www.elsevier.com / locate / bres
Research report
Rescue of ischemic brain injury by adenoviral gene transfer of glial
cell line-derived neurotrophic factor after transient global ischemia in
gerbils
a,e ,
*
a b c dTakashi Yagi
, Ikuyo Jikihara , Masayuki Fukumura , Kazuhiko Watabe , Toya Ohashi ,
d e a
Yoshikatsu Eto , Mitsuhiro Hara , Mitsuyo Maeda
a
First Department of Anatomy, Osaka City University Medical School, 1-4-3 Asahi-machi, Abeno-ku, Osaka 545-8585, Japan
b
DNAVEC Research Inc., Tsukuba, Japan
c
Department of Molecular Neuropathology, Tokyo Metropolitan Institute for Neuroscience, Tokyo, Japan
d
Department of Pediatrics and Institute of DNA Medicine, Jikei University School of Medicine, Tokyo, Japan
e
Department of Neurosurgery, Osaka City University Medical School, Osaka, Japan Accepted 12 September 2000
Abstract
Glial cell line-derived neurotrophic factor (GDNF), a member of the transforming growth factor (TGF)–bsuperfamily, is one of the most potent neurotrophic factors and promotes survival of many populations of cells. We examined neuroprotective effect of an adenoviral vector encoding glial cell line-derived neurotrophic factor (AxCAhGDNF) on the transient global ischemia. Gerbils received administration of AxCAhGDNF or an adenoviral vector encoding bacterial b-galactosidase gene (AxCALacZ) through the lateral ventricle. Two days later, occluding bilateral common carotid arteries for 5 min using aneurysm clips produced the transient global forebrain ischemia. Animals showed intense immunolabeling for GDNF in ependymal cells on 2, 4 and 7 days after the operation. The exogenous gene transducted by adenovirus in the same cells was detected by in situ hybridization. The treatment with AxCAhGDNF significantly prevented the loss of hippocampal CA1 pyramidal neurons 2 to 7 days after the operation, as compared to AxCALacZ treatment. Also terminal deoxynucleotidyl transferase-mediated dUTP-biotin in situ nick end labeling (TUNEL) staining was markedly reduced in the case with AxCAhGDNF treatment at 7 days after the operation. These results indicated that the adenovirus-mediated gene transfer of GDNF might prevent the delayed neuronal death of stroke and other disorders of the cerebral vasculature. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Ischemia
Keywords: GDNF; Adenoviral vector; Delayed neuronal death; Transient global ischemia
1. Introduction
ability to infect nerve cells, which are not its natural target,
and that the gene transfer and expression of Ad are highly
Numerous studies in recent years have reported that the
efficient and maintain long-term period both in vitro and in
adenovirus (Ad) expression vector is useful for application
vivo. In addition, its genome can accommodate foreign
to gene therapy [7,11–13,17,29,31,33,44,61]. The advan-
genes of up to 7.5 kb and high titers of the virus can be
tage for its use is that Ad vector is the replication-deficient
obtained easily [23].
recombinant adenovirus, which has low pathogenicity, due
Glial cell line-derived neurotrophic factor (GDNF), a
to lacking the E1A, E1B and E3 regions [40]. It has the
member of the transforming growth factor (TGF)–
b
superfamily [35], is one of the most potent neurotrophic
factors and promotes survival of many populations of cells,
*Corresponding author. Tel.: 181-6-6645-3701; fax: 1
81-6-6645-including brain tissues under the ischemic condition
3702.
E-mail address: [email protected] (T. Yagi).
[1,8,25,26,60], peripheral sensory neurons [46],
pal pyramidal neurons induced by kainic acid-mediated
used. The animals were maintained at constant temperature
seizures
[39],
spinal
motor
neurons
[4,5,17–
and 12:12 h light:dark cycle and allowed free access to
19,34,45,61,64,66], mesencephalic dopaminergic neurons
food and water until the investigations. The animals were
[6,7,10,12–14,22,29,31,38,50,53,54], and axotomized reti-
anesthetized with intraperitoneal injection of chloral
hy-nal ganglion cells [27,28]. GDNF is a target-derived
drate (300 mg / kg) and positioned in a stereotaxy frame.
neurotrophic factor [18,53] with a molecular weight of 15
These animals received a unilateral injection of either
10
kDa, forming a naturally occurring dimer and inducing
AxCAhGDNF
(5
m
l,
1
3
10
pfu / ml,
n
5
18)
or
10
glycosylation
[32,35].
GDNF
signaling
is
mediated
AxCALacZ (5
m
l, 1
3
10
pfu / ml, n
5
14) over a 6 min
through a two-component system consisting of the Ret
period into the left lateral ventricle using a 33-gauge 10
m
l
tyrosine kinase and a glycosyl-phosphatidyl-inositol-linked
Hamilton syringe. The Hamilton syringe was left in place
protein termed GFR
a
-1 or GFR
a
-2, which complexes with
for 3 min before removal. Injection coordinates relative to
GDNF and binds to and activates the tyrosine kinase
bregma were 1.5 mm posteriorly and 2.0 mm laterally to
receptor Ret [3,16,21,49,55,56]. In previous investigations,
the left side at a depth of 1.2 mm from cortical surface.
the Ret phosphorylation in response to GDNF results in
After the injection, the animals were again allowed free
activation of the mitogen-activated protein kinase (MAPK)
access to food and water. Two days later, injected animals
through the Ras-GTP activation [43,63] and of phospha-
were reanesthetized with intraperitoneal injection of
chlor-tidylinositol (PI)-3 kinase indirectly or directly, through
al hydrate (300 mg / kg). The bilateral CCA were exposed
the Ras-GTP activation or not [15,47,57,65]. It has been
through the midline skin incision in the neck and occluded
demonstrated in vitro that, PI-3 kinase activation can
for 5 min using aneurysm clips to produce transient
prevent apoptosis in rat oligodendrocytes and their pre-
forebrain global ischemia. Rectal temperature was kept as
cursors [58], rat pheochromocytoma PC-12 [65] and
close as possible to 38
8
C during and up to 30 min after
fibroblasts [24]. Wang [60] and Abe [1] have demonstrated
ischemia with the aid of a heating blanket and overhead
that GDNF can prevent ischemia-induced injury in cerebral
lamp [36,37]. After these procedures and their wounds
cortex, which is mainly a necrotic lesion. However, it is
closed, the animals were allowed to recover. The animals
not known whether GDNF can prevent the delayed neuro-
were sacrificed at 2 (six animals in hGDNF model and four
nal death induced by transient ischemia. In the present
animals in LacZ model), 4 (six animals in both models),
study, we investigated whether the treatment of intraven-
and 7 days (six animals in hGDNF model and four animals
tricular injection with Ad vector encoding GDNF cDNA
in LacZ model) after the operation as described above. The
can prevent the delayed neuronal death in hippocampal
experimental protocol and procedures conformed to that of
CA1 pyramidal neurons induced by occluding bilateral
the Animal Committee of the Osaka City University
common carotid arteries (CCA) for 5 min in Mongolian
School of Medicine.
gerbils.
2.3. Immunohistochemistry
2. Materials and methods
Neuropathological studies were undertaken in two
10
groups of animals: (1) AxCAhGDNF (5
m
l, 1
3
10
pfu /
10
2.1. Adenovirus preparation
ml)-treated group and (2) AxCALacZ (5
m
l, 1
3
10
pfu /
ml)-treated group had 18 and 14 gerbils, respectively.
Generation of the replication-defective recombinant Ad
Frozen sections of six (two animals of each model) of the
carrying human GDNF cDNA (AxCAhGDNF) and bac-
AxCAhGDNF-treated group and two (animals of 4 days
terial
b
-galactosidase gene (AxCALacZ) have been de-
model) of the AxCALacZ-treated group were used for
scribed elsewhere [48,61]. Briefly, the human GDNF
hGDNF staining and in situ hybridization, and the other
cDNA was derived from cultured human fetal astrocytes.
animals in both groups were subjected to paraffin section
This cDNA placed into a cassette cosmid carrying an
for other antibody staining. Animals were anesthetized
adenovirus type-5 genome lacking the E1A, E1B, and E3
with a lethal dose of pentobarbital sodium and
transcardial-region, which has Swa I cloning site flanked by the CAG
ly perfused with normal saline followed by 4%
paraformal-(cytomegalovirus-enhancer-chicken
b
-actin hybrid) pro-
dehyde in 0.1 M phosphate buffer, pH 7.4 (PB). The brain
moter on the 5
9
end and a rabbit globin poly (A) sequence
tissue was dissected and immersion fixed in the same
on the 3
9
end. These Ads were generated by in vivo
fixative. For paraffin section, the brain tissues were fixed
homologous recombination in 293 cells. The recombinant
for at least 24 h at 4
8
C, dehydrated, embedded in paraffin
Ad was propagated and isolated from 293 cells, and
wax, sliced coronally at the hippocampal levels into 3-
m
m-purified by two rounds of CsCl centrifugation [23]. Bioas-
thick sections and collected on glass slides coated with
say of AxCAhGDNF has been described elsewhere [61].
3-aminopropyl-triethoxy-silane (Silan). After
deparaffiniz-ing and rehydratdeparaffiniz-ing, sections were pretreated with 0.3%
2.2. Animals and surgical procedures
H O in phosphate-buffered saline (PBS), rinsed in PBS
2 2(RT), for blocking nonspecific binding. Sections were
Auto Wash (Research Genetics), counterstained with
He-incubated overnight at 4
8
C with a mouse monoclonal
matoxylin (Research Genetics) and coverslipped with
antibody to
b
-tubulin (Promega) or glial fibrillary acidic
Pristine Mount (Research Genetics).
protein (GFAP) (DAKO) at a dilution of 1:300 or 1:500,
respectively. They were then incubated with biotinylated
2.5. Tunel assay
anti-mouse IgG, at a dilution of 1:200, ABC reagent
(Vector) and visualized by 3,3
9
-diaminobenzidine tetrahy-
Histochemical staining for TUNEL at hippocampus 7
drochloride (DAB)-H O solution. Griffonia simplicifolia
2 2days after the operation was performed with a kit
B 4 isolectin (B4–lectin) (Sigma) staining was performed
(ApopTag
Peroxidase In Situ Apoptosis Detection Kit
by a method described elsewhere [52]. These sections were
[
S7100, Intergen). After a detection of double-strand
counterstained with Hematoxylin. For histopathological
breaks in genomic DNA with DAB-H O
2 2solution, the
analysis, Hematoxylin–Eosin or Toluidine Blue (TB) was
sections were counterstained with Methyl Green according
performed on some sections of each animal.
to the protocol in the kit.
For frozen section, the brain tissues were fixed for 2 h at
4
8
C, cryoprotected in 30% sucrose in 0.1 M PB and serial
2.6. Statistical analysis
sections (10-
m
m-thick) at hippocampal levels were made
by cryostat and collected on glass slides coated with Silan.
The number of neurons in the bilateral CA1 area was
For immunostaining against hGDNF, sections were pre-
counted on paraffin sections at two points each side from
treated with 0.3% H O in PBS, followed by preincubation
2 2each animal and expressed as neuronal cell density per
with 0.3% Triton X-100 in PBS (T-PBS) for 30 min at RT,
millimeter linear length. Hence, the four numbers of
rinsed in PBS three times and preincubated in 3% NGS in
neurons were made from one animal. Sections obtained
T-PBS for 1 h at RT. Sections were incubated overnight at
from control animals (AxCALacZ-treated group) were also
4
8
C with a rabbit polyclonal antibody to hGDNF (Santa
examined. Results are expressed as the mean
6
S.D. from
Cruz) at a dilution 1:100 in 3% NGS / T-PBS, followed by
four animals 2, 4 and 7 days after the operation in both
incubating with biotinylated anti-rabbit IgG, at a dilution
groups. Statistical significance was assessed by Mann–
of 1:100, ABC reagent (Vector) and visualized by DAB-
Whitney U-test. All comparisons were made between an
H O solution and counterstained with Hematoxylin.
2 2AxCAhGDNF-treated group and an AxCALacZ-treated
group.
2.4. In situ hybridization
The number of GFAP and B4-lectin positive cells
around hippocampal CA1 was counted on paraffin
sec-The oligonucleotide probe complementary to hGDNF
tions. Results are expressed as the mean
6
S.D. from four
(30-mer probe from bases 390–419, in the sequence
animals 2, 4 and 7 days after the operation in both groups.
deposited in GenBank, accession number L19063) was
In each group, a comparison of right and left was made.
synthesized and biotin labeled on the 3
9
end (Research
Comparison of the AxCALacZ-treated group and
Ax-Genetics, Huntsville, Alabama). This synthetic probe was
CAhGDNF-treated group for the same day was made for
lyophilized and reconstituted in 10 mM Tris, 1 mM EDTA
the same side. Statistical significance was assessed by
solution, pH 7.8, to make a 1
m
g /
m
l solution and stored at
paired t-test and Student’s t-test, respectively.
2
20
8
C prior to use. In situ hybridization (ISH) was
performed with the MicroProbe system (Fisher Scientific,
Pittsburgh, PA), utilizing the capillary action principle
3. Results
[9,20,41,59]. Ten micrometer sections (same brain tissue
for immunostaining against hGDNF) were cut, placed on
3.1. Change in hippocampal CA1 pyramidal neurons
ProbeOn Plus slides (Fisher Scientific) and air dried. The
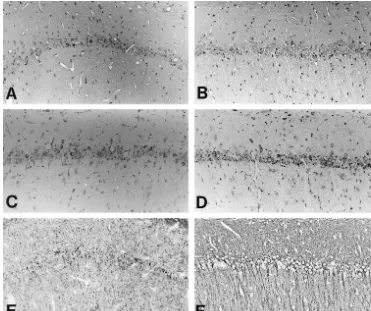
Fig. 1. Representative staining of Hematoxylin–Eosin (A, B), Toluidine Blue (C, D) andb-tubulin (E, F). Cerebral sections at 7 days after transient global ischemia from animals treated with AxCALacZ (A, C, E) or AxCAhGDNF (B, D, F). Counterstaining was with Hematoxylin (E, F). Animals treated with intraventricular administration of AxCALacZ (A, C) demonstrated numerous losses of hippocampal CA1 pyramidal neurons, as compared with animals treated with AxCAhGDNF (C, D). The dendrites of hippocampal CA1 pyramidal neurons were extensively disrupted (E), but they were almost preserved by AxCAhGDNF administration (F). Scale bar5100mm.
reduced the number of TUNEL-positive cells (Fig. 2B).
3.2. Changes in other cells around the hippocampus
Thus, it was considered that the AxCAhGDNF
administra-tion into ventricle could prevent the delayed neuronal
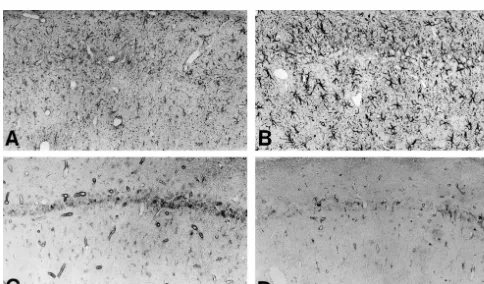
Immunostaining against GFAP, to which astrocytes were
death of the hippocampal CA1 pyramidal neurons induced
identified with antibody, demonstrated that astrocytes
by transient global ischemia.
around the hippocampus treated with AxCAhGDNF
Table 1
creased in cell body size and density of their processes
aChange in GFAP-positive cell numbers around hippocampal CA1
(Fig. 3A and B). Also, there were no differences in cell
2 days 4 days 7 days
numbers between both groups at 2 days, but at 4 and 7
†
days (Table 1). For microglia and macrophage, which were
AxCALacZ rt 122.0611.6 103.3625.1* 184.0632.2‡ ¶
identified with B4-lectin staining, around hippocampus
(n54) lt 146.5648.3 119.0625.2* 177.6644.2†
AxCAhGDNF rt 97.0635.4 191.0639.7 264.0672.4
there were no differences in cell numbers between Ax-
‡ ¶(n54) lt 106.8642.1 280.0625.6 258.8664.4
CAhGDNF and AxCALacZ administration at 2, 4 and 7
2(cells / mm )
days (in right side) (Table 2), but only in left side of 7
aThe number of GFAP-positive cells around hippocampal CA1 was
days (Fig. 3C and D, Table 2).
counted on paraffin sections. Results are expressed as the mean6S.D.
Only two reports have described the effect of GDNF on
from four animals 2, 4 and 7 days after the operation in both groups. Inastrocytes in vitro previously. Lin demonstrated that
each group a comparison of right and left was made and on the same dayGDNF was not effective on the number of astrocytes [35].
a comparison of AxCALacZ and AxCAhGDNF was made for the sameside.
In another study, Burke demonstrated that in the presence
†,‡ ¶
*P,0.05 (paired t-test); P,0.01; P,0.05 (Student’s t-test).
of astrocytes, GDNF had more effect on survival of
neurons [10]. However, both reports did not demonstrate
morphological changes and number of astrocytes in vivo.
the brain tissues treated with AxCALacZ, the presence of
Therefore our study is the first to report changes in
transcripts for hGDNF could not be detected (Fig. 4A).
astrocytes in the presence of GDNF in vivo.
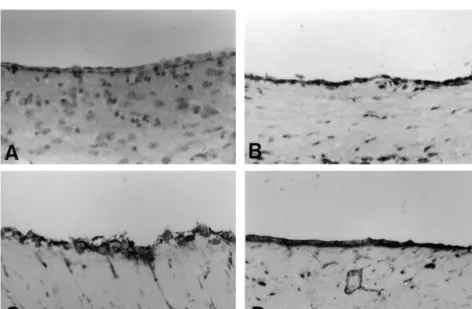
Immunostaining against hGDNF demonstrated that there
were the hGDNF-positive cells on the ventricle wall
3.3. Transduction by AxCAhGDNF vector
following intraventricular administration of AxCAhGDNF
(Fig. 5). The hGDNF-positive cells were mainly restricted
ISH of the brain tissues treated with AxCAhGDNF
to the bilateral lateral ventricle walls (Fig. 5) and the third
revealed the presence of transcripts for hGDNF (Fig. 4).
ventricle (not shown), and a few hGDNF-positive cells
At 2, 4 and 7 days, high levels of mRNA for hGDNF were
existed on the choroid plexus (not shown). The
hGDNF-presented on the lateral ventricle walls (Fig. 4B–D), but in
positive cells were observed at 2, 4 and 7 days and were
Table 2
not express the hGDNF protein after AxCAhGDNF
ad-Change in B4-lectin-positive cell number around hippocampal CA1ministration, including hippocampal CA1 neuron,
as-2 a
(cells / mm )
trocyte, and macrophage etc. These findings are in
agree-2 days 4 days 7 days
ment with Bajocchi [2] and Ohashi [44]. However, Martin
AxCALacZ rt 29.369.2 62.3624.1 65.2615.6
[39] demonstrated that positive immunostaining against
(n54) lt 29.3616.6 71.5615.6 55.464.2*
recombinant human GDNF (rhGDNF) is present in the
AxCAhGDNF rt 34.069.6 68.5616.9 82.061.6thalamus, hippocampus and cortical brain after
intraven-(n54) lt 37.5612.0 85.8628.6 70.069.8*2
tricular administration of rhGDNF itself. It is therefore
(cells / mm )
presumable that the hGDNF produced from the ependymal
*P,0.05 (Student’s t-test).
cells distributed extensively to the parenchyma, but the
aThe number of B4-lectin-positive cells around hippocampal CA1 was
amount of hGDNF might be below the sensitivity of
counted on paraffin sections. Results are expressed as the mean6S.D.
immunostaining against GDNF.
from four animals 2, 4 and 7 days after the operation in both groups. In each group, a comparison of right and left was made, and on the same
day, a comparison of AxCALacZ and AxCAhGDNF was made for the
3.4. Neuroprotective effect of AxCAhGDNF on
same side.hippocampal CA
1 neurons
localized primarily in the single cell layer lining the
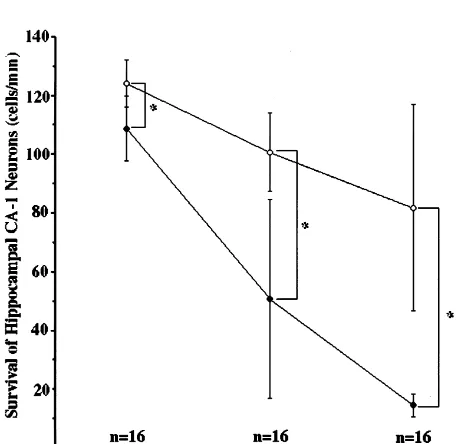
Two to seven days after the transient global ischemia
cerebral ventricles, and presumably are ependymal cells
treated with administration of AxCALacZ, there were
(Fig. 5). The immunostaining pattern of hGDNE was
marked loss of hippocampal CA1 pyramidal neurons, but
similar to ISH pattern. Therefore, we considered that
the treatment with AxCAhGDNF significantly prevented
AxCAhGDNF could infect the ependymal cells, and then
this loss (Fig. 6). At two days, the number of neurons of
the hGDNF protein was produced in the same cells.
hippocampal
CA1
treated
with
administration
of
Interestingly, other cells except for ependymal cells did
AxCALacZ declined by 12% as compared with treatment
Fig. 5. Representative staining of GDNF. Cerebral sections at 2 (B), 4 (C) and 7 (D) days after transient global ischemia from animal treated with AxCAhGDNF. (A) is the cerebral section treated with AxCALacZ at 4 days after the operation. Counterstaining was with Hematoxylin. The GDNF-positive cells were localized primarily in single cell layer lining the cerebral ventricles, presumably they are the ependymal cells (B–D). In A, there were no GDNF-positive cells. Scale bars525mm.
with AxCAhGDNF. The former declined by 50% and 81%
4. Discussion
at 4 and 7 days, respectively, as compared with the latter
(Fig. 6).
Previously, numerous studies have demonstrated that
GDNF can prevent the loss of neurons under the ischemic
condition [1,25,26,39,60]. These methods were the local
displacement [1,25,26] or the intraventricular
administra-tion [39,60] of GDNF protein itself, and the microgram
amount of GDNF protein was necessitated for the purpose
of the prevention of neuronal loss. However, these
meth-ods and amount of GDNF protein are not suitable for the
clinical condition. In the present study we considered a
method that was minimally invasive and able to maintain
the hGDNF protein produced, owing to the decrease of
amount of GDNF protein. Intraventricular delivery is less
traumatic and less invasive than any intraparenchymal
delivery. Moreover, the lateral ventricle would be a more
accessible intracranial target and would have great clinical
utility. Furthermore, it has been demonstrated that the
hippocampal CA1 pyramidal neurons can be prevented
from neuronal damage induced by kainic acid-mediated
seizures after intraventricular administration of GDNF
protein [39]. On the other hand, Ad vector has the ability
to infect nerve cells and the gene transfer and expression
of Ad are highly efficient and maintain long-term period
respectively in vivo [33], as well as having a low
patho-Fig. 6. Time-course of the survival of hippocampal CA1 neurons.
genicity because of the replication-deficient recombinant
Symbols indicated treatment with AxCAhGDNF (.) or AxCALacZ (æ),
adenovirus due to lacking the E1A, E1B and E3 regions
respectively. Statistical comparison was done by Mann–Whitney U-test
for the administration of AxCAhGDNF to the lateral
Acknowledgements
ventricle. Indeed, the ependymal cells infected with
Ax-CAhGDNF vector maintained the expression of hGDNF
We thank Mrs Kadono for her assistance with the animal
for at least nine days.
models and immunostaining procedures.
GDNF signaling is mediated through a two-component
system consisting of the Ret tyrosine kinase [16,56] and
GFR
a
-1 [21,55] or GFR
a
-2 [3,49], which binds GDNF.
References
The GDNF-GFR
a
-1 or 2 complex binds to and activates
the tyrosine kinase receptor Ret [16,56]. It has been
[1] K. Abe, T. Hayashi, Y. Itoyama, Amelioration of brain edema bydescribed that in the central nervous system (CNS), high
topical application of glial cell line-derived neurotrophic factor inlevels of GDNF mRNA are expressed in the developing rat
reperfused rat brain, Neurosci. Lett. 231 (1997) 37–40.[2] G. Bajocchi, S.H. Feldman, R.G. Crystal, A. Mastrangeli, Direct in
striatum, but in the adult rat hippocampus, there is little
vivo gene transfer to ependymal cells in the central nervous system
expression of GDNF mRNA [30,51]. Also, Lapchak
using recombinant adenovirus vectors [see comments], Nat. Genet. 3
described that in adult rat hippocampus, GDNF mRNA is
(1993) 229–234.upregulated following kainic-induced epileptic seizures
[3] R.H. Baloh, M.G. Tansey, J.P. Golden, D.J. Creedon, R.O.Heuc-[32]. Previously it has been described that in adult rat
keroth, C.L. Keck, D.B. Zimonjic, N.C. Popescu, E.M. Johnson, J. Milbrandt, TrnR2, a novel receptor that mediates neurturin andhippocampus, Ret mRNA [56], GFR
a
-1 mRNA [42,55]
GDNF signaling through Ret, Neuron 18 (1997) 793–802.
and GFR
a
-2 mRNA [49,62] are expressed. Interestingly,
[4] B.J. Baumgartner, H.D. Shine, Neuroprotection of spinal
Kokaia [30] described that under ischemic condition in
motoneurons following targeted transduction with an adenoviral
adult rat hippocampus, GDNF, Ret and GFR
a
-1 are
vector carrying the gene for glial cell line-derived neurotrophicunchanged but only GFR
a
-2 is upregulated. These ob-
factor, Exp. Neurol. 153 (1998) 102–112.[5] B.J. Baumgartner, H.D. Shine, Targeted transduction of CNS
servations that GDNF, Ret and GFR
a
-1, 2 mRNA are
neurons with adenoviral vectors carrying neurotrophic factor genes
expressed in the hippocampal CA1 pyramidal neurons
confers neuroprotection that exceeds the transduced population, J.
indicated that GDNF can act in an autocrine or paracrine
Neurosci. 17 (1997) 6504–6511.
manner in this area. On the other hand, the Ret phos-
[6] K.D. Beck, J. Valverde, T. Alexi, K. Poulsen, B. Moffat, R.A.phorylation in response to GDNF results in activation of
Vandlen, A. Rosenthal, F. Hefti, Mesencephalic dopaminergicPI-3 kinase indirectly or directly, through the Ras-GTP
neurons protected by GDNF from axotomy-induced degeneration in the adult brain [see comments], Nature 373 (1995) 339–341.activation or not [15,47,57,65]. It has also been
demon-[7] A. Bilang-Bleuel, F. Revah, P. Colin, I. Locquet, J.J. Robert, J.
strated that, in vitro, PI-3 kinase activation can prevent
Mallet, P. Horellou, Intrastriatal injection of an adenoviral vector
apoptosis in rat oligodendrocytes and their precursors [58],
expressing glial cell line-derived neurotrophic factor preventsdopa-rat pheochromocytoma PC-12 [65], and fibroblasts [24].
minergic neuron degeneration and behavioral impairment in a ratTherefore we speculated easily that survival of hippocam-
model of Parkinson disease, Proc. Natl. Acad. Sci. USA 94 (1997) 8818–8823.pal CA1 pyramidal neurons depend, at least partially, on a
[8] M.C. Bohn, A commentary on glial cell line-derived neurotrophic
PI-3 kinase, which was activated by GDNF-mediated
factor (GDNF). From a glial secreted molecule to gene therapy,
signaling pathway.
Biochem Pharmacol 57 (1999) 135–142.We could not demonstrate that there were GDNF-posi-
[9] C.D. Bucana, R. Radinsky, Z. Dong, R. Sanchez, D.J. Brigati, I.J.tive cells in hippocampal CA1 pyramidal neurons and
Fidler, A rapid colorimetric in situ mRNA hybridization technique using hyperbiotinylated oligonucleotide probes for analysis of mdr1other areas, except for the ependymal cells in the ventricle
in mouse colon carcinoma cells, J. Histochem. Cytochem. 41 (1993)
walls and choroid plexus, but demonstrated that astrocytes
499–506.
around hippocampus obviously changed morphology and
[10] R.E. Burke, M. Antonelli, D. Sulzer, Glial cell line-derivedneuro-cell numbers. We considered that these findings might be
trophic growth factor inhibits apoptotic death of postnatal substantiainfluenced
by
GDNF
protein
permeating
from
the
nigra dopamine neurons in primary culture, J. Neurochem. 71 (1998) 517–525.ependymal cells. We could therefore speculate that the
[11] A.P. Byrnes, R.E. MacLaren, H.M. Charlton, Immunological
in-GDNF protein might permeate from the ependymal cells to
stability of persistent adenovirus vectors in the brain: peripheral
hippocampus and activate a PI-3 kinase-mediated signal
exposure to vector leads to renewed inflammation, reduced genepathway, finally preventing the delayed neuronal death in
expression, and demyelination, J. Neurosci. 16 (1996) 3045–3055.hippocampal CA1 pyramidal neurons. However, further
[12] D.L. Choi-Lundberg, Q. Lin, Y.N. Chang, Y.L. Chiang, C.M. Hay, H. Mohajeri, B.L. Davidson, M.C. Bohn, Dopaminergic neuronsstudies are required to know whether PI-3 kinase is
protected from degeneration by GDNF gene therapy [see
com-activated indeed by GDNF in vivo under this model.
ments], Science 275 (1997) 838–841.
Overall, our data indicate that GDNF is a neuroprotec-
[13] D.L. Choi-Lundberg, Q. Lin, T. Schallert, D. Crippens, B.L.tant not only for the cerebral cortex after ischemia (mainly
Davidson, Y.N. Chang, Y.L. Chiang, J. Qian, L. Bardwaj, M.C.necrosis), but also for delayed neuronal death in the
Bohn, Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derivedhippocampal CA1 neurons (mainly apoptosis). Also, the
neurotrophic factor, Exp. Neurol. 154 (1998) 261–275.
Ad delivery system is suitable for the CNS. These findings
[14] E.D. Clarkson, W.M. Zawada, C.R. Freed, GDNF improves survival
appear to provide a new therapeutic approach to the
and reduces apoptosis in human embryonic dopaminergic neurons intreatment of stroke and other disorders of the cerebral
vitro, Cell Tiss. Res. 289 (1997) 207–210.T.J. Fahrner, R.O. Heuckeroth, J. Milbrandt, E. Johnson Jr., glial cell line-derived neurotrophic factor (GDNF): implications for Neurturin shares receptors and signal transduction pathways with GDNF as a therapeutic molecule for treating neurodegenerative glial cell line-derived neurotrophic factor in sympathetic neurons, diseases, Cell Tiss. Res. 286 (1996) 179–189.
Proc. Natl. Acad. Sci. USA 94 (1997) 7018–7023. [33] G. Le-Gal-La-Salle, J.J. Robert, S. Berrard, V. Ridoux, L.D. Stratfor-[16] P. Durbec, C.V. Marcos-Gutierrez, C. Kilkenny, M. Grigoriou, K. d-Perricaudet, M. Perricaudet, J. Mallet, An adenovirus vector for Wartiowaara, P. Suvanto, D. Smith, B. Ponder, F. Costantini, M. gene transfer into neurons and glia in the brain, Science 259 (1993) Saarma, H. Sariola, V. Pachnis, GDNF signalling through the Ret 988–990.
receptor tyrosine kinase [see comments], Nature 381 (1996) 789– [34] L. Li, W. Wu, L.F. Lin, M. Lei, R.W. Oppenheim, L.J. Houenou,
793. Rescue of adult mouse motoneurons from injury-induced cell death
[17] M. Gimenez-y-Ribotta, F. Revah, L. Pradier, I. Loquet, J. Mallet, A. by glial cell line-derived neurotrophic factor, Proc. Natl. Acad. Sci. Privat, Prevention of motoneuron death by adenovirus-mediated USA 92 (1995) 9771–9775.
neurotrophic factors, J. Neurosci. Res. 48 (1997) 281–285. [35] L.F. Lin, D.H. Doherty, J.D. Lile, S. Bektesh, F. Collins, GDNF: a [18] C.E. Henderson, H.S. Phillips, R.A. Pollock, A.M. Davies, C. glial cell line-derived neurotrophic factor for midbrain dopaminergic
Lemeulle, M. Armanini, L.C. Simmons, B. Moffet, R.A. Vandlen, neurons [see comments], Science 260 (1993) 1130–1132. V.E. Koliatsos, A. Rosenthal, GDNF: a potent survival factor for [36] M. Maeda, F. Akai, S. Nishida, T. Yanagihara, Intracerebral motoneurons present in peripheral nerve and muscle, Science 266 distribution of albumin after transient cerebral ischemia: light and
(1994) 1062–1064. electron microscopic immunocytochemical investigation, Acta
[19] L.J. Houenou, R.W. Oppenheim, L. Li, A.C. Lo, D. Prevette, Neuropathol. Berl. 84 (1992) 59–66.
Regulation of spinal motoneuron survival by GDNF during develop- [37] M. Maeda, T. Sugiyama, F. Akai, I. Jikihara, Y. Hayashi, H. Takagi, ment and following injury, Cell Tiss. Res. 286 (1996) 219–223. Single stranded DNA as an immunocytochemical marker for apo-[20] J.C. Iezzoni, J.H. Kang, K.T. Montone, J.A. Reed, D.J. Brigati, ptotic change of ischemia in the gerbil hippocampus, Neurosci. Lett.
Colorimetric detection of herpes simplex virus by DNA in situ 240 (1998) 69–72.
sandwich hybridization: a rapid, formamide-free, random oligomer- [38] D. Martin, G. Miller, N. Fischer, D. Diz, T. Cullen, D. Russell, Glial enhanced method, Nucl. Acids Res. 20 (1992) 1149–1150. cell line-derived neurotrophic factor: the lateral cerebral ventricle as [21] S. Jing, D. Wen, Y. Yu, P.L. Holst, Y. Luo, M. Fang, R. Tamir, L. a site of administration for stimulation of the substantia nigra Antonio, Z. Hu, R. Cupples, J.C. Louis, S. Hu, B.W. Altrock, G.M. dopamine system in rats, Eur. J. Neurosci. 8 (1996) 1249–1255. Fox, GDNF-induced activation of the ret protein tyrosine kinase is [39] D. Martin, G. Miller, M. Rosendahl, D.A. Russell, Potent inhibitory mediated by GDNFR-alpha, a novel receptor for GDNF, Cell 85 effects of glial derived neurotrophic factor against kainic acid
(1996) 1113–1124. mediated seizures in the rat, Brain Res. 683 (1995) 172–178.
[22] F.G. Kaddis, W.M. Zawada, J. Schaack, C.R. Freed, Conditioned [40] S. Miyake, M. Makimura, Y. Kanegae, S. Harada, Y. Sato, K. medium from aged monkey fibroblasts stably expressing GDNF and Takamori, C. Tokuda, I. Saito, Efficient generation of recombinant BDNF improves survival of embryonic dopamine neurons in vitro, adenoviruses using adenovirus DNA-terminal protein complex and a Cell Tiss. Res. 286 (1996) 241–247. cosmid bearing the full-length virus genome, Proc. Natl. Acad. Sci. [23] Y. Kanegae, M. Makimura, I. Saito, A simple and efficient method USA 93 (1996) 1320–1324.
for purification of infectious recombinant adenovirus, Jap. J. Med. [41] K.T. Montone, J.E. Tomaszewski, In situ hybridization protocol for Sci. Biol. 47 (1994) 157–166. overall preservation of mRNA in fixed tissues with a poly d (T) [24] A. Kauffmann-Zeh, P. Rodriguez-Viciana, E. Ulrich, C. Gilbert, P. oligonucleotide probe, J. Histotechnol. 16 (1993) 315–322.
Coffer, J. Downward, G. Evan, Suppression of c-Myc-induced [42] C.A. Nosrat, A. Tomac, B.J. Hoffer, L. Olson, Cellular and apoptosis by Ras signalling through PI (3)K and PKB, Nature 385 developmental patterns of expression of Ret and glial cell
line-(1997) 544–548. derived neurotrophic factor receptor alpha mRNAs, Exp. Brain Res.
[25] H. Kitagawa, K. Abe, T. Hayashi, Y. Mitsumoto, N. Koga, Y. 115 (1997) 410–422.
Itoyama, Ameliorative effect of glial cell line-derived neurotrophic [43] C. Nozaki, N. Asai, H. Murakami, T. Iwashita, Y. Iwata, K. Horibe, factor on brain edema formation after permanent middle cerebral R.D. Klein, A. Rosenthal, M. Takahashi, Calcium-dependent Ret artery occlusion in rats, Neurol. Res. 20 (1998) 333–336. activation by GDNF and neurturin, Oncogene 16 (1998) 293–299. [26] H. Kitagawa, T. Hayashi, Y. Mitsumoto, N. Koga, Y. Itoyama, K. [44] T. Ohashi, K. Watabe, K. Uehara, W.S. Sly, C. Vogler, Y. Eto, Abe, Reduction of ischemic brain injury by topical application of Adenovirus-mediated gene transfer and expression of human beta-glial cell line-derived neurotrophic factor after permanent middle glucuronidase gene in the liver, spleen, and central nervous system cerebral artery occlusion in rats, Stroke 29 (1998) 1417–1422. in mucopolysaccharidosis type VII mice, Proc. Natl. Acad. Sci. USA [27] N. Klocker, F. Braunling, S. Isenmann, M. Bahr, In vivo neuro- 94 (1997) 1287–1292.
trophic effects of GDNF on axotomized retinal ganglion cells, [45] R.W. Oppenheim, L.J. Houenou, J.E. Johnson, L.F. Lin, L. Li, A.C.
NeuroReport 8 (1997) 3439–3442. Lo, A.L. Newsome, D.M. Prevette, S. Wang, Developing motor
[28] P.D. Koeberle, A.K. Ball, Effects of GDNF on retinal ganglion cell neurons rescued from programmed and axotomy-induced cell death survival following axotomy, Vision Res. 38 (1998) 1505–1515. by GDNF [see comments], Nature 373 (1995) 344–346.
[29] H. Kojima, Y. Abiru, K. Sakajiri, K. Watabe, N. Ohishi, M. [46] M.S. Ramer, J.V. Priestley, S.B. McMahon, Functional regeneration Takamori, H. Hatanaka, K. Yagi, Adenovirus-mediated transduction of sensory axon into the adult spinal cord, Nature 403 (2000) with human glial cell line-derived neurotrophic factor gene prevents 312–316.
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopamine de- [47] P. Rodriguez-Viciana, P.H. Warne, R. Dhand, B. Vanhaesebroeck, I. pletion in striatum of mouse brain, Biochem. Biophys. Res. Com- Gout, M.J. Fry, M.D. Waterfield, J. Downward,
mun. 238 (1997) 569–573. Phosphatidylinositol-3-OH kinase as a direct target of Ras [see
[30] Z. Kokaia, M.S. Airaksinen, A. Nanobashvili, E. Larsson, E. comments], Nature 370 (1994) 527–532.
Kujamaki, O. Lindvall, M. Saarma, GDNF family ligands and [48] T. Sakamoto, K. Watabe, T. Ohashi, Y. Kawazoe, K. Oyanagi, K. receptors are differentially regulated after brain insults in the rat, Inoue, Y. Eto, Adenoviral vector-mediated GDNF gene transfer Eur. J. Neurosci. 11 (1999) 1202–1216. prevents death of adult facial motoneurons, NeuroReport 11 (2000) [31] P.A. Lapchak, D.M. Araujo, D.C. Hilt, J. Sheng, S. Jiao, Adenoviral 1857–1860.
cell-surface accessory proteins, Proc. Natl. Acad. Sci. USA 94 (1997) [58] G.S. Vemuri, F.A. McMorris, Oligodendrocytes and their precursors
6238–6243. require phosphatidylinositol 3-kinase signaling for survival,
De-[50] R. Schmidt-Kastner, A. Tomac, B. Hoffer, S. Bektesh, B. Rosen- velopment 122 (1996) 2529–2537.
zweig, L. Olson, Glial cell-line derived neurotrophic factor (GDNF) [59] J.Y. Wang, K.T. Montone, A rapid simple in situ hybridization mRNA upregulation in striatum and cortical areas after pilocarpine- method for herpes simplex virus employing a synthetic biotin-induced status epilepticus in rats, Mol. Brain Res. 26 (1994) 325– labeled oligonucleotide probe: a comparison with
immunohistoch-330. emical methods for HSV detection, J. Clin. Lab. Anal. 8 (1994)
[51] J.E. Springer, X. Mu, L.W. Bergmann, J.Q. Trojanowski, Expression 105–115.
of GDNF mRNA in rat and human nervous tissue, Exp. Neurol. 127 [60] Y. Wang, S.Z. Lin, A.L. Chiou, L.R. Williams, B.J. Hoffer, Glial cell
(1994) 167–170. line-derived neurotrophic factor protects against ischemia-induced
[52] W.J. Streit, An improved staining method for rat microglial cells injury in the cerebral cortex, J. Neurosci. 17 (1997) 4341–4348. using the lectin from Griffonia simplicifolia (GSA I-B4), J. Histoch- [61] K. Watabe, T. Ohashi, T. Sakamoto, Y. Kawazoe, T. Takeshima, K. em. Cytochem. 38 (1990) 1683–1686. Oyanagi, K. Inoue, Y. Eto, S.U. Kim, Rescue of lesioned adult rat [53] I. Stromberg, L. Bjorklund, M. Johansson, A. Tomac, F. Collins, L. spinal motoneurons by adenoviral gene transfer of glial cell line-Olson, B. Hoffer, C. Humpel, Glial cell line-derived neurotrophic derived neurotrophic factor, J. Neurosci. Res. 60 (2000) 511–519. factor is expressed in the developing but not adult striatum and [62] J. Widenfalk, C. Nosrat, A. Tomac, H. Westphal, B. Hoffer, L. stimulates developing dopamine neurons in vivo, Exp. Neurol. 124 Olson, Neurturin and glial cell line-derived neurotrophic factor
(1993) 401–412. receptor-beta (GDNFR-beta), novel proteins related to GDNF and
[54] A. Tomac, E. Lindqvist, L.F. Lin, S.O. Ogren, D. Young, B.J. GDNFR-alpha with specific cellular patterns of expression sug-Hoffer, L. Olson, Protection and repair of the nigrostriatal dopa- gesting roles in the developing and adult nervous system and in minergic system by GDNF in vivo [see comments], Nature 373 peripheral organs, J. Neurosci. 17 (1997) 8506–8519.
(1995) 335–339. [63] C.A. Worby, Q.C. Vega, Y. Zhao, H.H.-J. Chao, A.F. Seasholtz, J.E.
[55] J.J. Treanor, L. Goodman, F. de-Sauvage, D.M. Stone, K.T. Poulsen, Dixon, Glial cell line-derived neurotrophic factor signals through the C.D. Beck, C. Gray, M.P. Armanini, R.A. Pollock, F. Hefti, H.S. RET receptor and activates mitogen-activated protein kinase, J. Biol. Phillips, A. Goddard, M.W. Moore, A. Buj-Bello, A.M. Davies, N. Chem. 271 (1996) 23619–23622.
Asai, M. Takahashi, R. Vandlen, C.E. Henderson, A. Rosenthal, [64] Q. Yan, C. Matheson, O.T. Lopez, In vivo neurotrophic effects of Characterization of a multicomponent receptor for GDNF [see GDNF on neonatal and adult facial motor neurons [see comments],
comments], Nature 382 (1996) 80–83. Nature 373 (1995) 341–344.
[56] M. Trupp, E. Arenas, M. Fainzilber, A.S. Nilsson, B.A. Sieber, M. [65] R. Yao, G.M. Cooper, Requirement for phosphatidylinositol-3 kinase Grigoriou, C. Kilkenny, E. Salazar-Grueso, V. Pachnis, U. Arumae, in the prevention of apoptosis by nerve growth factor, Science 267 H. Sariola, M. Saarma, C.F. Ibanez, Functional receptor for GDNF (1995) 2003–2006.
encoded by the c-ret proto-oncogene [see comments], Nature 381 [66] A.D. Zurn, E.E. Baetge, J.P. Hammang, S.A. Tan, P. Aebischer,
(1996) 785–788. Glial cell line-derived neurotrophic factor (GDNF), a new
neuro-[57] D.H. van-Weering, J.L. Bos, Glial cell line-derived neurotrophic trophic factor for motoneurones, NeuroReport 6 (1994) 113–118. factor induces Ret-mediated lamellipodia formation, J. Biol. Chem.