Modification of flower color in torenia
(Torenia fournieri
Lind.) by
genetic transformation
Ryutaro Aida
a,*, Sanae Kishimoto
a, Yoshikazu Tanaka
b, Michio Shibata
aaNational Research Institute of Vegetables,Ornamental Plants and Tea,Ano,Mie514-2392,Japan bSuntory Limited,Institute for Fundamental Research,Shimamoto,Osaka618-8503,Japan
Received 28 September 1999; received in revised form 9 November 1999; accepted 11 November 1999
Abstract
We modified flower color in torenia (Torenia fournieri Lind.) by transferring the chalcone synthase (CHS) or the dihy-droflavonol-4-reductase (DFR) gene in sense or antisense orientation byAgrobacterium-mediated gene transfer. The modification patterns of flower color among the transformants formed three groups: (1) same color as the wild-type plant; (2) whole corolla changed to a uniformly light color; and (3) with greater degree of lightening in the tube than in the lip. Transformants incorporating antisense transgene(s) tended to become group 2 types, with no plants becoming group 3 type. Transformants harboring sense transgene(s) tended to become group 3 types, rather than group 2 types. Sense genes and antisense genes seemed to have different potential for changing the flower color. We also produced transformants with new characters in torenia flower color; for example, lines with pastel flowers, wavy patterned flowers and parti-colored flowers. We regard this system to be useful for flower color breeding in torenia and for studying gene expression. © 2000 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Anthocyanin; Gene silencing;Torenia fournieri; Transformation; Flower color
www.elsevier.com/locate/plantsci
1. Introduction
Flower color is one of the most important char-acters for ornamental plants. Creation of new flower colors is one of the important targets for breeding. By controlling expression levels of genes related to the biosynthetic pathway of flower color pigments we can breed novel varieties with respect to flower color.
Genetic transformation can be done by either introducing and expressing a new gene or re-intro-ducing an existing gene in sense or antisense orien-tation to inactivate an endogenous gene [1].
Flower color had been modified by transforma-tion in several ornamental plants by suppressing
the chalcone synthase (CHS) or the
dihy-droflavonol-4-reductase (DFR) gene that encode enzymes in the biosynthetic pathway of
an-thocyanins. The anthocyanins are important pig-ments for flower color. Introduction of the antisense CHS gene suppressed the formation of flower pigments in petunia and tobacco [2]. Intro-duction of the sense CHS gene [3] or sense DFR gene [4] caused the production of transgenic petu-nia plants with reduced flower color pigmentation. Introduction of the sense CHS gene in chrysanthe-mum caused the production of transgenic plants whose flowers changed from pink to white [5]. Introduction of the antisense CHS gene in gerbera caused the production of transgenic plants whose flowers changed from red to pink [6], and in lisianthus, produced transgenic plants whose flow-ers changed from purple to white [7].
Torenia fournieri Lind. (family
Scrophulari-aceae), commonly known as torenia, is the most important species in the genus for ornamental use. We introduced the DFR or CHS gene into torenia in sense or antisense orientation to modify flower color.
* Corresponding author. Fax: +81-59-268-1339.
E-mail address:[email protected] (R. Aida)
R.Aida et al./Plant Science153 (2000) 33 – 42 34
2. Materials and methods
2.1. Vectors
The full length cDNA encoding CHS (about 1.4 kb, AB012923), isolated from a torenia cv. ‘sum-merwave’ and fused with E12 V promoter [8] in
sense (pBETC6) or antisense (pBETC7) orienta-tion, was inserted into a binary vector plasmid pBinl9 [9] (Fig. 1(A)). A fragment of cDNA en-coding DFR (about 1.1 kb; lacking 5% side of the coding region, AB012924), isolated from the ‘sum-merwave’ and fused with E12V promoter in sense (pBETD10) or antisense (pBETD11) orientation, was also inserted into binary vector plasmid pBinl9 (Fig. 1(B)).
2.2. Plant materials and transformation
The experiments used 3 clonal laboratory lines: (1) a violet line selected from ‘crown mix’ (crown violet); (2) a reddish-purple line selected from ‘crown mix’ (crown reddish-purple); and (3) a violet line selected from ‘common violet’ (common violet). Transgenic plants were obtained by the
Agrobacterium-mediated transformation system
described previously [10]. Transformants were grown in vitro until flowering. Plants whose flower color was considered to have changed were trans-ferred to a closed greenhouse for further investiga-tion.
2.3. Southern blot analysis and northern blot
analysis
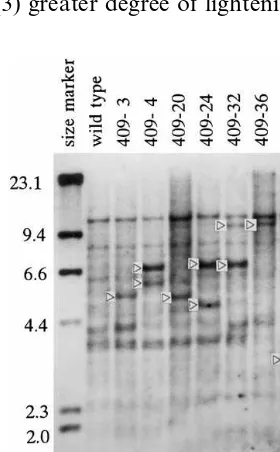
Seven putative transgenic plants transformed with pBETC6 derived from the crown reddish-purple line were used for Southern blot analysis. Total DNA was isolated as follows: leaf tissue was homogenized in a homogenization buffer contain-ing 15% sucrose, 50 mM EDTA, 250 mM NaCI, 50 mM Tris – HCI (pH 8.0) and 0.1% 2-mercap-toethanol. After centrifugation, DNA was ex-tracted from the pellet with ISOPLANT (Nippon
Gene, Toyama, Japan). About 10 mg of DNA
digested with HindIII was electrophoresed in a 0.6% agarose gel and transferred to a nylon mem-brane. HindIII cuts the plasmid pBETC6 at a single site outside the coding region of the CHS gene. The cloned region of the CHS gene was used as a probe. Blots were finally washed with 0.5×
SSC, 0.1% SDS, at 68°C.
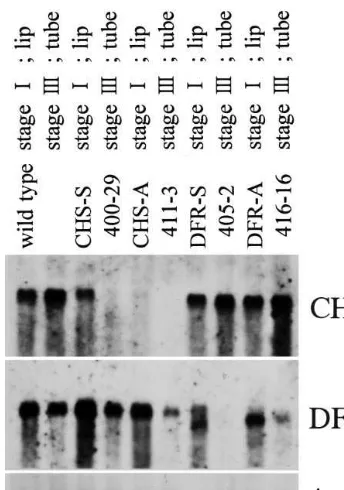
For northern blot analysis, we selected four transformants derived from the crown violet line as follows: a plant harboring sense CHS transge-ne(s): 400-29; a plant harboring antisense CHS transgene(s): 411-3; a plant harboring sense DFR transgene(s): 405-2; and a plant harboring anti-sense DFR transgene(s): 416-16. Each of those
Fig. 1. (A) The structure of the binary vectors pBETC6 and pBETC7. The full length cDNA encoding the torenia CHS gene (about 1.4 kb) was inserted into pBinl9 [9] with sense (pBETC6) or antisense (pBETC7) orientation. (B) The struc-ture of the binary vectors pBETD10 and pBETD11. A frag-ment of cDNA encoding the torenia DFR gene (about 1.1 kb; lacking 5%side of the coding region) was inserted into pBinl9
Table 1
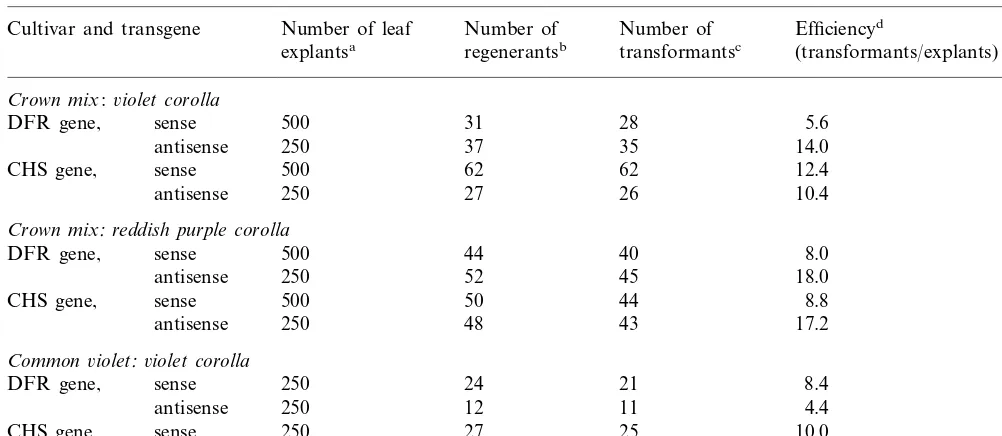
Transformation efficiency in torenia with vectors harboring a transgene related to flavonoid biosynthesis
Efficiencyd
Cultivar and transgene Number of leaf Number of Number of
explantsa regenerantsb transformantsc (transformants/explants) (%)
Crown mix:6iolet corolla
500 31 28 5.6
DFR gene, sense
250 37
antisense 35 14.0
500 62 62
CHS gene, sense 12.4
250 27 26
antisense 10.4
Crown mix:reddish purple corolla
500 44
antisense 48 43 17.2
Common6iolet:6iolet corolla
DFR gene, sense 250 24 21 8.4
250 12
antisense 11 4.4
250 27
CHS gene, sense 25 10.0
250 18
antisense 16 6.4
aLeaf explants were infected withAgrobacteriumand co-cultured for 7 days on a medium containing 0.5 mg/l BA, 0.1 mg/l IAA
and 100mM acetosyringon. After co-culturing, they were further cultured on a selection medium containing 1.0 mg/l BA, 100 mg/l carbenicillin and 300 mg/l kanamycin.
bRegenerated shoots were collected 13 weeks afterAgrobacteriuminfection. Only a single shoot was collected from each callus
to obtain independent transformants.
cTransformation was confirmed by leaf tests. dNumber of transformants/explants.
four plants showed typical modification pattern among its group. Total RNA was extracted from the flowers excluding sepals during the period of lip coloring (stage I; about 9 days before flower-ing) or during the period of tube coloring (stage III; about 3 days before flowering) with ISOGEN (Nippon Gene) according to the manufacturer’s directions. The RNA was electrophoresed and transferred to a nylon membrane. The cloned re-gion of the CHS or DFR gene was used as a probe. An actin probe was used to estimate the load of RNA. Blots were finally washed with 0.1×SSC, 0.1% SDS at 68°C.
Southern and northern blotting was performed using a DlG-High Prime and DIG Luminescent Detection Kit for nucleic acids (Boehringer, Mannheim, Germany).
2.4. E6aluation of flower color and measurement
of anthocyanin contents
Flower color of all the transformants was ob-served by eye. We measured anthocyanin contents in the lips and tubes of corollas of some transfor-mants. The experiments used plants derived from
the crown violet line as follows: plants harboring sense CHS transgene(s) (CHS-S): 387-2, 387-15, 400-2, 400-26, 400-27; plants harboring antisense CHS transgene(s) (CHS-A): 411-3, 411-9, 411-10, 411-18, 411-22; plants harboring sense DFR trans-gene(s) (DFR-S): 405-2, 405-14; and plants har-boring antisense DFR transgene(s) (DFR-A): 416-8, 416-16, 416-18, 416-20, 416-34. The corolla was divided into lip and tube parts, and an-thocyanins were extracted from each part with 1% hydrochloric acid in methanol. Concentrations were determined by absorbance measurements at 530 nm.
3. Results
R.Aida et al./Plant Science153 (2000) 33 – 42 36
contaminating plasmid in the tissues should be detected by the presence of a single 14.1 kb band. The plants showed 1 or 2 extra bands of differing sizes, indicating single- or multiple-copy integra-tion of the CHS transgene into the genome. We considered that the extra bands represent the exis-tence of transgene(s). The intensity of the extra bands was varied even in a same line. It is not clear why the intensity is not equal. Some transge-nes might be tandem-repeat structures that make strong signal, or some transgene might be partial structures because of incomplete integration that makes a weak signal.
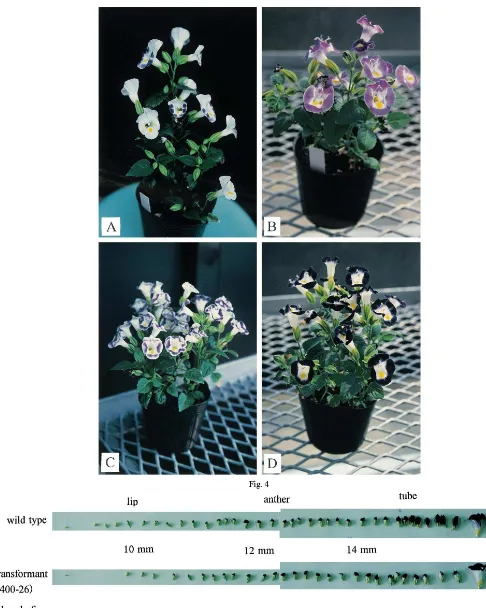
Photographs of corollas from crown violet transformants are shown in Fig. 3. Some transfor-mants had a pastel flower color (e.g. 411-1, 411-3, 411-9, 416-18 and 416-20); one had a wavy pattern on the flower lip (411-7); another had parti-col-ored flowers (403-20, Fig. 4(A)). These patterns do not exist in normal cultivars.
Flower color of the transformants was classified by eye into three groups: (1) same color as the wild-type plant; (2) whole corolla changed to a uniformly light color (e.g. 411-1, 411-3 and 416-20); and (3) greater degree of lightening of color in
the tube than in the lip, creating strong contrast between colored and white parts of the corolla (e.g. 387-2, 400-26 and 405-2). Relationships be-tween the plant lines/transgenes and type of flower color change are shown on Table 2. The color changed in most combinations at a rate of 18 – 89%. Transformants harboring antisense transge-ne(s) tended to become group 2 types (0 – 39% of plants harboring DFR transgene(s), and 56 – 89% of plants harboring CHS transgene(s); no plants became group 3 type. However, transformants harboring sense transgene(s) tended to become group 3 types (18 – 33% of plants harboring DFR transgene(s), and 32 – 46% of plants harboring CHS transgene(s); fewer became group 2 types (from 0 to 18% of plants harboring DFR transge-ne(s), and 2 – 20% of plants harboring CHS trans-gene(s). These results show that each sense and antisense transgene has a unique potential for changing flower color.
Northern blot analysis revealed that transfor-mants harboring the CHS transgene showed re-duced CHS mRNA level and normal DFR mRNA level; transformants harboring the DFR transgene showed reduced DFR mRNA level and normal CHS mRNA level (Fig. 5). Thus, the CHS gene and the DFR gene were independently inacti-vated in the torenia transformants. The manner of reduction of the mRNA level was different be-tween a plant harboring sense transgene and a plant harboring antisense transgene. The transfor-mants harboring sense transgenes had less mRNA at the stage for coloring tube (stage III; about 3 days before flowering) than at the stage for color-ing lip (stage I; about 9 days before flowercolor-ing). The mRNA was hardly detected at the stage III on transformants harboring sense transgenes. On the contrary, the transformants harboring anti-sense CHS transgenes had little mRNA at both stages and the transformants harboring antisense DFR transgenes had some amount of mRNA at both stages. There were additional DFR mRNA bands at a lower molecular weight than the con-trol on the transformants harboring DFR transge-nes. The additional bands might be produced from transgenes because the DFR transgene lacked 5% side of the coding region.
Anthocyanin contents in lip and tube parts of corollas of the transformants derived from the crown violet line are shown in Fig. 6. In plants harboring either DFR or CHS sense transgene(s),
Fig. 2. Southern blot analysis of the CHS gene in the torenia plants transformed with pBETC6. Total DNA digested with
Aida
et
al
.
/
Plant
Science
153
(2000)
33
–
42
37
R.Aida et al./Plant Science153 (2000) 33 – 42 38
Fig. 4. (A) Parti-colored flowers on a transformant (403-20) harboring the sense CHS transgene derived from the Common violet line. (B – D) Uniformly colored flowers on transformants 411-3, 411-7 (harboring the antisense CHS transgene derived from the Crown violet), and 405-2 (harboring the sense DFR transgene derived from the Crown violet), respectively.
Table 2
Patterns of flower color observed within transgenic torenia plants
Patterns of flower colorb
Number of transgenic plantsa
Cultivar and transgene
(number of plants (%)c)
1 2 3
Crown mix:6iolet corolla
24 17(71) 0(0) 7(29)
DFR gene, sense
28 17(71)
antisense 11(25) 0(0)
CHS gene, sense 47 31(61) 1(2) 15(32)
17 2(12) 15(88)
antisense 0(0)
Crown mix:reddish purple corolla
33 16(48)
antisense 41 18(44) 23(56) 0(0)
Common6iolet:6iolet corolla
11
DFR gene, sense 9(82) 0(0) 2(18)
4 4(100)
antisense 0(0) 0(0)
15 8(53)
sense 1(7)
CHS gene, 6(40)
antisense 9 1(11) 8(89) 0(0)
aNumber of transgenic plants in which flower color has been evaluated.
b(1) Same color as the wild-type plant, (2) whole corolla changed to a uniformly light color, (3) greater degree of lightening
of color in the tube than in the lip, creating strong contrast between colored and white parts of the corolla.
cPercentages rounded off to whole numbers.
there was greater reduction of anthocyanin con-tent in the tube than in the lip. In plants harboring antisense CHS transgene(s), the degree of reduc-tion was almost the same in the tube and lip. In plants harboring antisense DFR transgene(s), there was greater reduction in the lip than in the tube. Thus, flower color change was associated with reduction of anthocyanin content.
4. Discussion
The inactivating mechanisms are thought to be different for the two gene types. Antisense genes produce complementary RNA to the target RNA, and binding of the two RNAs inhibits protein production [11]. While there are many examples of gene suppression by sense genes (i.e. sense sup-pression or homology-dependent gene silencing), the mechanism of the suppression is not yet clear [12 – 14].
There are many reports on modification of flower color using these techniques. The antisense CHS gene has been used to suppress formation of flower pigments in petunia and tobacco [2], ger-bera [6], and lisianthus [7]. The sense CHS gene
has been used to reduce flower pigments in petunia [3] and chrysanthemum [5], as has the sense DFR gene to reduce flower pigments in petunia [4]. Many transformants have been obtained and ex-amined in petunia, but few in other plant species. We obtained 76 phenotypically altered sense-intro-duced plants and 64 phenotypically altered anti-sense-introduced plants of torenia, matching the number of petunia transformations.
In this report, all the torenia transformants that were considered to have modified flower color had lighter color than that of wild-type plants. The mRNA levels of the CHS or DFR gene were reduced (Fig. 5), and anthocyanin contents of the corolla were also reduced (Fig. 6). These results suggest that the modification of flower color was caused by gene suppression and resultant reduc-tion of flower pigments. Further investigareduc-tions would be clarify the reason for the flower color modification in the aspect of actual pigment composition.
R.Aida et al./Plant Science153 (2000) 33 – 42 40
Fig. 5. Northern blot analysis of the CHS and DFR gene in the torenia transformants. Total RNA was extracted from the flowers excluding sepals during the period of lip coloring (stage I; about 9 days before flowering) or during the period of tube coloring (stage III; about 3 days before flowering). The RNA was electrophoresed and transferred to a nylon membrane. The cloned region of the CHS or DFR gene was used as a probe. An actin probe was used to estimate the load of RNA. Blots were finally washed with 0.1×SSC, 0.1% SDS at 68°C. Transformants harboring the CHS transgene showed reduced CHS mRNA level and normal DFR mRNA level while transformants harboring the DFR transgene showed reduced DFR mRNA level and normal CHS mRNA level. The transformants harboring sense transgenes had less mRNA in stage III than in stage I.
to become group 3 types. These results suggest a common pattern in sense-gene-introduced plants, and another common pattern in antisense-gene-in-troduced plants, whether the inantisense-gene-in-troduced gene was CHS or DFR. The sense and antisense genes seem to have different potential for changing the flower color. Breeding of flower color by genetic transfor-mation would be more effective by proper use of the sense and antisense genes.
Jorgensen et al. [15] compared the modification patterns of flower color between sense- and anti-sense-gene-introduced petunia plants in transfor-mants harboring the CHS transgene. They re-ported that the sense gene produced several patterns (e.g. junction and Cossack dancer) that the antisense gene did not, and that the tube was not affected in antisense-gene-introduced plants. Our results on torenia demonstrate similar pheno-typic changes: torenia transformants harboring the sense gene produced high contrast corollas (group 3) that were not observed in antisense-gene-intro-duced plants, and the tube had a greater tendency to become lighter in color with the sense transgene than with the antisense transgene. However, some torenia transformants had high contrast corollas and there was no petunia-like white sector pattern (junction or Cossack dancer type). Nevertheless, it seems that transgene-mediated modification of flower color in both torenia and petunia are caused, at least in part, by the same gene-silencing mechanisms.
Petunia plants that harbor CHS transgene(s) often show clearly differently-colored flowers on
Fig. 8. Model for gene regulation through flower development in torenia transformants. I: the lip coloring period; II: the anther coloring period; III: the tube coloring period.
whose transcriptional rates might be higher than that of wild-type plants, may exceed a threshold level during flower development, causing silencing of the gene and resultant reduction of flower pig-ments (Fig. 8). In antisense-gene-introduced plants, in which the degree of color lightening was similar for the whole corolla, the reduced level of flower pigment production may have been rela-tively constant throughout the flower develop-ment.
We have produced novel flower colors in tore-nia, for example, pastel, wavy patterned and parti-colored flowers. Genetic transformation is a useful method for breeding torenia flower color. In the future, breeding flower color by genetic transfor-mation will be even more effective through pro-gress in studies on construction of and synthetic pathways of flower pigments.
We expect that this experimental system will be a good model for analysis of gene expression, because examination can be performed by eye. There are many studies on gene expression in petunia plants harboring CHS or DFR transgenes because of the advantages mentioned above [15,18 – 22]. Torenia would also useful as an exper-imental plant for such studies, because it is easy to transform and it is taxonomically different from petunia. Another advantage of torenia is that it can easily flower in vitro [23], making experimen-tation more efficient.
Acknowledgements
We thank Kazuko Nakagawa and Kumiko Bessho for their skilful assistance. This research was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan, within the framework of the Pioneer Research Project.
References
[1] J.M. Kooter, J.N.M. Mol, Trans-inactivation of gene expression in plants, Curr. Opin. Biotechnol. 4 (1993) 166 – 171.
[2] A.R. van der Krol, P.E. Lenting, J. Veenstra, I.M. van der Meer, R.E. Koes, A.G.M. Gerats, J.N.M. Mol, A.R. Stuitje, An anti-sense chalcone synthase gene in trans-genic plants inhibits flower pigmentation, Nature 333 (1988) 866 – 869.
the same plant [16]. We obtained only a single transformant that showed such a phenotype, i.e. parti-colored flowers (403-20; Fig. 4(A)). All the other plants seemed to show flowers similar in color on the whole plant (e.g. 411-3, 411-7 and 405-2; Fig. 4(B – D)). We do not know the reasons for this difference. Torenia flowers are uniformly colored even in transformants, and this is one of the advantages of torenia for analysis of gene expression mechanisms using this experimental system; many identically conditioned flowers can be obtained. We are investigating the stability of the modified flower color in the following genera-tion now.
There were many sense-gene-introduced plants in which the degree of color lightening of the flower was higher in the tube than in the lip. In these plants, there was greater reduction of an-thocyanin content in the tube than in the lip (Fig. 6). The coloring pattern of the torenia corolla is developmentally regulated: the lip, anther and tube of the corolla become colored about 9, 6 and 3 days before flowering, respectively (Fig. 7).
R.Aida et al./Plant Science153 (2000) 33 – 42 42
[3] C. Napoli, C. Lemieux, R. Jorgensen, Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans, Plant Cell 2 (1990) 279 – 289.
[4] A.R. van der Krol, L.A. Mur, M. Beld, J.N.M. Mol, A.R. Stuitje, Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression, Plant Cell 2 (1990) 291 – 299. [5] N. Couryney-Gutterson, C. Napoli, C. Lemieux, A.
Morgan, E. Firoozabady, K.E.P. Robinson, Modifica-tion of flower color in florist’s chrysanthemum: produc-tion of a white-flowering variety through molecular genetics, Biotechnology 12 (1994) 268 – 271.
[6] P. Elomaa, J. Honkanen, R. Puska, P. Seppanen, Y. Helariutta, M. Mehto, M. Kotilainen, L. Nevalainen, T.H. Teeri,Agrobacterium-mediated transfer of antisense chalcone synthase cDNA to Gerbera hybrida inhibits flower pigmentation, Biotechnology 11 (1993) 508 – 511. [7] S. Deroles, M. Bradley, K. Davies, K. Schwinn, D.
Manson, Generation of novel patterns in lisianthus flow-ers using an antisense chalcone synthase gene, Acta Hortic. 420 (1995) 26 – 28.
[8] I. Mitsuhara, M. Ugaki, H. Hirochika, M. Ohshima, T. Murakami, Y. Gotoh, Y. Katayose, S. Nakamura, R. Honkura, S. Nishimiya, K. Ueno, A. Mochizuki, H. Tanimoto, H. Tsugawa, Y. Otsuki, Y. Ohashi, Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants, Plant Cell Physiol. 37 (1996) 49 – 59.
[9] M. Bevan, BinaryAgrobacteriumvectors for plant trans-formation, Nucl. Acids Res. 12 (1984) 8711 – 8721. [10] R. Aida, M. Shibata, Transformation in torenia, Y.P.S.
Bajaj (Ed.), Biotechnology in Agriculture and Forestry, Springer Verlag, in press.
[11] P.J. Green, O. Pines, M. Inoue, The role of antisense RNA in gene regulation, Ann. Rev. Biochem. 55 (1986) 569 – 597.
[12] M.A. Matzke, A.J.M. Matzke, How and why do plants inactivate homologous (trans)genes?, Plant Physiol. 107 (1995) 679 – 685.
[13] P. Meyer, H. Saedler, Homology-dependent gene
silenc-ing in plants, Ann. Rev. Plant Physiol. Plant Mol. Biol. 47 (1996) 23 – 48.
[14] M. Stam, J.N.M. Mol, J.M. Kooter, The silence of genes in transgenic plants, Ann. Bot. 79 (1997) 3 – 12. [15] R.A. Jorgensen, P.D. Cluster, J. English, Q. Que, C.A.
Napoli, Chalcone synthase cosuppression phenotypes in petunia flowers: comparison of sense versus antisense constructs and single-copy versus complex T-DNA, Plant Mol. Biol. 31 (1996) 957 – 973.
[16] R.A. Jorgensen, Cosuppression, flower color patterns, and metastable gene expression states, Science 268 (1995) 686 – 691.
[17] H.A. Smith, S.L. Swaney, T.D. Parks, E.A. Wernsman, W.G. Doughety, Transgenic plant virus resistance medi-ated by untranslatable sense RNAs: expression, regula-tion, and fate of nonessential RNAs, Plant Cell 9 (1994) 1441 – 1453.
[18] R. van Blokland, N. van der Geest, J.N.M. Mol, J.M. Kooter, Transgene-mediated suppression of chalcone synthase expression in Petunia hybrida results from an increase in RNA turnover, Plant J. 6 (1994) 861 – 877. [19] P. Elomaa, Y. Helariutta, R.J. Griesbach, M. Kotilainen,
P. Seppanen, T.H. Teeri, Transgene inactivation inPetu
-nia hybridais influenced by the properties of the foreign gene, Mol. Gen. Genet. 248 (1995) 649 – 656.
[20] M. Metzlaff, M. O’Dell, P.D. Cluster, R.B. Flavell, RNA-mediated RNA degradation and chalcone synthase A silencing in petunia, Cell 88 (1997) 845 – 854.
[21] M. Stam, R. de Bruin, S. Kenter, R.A.L. van der Hoorn, R. van Blokland, J.N.M. Mol, J.M. Kooter, Post-tran-scriptional silencing of chalcone synthase in Petunia by inverted transgene repeats, Plant J. 12 (1997) 63 – 82. [22] Q. Que, H.-Y. Wang, J.J. English, R.A. Jorgensen, The
frequency and degree of cosuppression by sense chalcone synthase transgenes are dependent on transgene pro-moter strength and are reduced by premature nonsense codons in the transgene coding sequence, Plant Cell 9 (1997) 1357 – 1368.
[23] S. Tanimoto, H. Harada, Wishbone flower (chapter 31), in: P.V. Ammirato, D.A. Evans, W.R. Sharp, Y.P.S. Bajaj (Eds.), Handbook of Plant Cell Culture, Vol. 5 Ornamental Species, McGraw-Hill, New York, 1990, pp. 763 – 782.
.
![Fig. 1. (A) The structure of the binary vectors pBETC6 andpBETC7. The full length cDNA encoding the torenia CHSpromoter [8]; NPTII equals the coding region of neomycinphosphotransferaseII gene; B, E, H, X andwith sense (pBETD10) or antisense (pBETD11) orie](https://thumb-ap.123doks.com/thumbv2/123dok/1035001.928959/2.612.75.242.149.309/structure-vectors-andpbetc-encoding-torenia-chspromoter-neomycinphosphotransferaseii-antisense.webp)