,30,38]. Moreover, as a novel finding we strongly suggest that PRL secretion may not be modified found that a 3-day constant infusion of leptin to fasted rats by acute hyperleptinemia in the rat. at such a dose (75 mg / kg BW/ day) which maintained It was reported by Yu et al. [39] that leptin was able to plasma leptin levels similar to those of normally fed rats, significantly stimulate PRL release in vitro from the reverted plasma PRL levels to normal. Furthermore, the anterior pituitary of male rats after a 3-h incubation. 3-day infusion of three times the larger dose (225mg / kg However, this effect was observed only at extremely high BW/ day) of leptin led to a further elevation of plasma
27 25
concentrations of leptin such as 10 –10 M, which are PRL as well as leptin. These results demonstrate a dose-3 5
10 –10 times higher than the circulating level of leptin in dependent stimulation of PRL secretion by chronic leptin 210
The elevation of PRL levels by chronic leptin infusion by rendering these cells much more responsive to physio-implies that hyperleptinemic subjects may have hyper- logically relevant PRL secretagogues [16,22]. This concept prolactinemia. It has been reported that obesity per se is may not disagree with our present data that only the not usually associated with increased PRL concentrations chronic, but not acute, administration of leptin was able to [25]. However, in a large population study, a weak, but stimulate PRL secretion. We speculate so, because the significant, correlation was revealed between serum PRL mammotrope may need to be exposed to a-MSH for a levels and body weight [34]. In addition, it was also sustained, but not a brief, period of time in order for reported that regularly menstruating women who attained a a-MSH to manifest its mammotrope-priming action. sizable amount of weight gain over a short period of time, In summary, in this study we demonstrated that chronic, had significantly higher levels of serum PRL than the but not acute, administration of leptin stimulates PRL controls [8]. Thus, it is possible that hyperleptinemia, secretion in male rats. PRL seems to be another pituitary which accompanies obesity and weight gain, leads to hormone which is regulated by the adipocyte-derived hyperprolactinemia also in humans. hormone.
An important question which reasonably emerges from the present data is the neuroendocrine mechanism whereby
leptin stimulates PRL secretion. As already discussed Acknowledgements above, it is unlikely that leptin directly stimulates PRL
release from the pituitary at least at the concentrations We wish to thank the National Hormone and Pituitary which we produced in the plasma by constant leptin Program of NIDDK and Dr. A.F. Parlow for the generous infusion. Thus, a probable site where leptin may primarily donation of leptin and reagents for rat PRL RIA. We are act to cause a series of events which eventually culminate also grateful to Dr. H. Kodama (Exploratory Research in stimulated PRL release, is the hypothalamus. A decrease Laboratories, Fujisawa Pharmaceutical Co. Ltd., Tsukuba, in the secretion of PRL-releasing factors, e.g. dopamine Ibaraki, Japan) for the measurement of leptin.
[1], or an increased release of PRL-releasing factors, such as thyrotropin-releasing hormone and vasoactive intestinal peptide [1], may play an intermediary role. However,
References because of the following reasons, we speculate it more
likely that several peptides which originate in the
hypo-[1] N. Ben-Jonathan, L.A. Arbogast, J.F. Hyde, Neuroendocrine
regula-thalamic arcuate nucleus [18] may play a primary role in tion of prolactin release, Prog. Neurobiol. 33 (1989) 399–447. causing the leptin-stimulated PRL secretion. It is well [2] M. Bergendahl, A. Perheentupa, I. Huhtainemi, Starvation-induced
suppression of pituitary-testicular function in rats is reversed by
established that the arcuate nucleus is such a site in the
pulsatile gonadotropin-releasing hormone substitution, Biol. Reprod.
hypothalamus that shows the most abundant expression of
44 (1991) 413–419.
the leptin receptor [21,26]. The arcuate nucleus contains a
[3] C.A. Campbell, M. Kurcz, S. Marshall, J. Meites, Effects of
high density of neurons that produce neuropeptide Y starvation in rats on serum levels of follicle stimulating hormone, (NPY), a-melanocyte stimulating hormone (a-MSH), b- luteinizing hormone, thyrotropin, growth hormone and prolactin;
endorphin (b-END), agouti-related protein, and several response to LH-releasing hormone and thyrotropin-releasing hor-mone, Endocrinology 100 (1977) 580–587.
other appetite-regulating factors [18]. Both a-MSH and
[4] L.A. Campfield, F.J. Smith, Y. Guisez, R. Devos, P. Burn,
Recombi-b-END are the products of the proopiomelanocortin
nant mouse OB protein: evidence for a peripheral signal linking
(POMC) gene [31]. It has been reported that the expres- adiposity and central neural networks, Science 269 (1995) 546–549. sion of the NPY and POMC genes in the arcuate nucleus is [5] F.F. Casanueva, C. Dieguez, Neuroendocrine regulation and actions
inhibited [27,32] or stimulated [28] by leptin, respectively. of leptin, Front. Neuroendocrinol. 20 (1999) 317–363.
[6] F.F. Chehab, M.E. Lim, R. Lu, Correction of the sterility defect in
Although NPY does not seem to play a significant role in
homozygous obese female mice by treatment with the human
the physiological regulation of PRL secretion [17], both
recombinant leptin, Nat. Genet. 12 (1996) 318–320.
a-MSH [15,16,22] and b-END [19,29] are considered as [7] R.G. Dyer, S. Mansfield, H. Corbet, A.D.P. Dean, Fasting impairs exerting an excitatory input on PRL release. Thus, it is LH secretion in female rats by activating an inhibitory opioid
conceivable that leptin’s stimulatory action on PRL secre- pathway, J. Endocrinol. 105 (1985) 91–97.
[8] M.F. Ferreira, L.G. Sobrinho, J.S. Pires, M.E.S. Silva, M.A. Santos,
tion is mediated by these POMC gene products. We
M.F.F. Sousa, Endocrine and psychologiocrine and psychological
recently obtained data suggesting that of the two
POMC-evaluation of women with recent weight gain,
Psychoneuroen-derived peptides, onlya-MSH, but notb-END, may play a docrinology 20 (1995) 53–63.
role in mediating the leptin stimulation of PRL secretion in [9] R.C. Frederich, A. Hamann, S. Anderson, B. Lollmann, B.B.
the rat. We found that repeated icv administrations of Lowell, J.S. Flier, Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action, Nat. Med. 1
neutralizing antibody againsta-MSH, but not that against
(1995) 1311–1314.
b-END, significantly lowered plasma PRL levels which
[10] L.C. Gonzalez, L. Pinilla, M. Tena-Sempere, E. Aguilar, Leptin
were elevated by chronic leptin infusion (H. Watanobe and (116–130) stimulates prolactin and luteinizing hormone secretion in ¨
H.B. Schioth, unpublished observations). Recent studies fasted adult male rats, Neuroendocrinology 70 (1999) 213–220.
leptin-related synthetic peptides on body weight and food intake in Licata, A. Notarbartolo, Thyroid function and release of thyroid-female ob / ob mice: localization of leptin activity to domains stimulating hormone and prolactin from the pituitary in obesity, J. between amino acid residues 106–140, Endocrinology 138 (1997) Int. Med. Res. 19 (1991) 389–394.
1413–1418. [26] M.W. Schwartz, R.J. Seeley, L.A. Campfield, P. Burn, D.G. Baskin, [12] P. Grasso, D.W. White, L.A. Tartaglia, M.C. Leinung, D.W. Lee, Identification of targets of leptin action in rat hypothalamus, J. Clin.
Inhibitory effects of leptin-related synthetic peptide 116-130 on food Invest. 98 (1996) 1101–1106.
intake and body weight gain in female C57BL / 6J ob / ob mice may [27] M.W. Schwartz, D.G. Baskin, T.R. Bukowski, J.L. Kuijper, D. not be mediated by peptide activation of the long isoform of the Foster, G. Lasser, D.E. Prunkard, D. Porte, S.C. Woods, R.J. Seeley, leptin receptor, Diabetes 48 (1999) 2204–2209. D.S. Weigle, Specificity of leptin action on elevated blood glucose [13] J.L. Halaas, K.S. Gajiwala, M. Maffei, S.L. Cohen, B.T. Chait, D. levels and hypothalamic neuropeptide Y gene expression in ob / ob
Rabinowitz, R.L. Lallone, S.K. Burley, J.M. Friedman, Weight- mice, Diabetes 45 (1996) 531–535.
reducing effects of the plasma protein encoded by the obese gene, [28] M.W. Schwartz, R.J. Seeley, S.C. Woods, D.S. Weigle, L.A. Cam-Science 269 (1995) 543–546. pfield, P. Burn, D.G. Baskin, Leptin increases hypothalamic pro-[14] B.A. Henry, J.W. Goding, W.S. Alexander, A.J. Tilbrook, B.J. opiomelanocortin mRNA expression in the rostral arcuate nucleus,
Canny, F. Dunshea, A. Rao, A. Mansell, I.J. Clarke, Central Diabetes 46 (1997) 2119–2123.
administration of leptin to ovariectomized ewes inhibits food intake [29] M. Selmanoff, K.A. Gregerson, Suckling-induced prolactin release without affecting the secretion of hormones from the pituitary gland: is suppressed by naloxone and stimulated by beta-endorphin, evidence for a dissociation of effects on appetite and neuroendocrine Neuroendocrinology 42 (1986) 255–259.
function, Endocrinology 140 (1999) 1175–1182. [30] A.M. Sirek, E. Horvath, C. Ezrin, K. Kovacs, Effect of starvation on ¨
[15] J.B. Hill, E.R. Lacy, G.M. Nagy, T.J. Gorcs, L.S. Frawley, Does pituitary growth hormone cells and blood growth hormone and alpha-melanocyte-stimulating hormone from the pars intermedia prolactin levels in the rat, Nutr. Metab. 20 (1976) 67–75. regulate suckling-induced prolactin release? Supportive evidence [31] A.I. Smith, J.W. Funder, Proopiomelanocortin processing in the from morphological and functional studies, Endocrinology 133 pituitary, central nervous system, and peripheral tissues, Endocr.
(1993) 2991–2997. Rev. 9 (1988) 159–179.
[16] J.B. Hill, G.M. Nagy, L.S. Frawley, Suckling unmasks stimulatory [32] T.W. Stephens, M. Basinski, P.K. Bristow, J.M. Bue-Vallesky, S.G. effect of dopamine on prolactin release: possible role for a- Burgett, L. Craft, J. Hale, J. Hoffmann, H.M. Hsiung, A. melanocyte-stimulating hormone as a mammotrope responsiveness Kriauciunas, M. Mackellar, P.R. Rosteck, B. Schnener, D. Smith, factor, Endocrinology 129 (1991) 843–847. F.C. Tinsley, X.-Y. Zhang, M. Heiman, The role of neuropeptide Y [17] S.P. Kalra, W.R. Crowley, Neuropeptide Y: a novel neuroendocrine in the antiobesity action of the obese gene product, Nature 377
peptide in the control of pituitary hormone secretion, and its relation (1995) 530–532.
´ ´
to lutenizing hormone, Front. Neuroendocrinol. 13 (1992) 1–46. [33] M. Tena-Sempere, L. Pinilla, L.C. Gonzalez, C. Dieguez, F.F. [18] S.P. Kalra, M.G. Dube, S. Pu, B. Xu, T.L. Horvath, P.S. Kalra, Casanueva, E. Aguilar, Leptin inhibits testosterone secretion from
Interacting appetite-regulating pathways in the hypothalamic regula- adult rat testis in vitro, J. Endocrinol. 161 (1999) 211–218. tion of body weight, Endocr. Rev. 20 (1999) 68–100. [34] D.Y. Wang, B.L. De Stavola, R.D. Bulbrook, D.S. Allen, H.G. Kwa, [19] L. Kehoe, R. Parman, J. Janik, P. Callahan, Opiate receptor subtype A.A. Verstraeten, J.W. Moore, I.S. Fentiman, M. Chaudary, J.L. involvement in the stimulation of prolactin release by beta-en- Hayward, I.H. Gravelle, The relationship between blood prolactin dorphin in female rats, Neuroendocrinology 57 (1993) 875–883. levels and risk of breast cancer in premonopausal women, Eur. J. [20] A. Kohsaka, H. Watanobe, Y. Kakizaki, S. Habu, T. Suda, A Cancer Clin. Oncol. 23 (1987) 1541–1548.
¨
significant role of leptin in the generation of steroid-induced [35] H. Watanobe, H.B. Schioth, J.E.S. Wikberg, T. Suda, The luteinizing hormone and prolactin surges in female rats, Biochem. melanocortin 4 receptor mediates leptin stimulation of luteinizing Biophys. Res. Commun. 254 (1999) 578–581. hormone and prolactin surges in steroid-primed ovariectomozed rats, [21] J.G. Mercer, N. Hoggard, L.M. Williams, C.B. Lawrence, L.T. Biochem. Biophys. Res. Commun. 257 (1999) 860–864.
Hannah, P. Trayhurn, Localization of leptin receptor mRNA and the [36] H. Watanobe, T. Suda, A detailed study on the role of sex steroid long form splice variant (Ob-Rb) in mouse hypothlamus and milieu in determinig plasma leptin concentrations in adult male and adjacent brain regions by in situ hybridization, FEBS Lett. 387 female rats, Biochem. Biophys. Res. Commun. 259 (1999) 56–59.
¨
(1996) 113–116. [37] H. Watanobe, T. Suda, J.E.S. Wikberg, H.B. Schioth, Evidence that [22] L. Nunez, L.S. Frawley, Alpha-MSH potentiates the responsiveness physiological levels of circulating leptin exert a stimulatory effect
21
of mammotropes by increasing Ca entry, Am. J. Physiol. 274 on luteinizing hormone and prolactin surges in rats, Biochem.
(1998) E971–977. Biophys. Res. Commun. 263 (1999) 162–165.
[23] M.A. Pelleymounter, M.J. Cullen, M.B. Baker, R. Hecht, D. [38] Q.W. Xie, Experimental studies on changes of neuroendocrine Winters, T. Boone, F. Collins, Effects of the obese gene product on functions during starvation and refeeding, Neuroendocrinology 53 body weight regulation in ob / ob mice, Science 269 (1995) 540– (Suppl. 1) (1991) 52–59.
543. [39] W.H. Yu, M. Kimura, A. Walczewska, S. Karanth, S.M. McCann,
[24] W.K. Samson, T.C. Murphy, D. Robinson, T. Vargas, E. Tau, J.-W. Role of leptin in hypothalamic-pituitary function, Proc. Natl. Acad. Chang, A 35 amino acid fragment of leptin inhibits feeding in the Sci. USA 94 (1997) 1023–1028.
rat, Endocrinology 137 (1996) 5182–5185. [40] Y. Zhang, R. Proenca, M. Maffei, M. Barone, L. Leopold, J.M. [25] R. Scaglione, M.R. Averna, M.A. Dichiara, C.M. Barbagallo, G. Friedman, Positional cloning of the mouse obese gene and its human