Summary Small Shorea stenoptera Burck. (Dipterocar-paceae) trees of reproductive age growing in an arboretum in west Java were studied to determine the pattern of vegetative shoot development, the time and method of floral initiation and the effect of paclobutrazol on floral enhancement. Vegetative buds were enclosed by two stipules between which was a leaf primordium, a small axillary vegetative bud and another pair of stipules. This sequence was reiterated five to seven times before the vegetative apex was visible. At the time of floral initiation, axillary buds developed into floral spikes and com-pound inflorescences formed at the end of drooping branches. A compound inflorescence might bear many floral spikes and each floral spike bore many flowers. The compound inflores-cence was a modification of the reiterative developmental pattern observed in vegetative shoots. The time of floral initia-tion began in late June or early July and continued until about November. Floral enhancement using paclobutrazol as a soil drench was attempted in mid-July, but this was later found to be after the onset of floral initiation, and the treatment failed to enhance flowering; however, it appeared to enhance the rate of floral and fruit development. The similarity in vegetative bud development among dipterocarp genera suggests that the time of floral initiation may be easily determined in many species based on simple dissection techniques.
Keywords: dipterocarp, floral enhancement, shoot develop-ment.
Introduction
The family Dipterocarpaceae may account for 10% of all tree species and 80% of all emergent trees in tropical rain forests (Ashton 1982). Dipterocarps occur in both aseasonal lowland rain forests where they are evergreen, and in seasonal upland or more xerophytic forests where they are deciduous (Smiti-nand et al. 1980).
A striking feature of many dipterocarp forests is the phe-nomenon of mass flowering followed by mass fruiting (Ashton et al. 1988). Mass flowering occurs at intervals of two to 10 years, over a period of a few weeks to a few months in either small areas or large regions such as the Malaysian Peninsula.
Large dipterocarps may produce up to four million flowers and 120,000 fruits (Ashton 1988). Although the fruits are poorly protected and are eaten in vast numbers by wild pigs and heavily parasitized by weevils (Chan 1980, Ashton 1988), the forest floor following a mass fruiting is often blanketed by dipterocarp seedlings. In some regions, reforestation is achieved by lifting and transplanting the wild seedlings.
Managing dipterocarps for seed production is difficult. First flowering commonly does not occur until trees are 20 to 30 years old (Ng 1966), by which time most are too large for easy management or seed harvest. Fruit production is episodic, and because seeds of most species are recalcitrant and store poorly (Sasaki 1980), there can be long intervals during which seed for reforestation is unavailable. Although flowers have three locules and two ovules per locule, normally only one indehis-cent seed develops per fruit. To overcome these obstacles, methods are needed to induce flowering at regular intervals in relatively small trees.
Effective control of flowering, requires knowledge of the time of floral initiation and the development of methods of floral induction or enhancement (Owens and Blake 1985). The time of natural floral initiation in dipterocarps has never been determined developmentally or experimentally. Dipterocarps show episodic shoot growth with distinct periodic vegetative flushes, followed by periods of shoot elongation. Flushes com-monly precede or coincide with flowering, and inflorescences tend to be borne near the ends of shoots (Burgess 1972). Stimuli that induce flushing and flowering in dipterocarps are not known. Ashton et al. (1988) proposed that flowering is controlled by an endogenous rhythm, perhaps related to epi-sodic shoot flushes and triggered by an external cue. Wycher-ley (1973) suggested that temperature may be responsible for floral induction in dipterocarps. Ashton et al. (1988) found no close correlation between flowering intensity of Shorea spe-cies and photoperiod or water availability, and proposed that exposure to a 2 °C decrease in minimum nighttime tempera-ture for several nights may be sufficient to induce flowering in some dipterocarps.
Increased temperature and drought, usually in conjunction with gibberellin A4/7 application, are common cultural
treat-ments applied to induce cones in Pinaceae (Owens and Blake
Time and method of floral initiation and effect of paclobutrazol on
flower and fruit development in Shorea stenoptera (Dipterocarpaceae)
DIDA SYAMSUWIDA
1and JOHN N. OWENS
21
Forest Tree Improvement Research and Development Institute, Jl. A.M. Sangaji Km. 15, Purwobinangun, Pakem, Sleman, DI Yogyakarta 55582, Indonesia
2
Centre for Forest Biology, University of Victoria, Victoria, B.C. V8W 2Y2, Canada
Received January 11, 1996
1985). The mode of action of these treatments, however, has yet to be demonstrated. One hypothesis is that these cultural treatments reduce vegetative growth, and the resulting excess gibberellins promote flowering (Pharis 1976). Gibberellins have not been found to enhance flowering in hardwood forest trees. However, flowering in several temperate fruit trees (Lever 1986, Steffens et al. 1993), clove (Martin and Dabek 1988) and Eucalyptus (Griffin et al. 1993) is enhanced when the growth retardant paclobutrazol is applied as a foliar spray, stem injection or soil drench.
The objectives of this study were to determine the time and method of floral initiation and to investigate the use of paclobutrazol as a method of enhancing flowering in young, reproductively mature Shorea stenoptera Burck. trees.
Materials and methods
In June 1994, 27 reproductively mature S. stenoptera trees that had previously flowered were selected from a stand of over 100 trees at the Kebun Percorbaan Arboretum in west Java, Indo-nesia (lat. 6° N, long. 106° E). Trees were grouped into three categories, with nine trees in each group, on the basis of circumference at breast height: Group I, 51--55 cm; Group II, 46--50 cm; Group III, 41--45 cm. Three trees of each size class were randomly assigned to each of three paclobutrazol treat-ments: A, high concentration; B, low concentration; and C, control.
Floral initiation
From mid-July through October, branches were sampled from several trees every month. The terminal bud on each branch was removed and placed in a vial with formalin/acetic acid/al-cohol (FAA) fixative (Johansen 1950). Buds were stored in FAA and taken to the University of Victoria where a central longitudinal section 1--2 mm was embedded in ‘‘Tissuemat’’ (Fisher Scientific, Ottawa, Canada) using the tertiary-butyl alcohol dehydration series of Johansen (1950). Embedded specimens were softened in Gifford’s softening reagent (Gif-ford 1950) for 2 to 4 weeks and then serially sectioned longi-tudinally in increments of 6 µm. The median sections were mounted on microscope slides and stained with safranin and fast green and examined to determine whether they were vege-tative or reproductive.
Other buds were dissected by removing successive stipules until the terminal bud or apex and the axillary vegetative buds or inflorescence primordia were visible. The shoot tips, or portions of them, were dehydrated in an ethanol series, critical point dried, mounted on aluminum stubs, sputter-coated with gold and observed with a JOEL JSM-35 scanning electron microscope (SEM) (JOEL, Boston, MA) at 10 kV. Other buds were dissected, sketched and photographed with a Wild M 400 Photomacroscope (Leica Canada Inc., Willowdale, Canada) to show bud morphology. In all three types of specimen observa-tion, vegetative buds and buds that had undergone floral initia-tion and various stages of floral development were observed and photographed.
Floral enhancement
In mid-July, sample trees were treated once with one of three paclobutrazol treatments. A shallow circular trench (about 10-cm-deep) was dug at a radius of 1 m from the base of each tree. Paclobutrazol in the water soluble form ‘‘Cultar’’ was obtained from ICI (Indonesian Operations, Jakarta). Concen-trations and application methods were based on successful experiments with Eucalyptus (Griffin et al. 1993, Moncur et al. 1994). Paclobutrazol treatments were either high concentra-tion (1.0 g of paclobutrazol per 15 cm of trunk circumference), low concentration (0.5 g of paclobutrazol per 15 cm of trunk circumference) or a control (water only). The appropriate amount of paclobutrazol was added to 4 liters of water, which was then mixed and poured into the trench around each tree.
Once per month in September, November, December and January 1994, trees were climbed and the numbers of inflores-cences with flowers and with developing fruits counted. The number of flowering compound inflorescences and number of fruiting compound inflorescences were counted on each obser-vation date. Numbers of flowers or fruits per compound inflo-rescence or per spike were not counted. Because trees varied in size, numbers of branches and sites in which inflorescences formed and the number of primary branches per tree were counted to determine the percentage of branches bearing inflo-rescences.
To observe any treatment carry-over effect, trees were as-sessed for frequency of flowering in November 1995, i.e., the year following paclobutrazol treatment.
Average rainfall per month was obtained from the weather station at the study site.
Figures 1--8. Vegetative and reproductive shoots and buds and fruits of
Shorea stenoptera.
Figure 1. Vegetative branch of mature tree showing red stipules (S).
Figure 2. Close-up of vegetative branch showing stipules and emerg-ing leaf (L).
Figure 3. Partially dissected vegetative bud with outer stipule removed to reveal the leaf primordium, axillary buds (A) and small green stipule inside.
Figure 4a. Vegetative bud with one stipule removed to show leaf primordium and stipule within and axillary bud at the onset of floral initiation. Figure 4b. Compound inflorescence with outer stipule re-moved to show enlarged axillary inflorescence (AI).
Figure 5. Later stage of a compound inflorescence with two stipules removed to show two stages of axillary inflorescence development.
Figure 6. Compound inflorescence during flowering showing red stipules at the base of each spike (SP), and developing fruits ( ).
Figure 7. Distal portion of a compound inflorescence showing several spikes. Most flowers (F) have been shed and one is aborting ( ) leaving the subtending bracts (B). Most stipules have been shed.
Statistical analysis
Numbers of inflorescences bearing flowers and fruits were analyzed separately, or summed for each date and analyzed by a balanced two-factor analysis of variance (ANOVA) design with size class and paclobutrazol dosage as fixed effects. Because size class did not influence the number of branches bearing flower or fruit inflorescences (α = 0.05), all size classes were pooled and subjected to a single-factor ANOVA for each date. Significant effects were further analyzed with the Duncan’s multiple range test (Zar 1984).
Observations and results
Shorea stenoptera formed pendant compound infloresences at the ends of long, often drooping branches in younger trees (Figure 1), and on shorter secondary and tertiary branches in older trees. The inflorescences, which were initiated and un-derwent early development within the terminal, partially fused, protective, stipules on the branch (Figures 2 and 3), commonly had seven to 15 floral spikes (Figure 6). Spikes were spirally arranged but appeared alternate, and were sepa-rated by long internodes (Figure 7). Spikes matured acropet-ally on the branch and flowers matured acropetacropet-ally on each spike (Figure 6). Each spike was enclosed by two stipules during early floral development (Figures 4 and 5), and bore one to usually many spirally arranged flowers (Figure 7), each on a short pedicel. Each flower had two opposite subtending bracts. Most flowers abscised at the base of the pedicel, leaving the two bracts that usually abscised a few days later (Figure 7). Rarely did more than one fruit develop per spike. Fruits rapidly enlarged, and the five sepals elongated, enclosing the globose fruit at their base, and forming three large and two small, broad flat wings. Spikes were pendant and the wings hung downward (Figure 8).
Vegetative bud development
Development within the vegetative bud provided a remarkable example of reiteration. Each bud was enclosed by two large
spoon-shaped red stipules, each of which attached around half of the stem and the base of a leaf. The two stipules adhered along their margins. Between the pair of stipules was a leaf, an axillary bud and another pair of smaller green stipules (Figures 2, 3 and 4a). Within these stipules was, again, a leaf, an axillary bud and a yet smaller pair of stipules. These structures were often reiterated five to seven times (Figure 9) as revealed by dissection (Figures 3, 4 and 10) and SEM, before the shoot apical meristem was visible (Figure 11). The inner and, to a lesser extent, the outer surfaces of the young stipules were covered by long epidermal hairs (Figure 11) that stuck to the leaf primordia and other stipules.
The apical meristem was a low dome with an oval cross section, from which successive leaf primordia arose at oppo-site ends (Figure 12). Soon after a leaf primordium was initi-ated, two ridges of meristematic tissue arose at the primordium base along the sides of the oval (Figure 12). These ridges extended around the apical meristem and base of the leaf primordium, and elongated upward to form two opposite stipule primordia (Figure 13). As the stipule primordia elon-gated, they became spoon-shaped and contacted one another along their margins. Epidermal hairs formed on the stipules, causing them to stick together along most of their margins, completely enclosing the leaf primordium and apical meristem within (Figure 11). As the leaf primordium elongated and a leaf blade began to form, a lateral bud primordium arose in the axil of the leaf primordium (Figure 12). By this time, the next leaf primordium and its stipules had been initiated (Figures 12--14).
Axillary buds on most vegetative shoots remained small. The axillary apex initiated two scales (Figure 12) that elon-gated and enclosed the apex (Figure 11). Development may be arrested at this stage (Figure 15) or the apical meristem may initiate leaf primordia and stipules. In the smaller trees, the primary branches generally lacked secondary branches so ax-illary buds appeared to become latent (Figure 15). In larger trees, secondary and tertiary branches formed from the axillary buds.
Figure 9. Diagram of a Shorea
stenoptera vegetative bud with
Floral initiation and development
Inflorescence initiation was observed in some buds collected in July and in all subsequent collections through November. At inflorescence initiation, the vegetative apex underwent transi-tion to an inflorescence apex. The terminal inflorescence apex was dome-shaped and initiated a spiral sequence of stem units consisting of a leaf primordium, two stipules and an axillary apex (Figure 16). Development of the components of the stem units differed depending on the time of inflorescence initia-tion, with leaf primordia being smaller or absent in stem units initiated later, whereas stipules and internodes were longer and
axillary buds enlarged more rapidly (Figures 16--18). All axil-lary buds initiated in the inflorescence developed into floral spikes (Figure 18).
An axillary bud that developed into a floral spike was en-closed by two opposite stipules (Figures 4, 5 and 19). A leaf primordium was present in the first few stem units, which were reproductive (Figures 4 and 5), but was absent in subsequent more distal stem units (Figures 6 and 7). Spike primordia elongated and initiated a series of lateral apices. Each lateral apex initiated two large bract primordia (Figures 19--21), then five sepals that remain fused at the base (Figures 20--22), five
Figures 10--15. Vegetative Shorea
stenoptera buds using light (LM)
and scanning electron microscopy (SEM).
Figure 10. Fixed and partially dis-sected bud viewed with a dissect-ing LM. Outer stipules were removed to show an inner stipule (S3), its leaf primordium (L3) and
a bud (A4) in the axil of each leaf.
Different subscripts indicate stages of development.
Figure 11. Enlarged SEM of a bud as in Figure 10. The stipules, leaves and axillary buds having the same subscript as in Figures 10 and 11 are at the same stage. The stipule (S2) has been removed
to reveal its leaf (L2), the apical
meristem (arrow) and the young-est leaf primordium (L1).
Figure 12. Enlarged SEM of the apical meristem ( ) and leaf pri-mordia (L1, L2, L3) and the
axil-lary bud (A3) as shown in Figure
11. Two stipules (S1) are those
that belong to L1. Outer stipules
(S2) have been removed. An
axil-lary bud (A2) has just begun to be
initiated in the axil of L2. Axillary
bud (A3) has two lateral scales
(ar-rowhead).
Figure 13. A later stage of stipule development to that shown in Fig-ure 12. Stipules (S1) for L1 have
enlarged covering the apex. The next leaf primordium (L0) is being
initiated.
Figure 14. Median longitudinal section of a vegetative bud as in Figure 13 showing the apical mer-istem ( ) leaf primordia (L1, L2)
and the axillary bud (A3) for leaf
three.
petals fused along most of their length forming a coronate flower bud (Figures 20--22), fifteen stamens (Figure 23) fused to the base of the petals, and finally, a central pistil with three locules and a single style. Proximal spikes were short with only one or few flowers, central spikes bore many flowers and distal spikes decreased in length and number of flowers acropetally
until the most distal spikes usually bore only one flower (Fig-ure 7). The inflorescence usually terminated in a single or pair of flowers (Figure 7). Occasionally, the inflorescence apex reverted back to a vegetative apex and resumed leaf initiation. This resulted in one or more floral spikes along an otherwise vegetative branch. Floral spikes had no arrested period of
Figure 21. SEM of newly initiated floral spike showing distal spike apex (arrowhead), paired bract primordia (one removed) and flower primordium showing five sepals and onset of petal initiation.
Figure 22. SEM of flower primordium at slightly later stage than in Figure 21, showing five sepal primordia ( ) and five petal primordia on star-shaped flower primordium.
Figure 23. SEM of a flower bud at latest stage shown in Figure 20 but with bracts, sepals and petals removed to show the 15 stamen primordia. Figures 16--23. Inflorescence de-velopment in Shorea stenoptera using light (LM) and scanning electron microscopy (SEM).
Figure 16. Fixed and partially dis-sected compound inflorescence bud viewed with a dissecting LM after outer stipules were removed to reveal inner stipule (S), leaf pri-mordia (L1--L4), large axillary
flo-ral spike buds (SP) and terminal inflorescence bud (arrowhead).
Figure 17. LM of median longitu-dinal section of a young floral spike in the axil of a leaf (L) showing bract (B) primordia. Ax-illary flower primordia are out of the plane of view or not yet initi-ated.
Figure 18. LM of median longitu-dinal section of an inflorescence bud showing floral spike primor-dia at various stages of develop-ment.
Figure 19. SEM of a floral spike primordium elongating beyond basal stipules showing bract pri-mordia with axillary flower api-ces (arrows) and the distal spike apex (arrowhead) starting to initi-ate more bracts.
development. Within an inflorescence, they developed, elon-gated and burst from the stipules in an acropetal manner over several weeks (Figure 6).
Floral enhancement
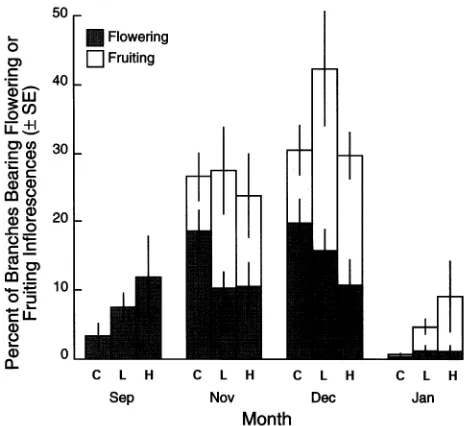
Dissections of buds collected in July showed that floral initia-tion had begun in some branches of some trees before the paclobutrazol treatments were applied in mid-July. In Septem-ber, nonfruiting inflorescences were visible, but there were no significant differences among treatments. No observations were made in October. In November, there were abundant flowering and fruiting inflorescences per tree. Statistical analyses showed that paclobutrazol concentration had a sig-nificant effect (P = 0.0463) on the number of branches bearing flowering inflorescences in November only. The Duncan’s multiple range test (α = 0.05) verified that paclobutrazol ap-peared to depress the number of branches bearing flowering inflorescences relative to the control treatment. However, the numbers of fruiting and flowering inflorescences combined were not influenced by paclobutrazol on any observation date. The treatment differences in November suggest that, although paclobutrazol did not influence the total number of flowering and fruiting inflorescences, it did hasten fruit development so that control trees had significantly more flowering inflores-cences but fewer fruiting infloresinflores-cences than paclobutrazol-treated trees. In January, there was a marked decrease in flowering and fruiting inflorescences because flowering had ceased and many inflorescences had shed their fruits (Fig-ure 24).
There was no carry-over effect of paclobutrazol in the 1995 flowering--fruiting season. Flowering was sparse and random among the control and treated trees.
Discussion
The development of vegetative buds and the initiation or devel-opment of inflorescences have not been described for any dipterocarp. Based on our observations of species of Diptero-carpus, Hopea, and Shorea, at least these major genera have very similar vegetative bud and shoot development. Shorea stenoptera vegetative buds were enclosed by two large, red, opposite, obtuse stipules, the bases of which encircled the stem just below the leaf axil. These stipules adhered along their margins, and opened as the leaf, shoot axis and reiterated terminal bud inside elongated. Five to seven reiterative buds were enclosed within the outermost stipules (Figure 9). Reit-eration is a well documented developmental pattern in many woody perennials, and is well represented in the above genera and easily observed in S. stenoptera. The predictability of this pattern of development means that the position of potential inflorescence primordia as axillary buds is easily seen and the time of floral initiation can be easily determined by dissecting terminal buds.
We found that shoot elongation and flowering in S. stenop-tera began during the dry season, June through September, but continued through the rainy season, December and January (Figure 25). This agrees with the results of van Schaik et al. (1993) who found that peaks of flushing and flowering in tropical forests occur preceding and during the early rainy period. Water availability is thought to be important in control-ling the phenologies of many tropical forest trees (Reich and Borchert 1984). Vegetative phenology, in turn, may affect floral initiation as it does in temperate trees (Owens and Blake 1985).
Floral initiation began in June or July and continued at least into December. Inflorescences were at all stages of develop-ment on a tree, and many stages of developdevelop-ment were apparent within an inflorescence. Therefore, a single time of floral initiation, as found in most temperate trees (Owens and Blake 1985), does not exist in S. stenoptera. As a result, it is difficult to determine particular climatic factors causing flowering. Several factors may be involved. Even in temperate trees, in which climate is seasonal and time of initiation precise, many climatic factors, including rainfall, temperature and light in-tensity, affect and interact to stimulate floral initiation. This commonly occurs during or after lateral branch elongation, the branches on which flowers are initiated (Owens and Blake 1995). Because floral initiation in S. stenoptera begins and predominantly occurs during the early rainy season, the pre-ceding drier period could be a factor. Vegetative growth, as shoot elongation, may be reduced during the dry season. Dur-ing the rainy season, floral initiation stops but flowerDur-ing con-tinues, though less abundantly. van Schaik et al. (1993) demonstrated major roles for irradiance and water stress in the control of phenology of tropical woody plants. Chaikiattiyos et al. (1994) describe how floral induction in tropical trees follows a check in vegetative growth, which normally occurs during the dry season. Ashton et al. (1988) considered drought to be an unlikely factor in floral initiation in dipterocarps; but, until shoot water potentials are measured, as has been done in some tropical fruit trees (Chaikiattiyos et al. 1994), drought
cannot be ruled out as a factor in floral initiation in diptero-carps. The dry season is also probably accompanied by in-creased periods of sunshine because of less cloudy conditions. Ng (1977) suggested that an increase of 2 h per day of sunshine could induce flowering in dipterocarps. Less cloud may also result in slight increases and decreases in day and night tem-perature, respectively.
Although we were unable to identify the mechanism by which environmental cues affect floral initiation in diptero-carps, we demonstrated that the time of floral initiation can be determined by bud dissection. Floral spikes arose from the axillary buds and the latter were always present within the reiterated buds. Also, the early axillary inflorescence buds were easily distinguished from axillary vegetative buds. Al-though bud dissection will reveal only after the fact that floral initiation has begun, this information is useful because floral initiation likely occurs at about the same time each year and over several months. Therefore, floral enhancement may be possible before or during the time when inflorescence buds become visible.
Paclobutrazol affected flowering and fruiting, but not in the manner we expected. In September, there was a trend toward increased flowering with increasing concentration of paclobu-trazol (Figure 24); however, this trend was not statistically significant. In November, control trees had more flowers than trees in either paclobutrazol treatment, but no significant treat-ment effects were found when numbers of flowering and fruiting inflorescences were combined. This, together with our developmental data, leads us to conclude that the application of paclobutrazol was either too late or at too low a concentra-tion to affect floral initiaconcentra-tion, but that it hastened floral and fruit development. This is consistent with effects of paclobutrazol on fruit trees where it acts as a growth retardant by suppressing gibberellin production, thereby reducing cell division and cell expansion (Dalziel and Lawrence 1984). Reduction in vegeta-tive growth allows greater partitioning of assimilates to repro-ductive growth, flower and fruit formation and fruit growth (Lever 1986). In apple (Quinlan and Richardson 1986), peach (Erez 1986), clove (Martin and Dabek 1988) and Eucalyptus (Griffin et al. 1993, Moncur et al. 1994), paclobutrazol reduced
vegetative growth and enhanced fruit production, but in all cases, the time of treatment was important. Our results together with those listed above indicate that paclobutrazol may be useful for enhancing fruit production in some dipterocarps. It may be especially beneficial if applied before floral initiation to young dipterocarps thereby reducing vegetative growth over several years and thus making the trees a more manageable size for seed collection (Erez 1986).
Acknowledgments
This research was supported by the ASEAN Forest Tree Seed Centre Project (Muak Lek, Saraburi, Thailand), the Forest Tree Improvement Research and Development Institute (Yogyakarta, Indonesia) and through a Canadian NSERC Operating Grant (No. 1982) to J.N.O. Appreciation is extended to Parlin Tambunan for collection of speci-mens and flowering data, to the staff at The Kebun Percorbaan Arbo-retum, west Java, Indonesia for use of the trees and assistance in collection of specimens, Glenda Catalano for technical assistance, Dr. Derek Harrison for statistical assistance and Diane Gray for typing the manuscript.
References
Ashton, P.S. 1982. Dipterocarpaceae. In Flora Malesiana Ser. I. Sper-matophyta. Ed. C.G.G.J. van Steenis. 9:237--552.
Ashton, P.S. 1988. Dipterocarp biology as a window to the under-standing of tropical forest structure. Annu. Rev. Ecol. Syst. 19:347--370.
Ashton, P.S., T.J. Givnish and S. Appanah. 1988. Staggered flowering in the Dipterocarpaceae: new insights into floral induction and evolution of mass fruiting in the aseasonal tropics. Am. Nat. 132:44--66.
Burgess, P.F. 1972. Studies of the regeneration of the hill forests of the Malay Peninsula. Malay. For. 35:103--123.
Chan, H.T. 1980. Reproductive biology of some Malaysian diptero-carps. II. Fruiting biology and seedling studies. For. Ecol. 43:438--451.
Chaikiattiyos, S., C.M. Menzel and T.S. Rasmussen. 1994. Floral induction in tropical fruit trees: effects of temperature and water supply. J. Hortic. Sci. 69:397--415.
Dalziel, J. and D.K. Lawrence. 1984. Biochemical and biological effects of kaurene oxidase inhibitors such as paclobutrazol. In Biochemical Aspects of Synthetic and Natural Occurring Plant Growth Regulators. British Plant Growth Regulator Group Monogr. 11:43--57.
Erez, A. 1986. Effect of soil applied paclobutrazol in drip irrigated peach orchards. Acta Hortic. 179:513--520.
Gifford, E.M. 1950. Softening refractory plant material embedded in paraffin. Stain Technol. 25:161--162.
Griffin, A.R., P. Whiteman, T. Rudge, I.P. Burgess and M. Moncur. 1993. Effect of paclobutrazol on flower-bud production and vegeta-tive growth in two species of Eucalyptus. Can. J. For. Res. 23:640--647.
Johansen, D.A. 1950. Plant embryology. Chronica Botanica Co., Waltham, MA, 305 p.
Lever, B.G. 1986. ‘‘Cultar’’----A technical overview. Acta Hortic. 179:459--466.
Martin, P.J. and A.J. Dabek. 1988. Effects of paclobutrazol on the vegetative growth and flowering of young clove trees. Trop. Agric. (Trinidad) 65:25--28.
Moncur, M.W., G.F. Rasmussen and O. Hasan. 1994. Effect of paclobutrazol on flower-bud production in Eucalyptus nitens espal-ier seed orchards. Can. J. For. Res. 24:46--49.
Ng, F.S.P. 1966. Age at first flowering in Dipterocarps. Malays. For. 19:290--295.
Ng, F.S.P. 1977. Gregarious flowering of dipterocarps in Kepong, 1976. Malay. For. 40:126--137.
Owens, J.N. and M.D. Blake. 1985. Forest tree seed production. A review of literature and recommendations for future research. Can. For. Serv. Inf. Rep. PI-X-53, 161 p.
Pharis, R.P. 1976. Manipulation of flowering in conifers through use of plant hormones. In Modern Methods in Forest Genetics. Ed. M.P. Miksche. Springer-Verlag, New York, pp 265−282.
Quinlan, J.D. and P.J. Richardson. 1986. Uptake and translocation of paclobutrazol and implications for orchard use. Acta Hortic. 179:443--451.
Reich, P.B. and R. Borchert. 1984. Water stress and tree phenology in a tropical dry forest in the low lands of Costa Rica. J. Ecol. 72:61--74.
Sasaki, S. 1980. Storage and germination of dipterocarp seeds. Malay. For. 43:290--308.
Smitinand, T., T. Santisuk and C. Phengklai. 1980. The manual of Dipterocarpaceae of mainland south-east Asia. The Forest Herbar-ium, Royal Forestry Dept., Bangkok, Thailand, 133 p.
Steffens, G.L., F.W. Jacobs and M.E. Engelhaupt. 1993. Size, flower-ing and fruitflower-ing of maturflower-ing own-rooted ‘‘Gala’’ apple trees treated with paclobutrazol sprays and trunk drenches. Sci. Hortic. 56:13--21.
van Schaik, C.P., J.W. Terborgh and S.J. Wright. 1993. The phenology of tropical forests: adaptive significance and consequences for pri-mary consumers. Annu. Rev. Ecol. Syst. 24:353--377.
Wycherley, P.R. 1973. The phenology of plants in the humid tropics. Micronesia 9:75--96.