47
E
MBOLISMS AND REFILLING IN THE MAIZE LEAFLAMINA
,
AND THE ROLE OF THE PROTOXYLEMLACUNA1
M
ARTINJ. C
ANNYResearch School of Biological Sciences, Australian National University, PO Box 475, Canberra 2601, Australia
The proportion of embolized vessels in the veins of maize leaf laminas was measured during 24 h by direct counting in snap-frozen samples in the cryo-scanning electron microscope. All vessels were sap filled at night. Vessels of intermediate and small veins, and the small tracheary elements of lateral veins, were sap filled throughout the 24 h. The large metaxylem vessels of lateral veins were embolized during the day. The percentage of these vessels embolized was maximum (.70%) at 1400, and declined during the afternoon to 20% at dusk. Leaf water potential reached a minimum (21.2 MPa) at dusk. The protoxylem lacuna of the lateral veins was much less embolized than the large vessels, although it was of comparable diameter. The observations are interpreted in terms of the refilling hypothesis that is part of the compensating pressure theory of water transport.
Key words: cavitation; compensating pressure theory; embolism refilling; leaf veins; protoxylem lacuna; transpiration; xylem vessels; Zea mays.
Recent measurements of embolisms by direct observation of quick-frozen, intact plant parts have shown consistent daily patterns: rapid increase in embolisms soon after sunrise, a peak close to midday, a decline during transpiration in the afternoon to reach very low values at night, in petioles (Canny, 1997a, b), and in roots (Buchard, McCully, and Canny, 1999; Mc-Cully, Huang, and Ling, 1998; McMc-Cully, 1999; Pate and Can-ny, 1999; Shane and McCully, 1999). This pattern of embo-lism during day and night has been so generally found that it may be distinguished as the typical daily pattern. Also, in these observations the small vessels were less embolized than the large ones. An explanation of these observations was elabo-rated in terms of fast refilling of embolisms by supplementary water pushed into vessels by tissue-pressure-driven reverse os-mosis (Canny, 1998b). The observations stimulate the question of whether similar changes are occurring in the laminas of leaves, where the tension in the xylem sap is greatest, where the supply of water outside the vessels seems limited, and where the arrangement of tissues and air spaces might be thought inadequate to generate and contain tissue pressure. This paper reports an investigation of this question.
The vein system of leaf laminas comprises two types of vein: large veins with large vessels, which distribute water quickly all over the lamina, and small veins with small vessels, which draw water from the large veins and distribute it locally to the mesophyll from which it evaporates (Canny, 1990, 1993). The tension in the small veins at the end of the flow path must be greatest and, if both types are equally liable to embolism, might be expected to produce more embolisms there than in the large veins. Embolisms blocking the few small vessels in fine veins would produce patches of leaf under high water stress and might lead to local closures of stomata. A monocotyledon leaf with parallel veins was selected
be-1Manuscript received 26 October 1999; revision accepted 16 March 2000.
The author thanks Cheng Huang and Lew Ling of the Carleton University Research Facility for Electron Microscopy for their preparation of the samples for microscopy, Marilyn Ball for the provision of laboratory facilities and for helpful discussions, Adam Baker for making the plate and Jack Egerton for the figure, Margaret McCully for critical advice, and the Natural Sciences and Engineering Research Council of Canada for an operating grant.
cause of its geometrical simplicity. Any cross section of such a leaf provides right cross sections of the large and small par-allel veins, but not, of course, of the transverse veins that con-nect them. In such a leaf, large veins with large vessels and a protoxylem lacuna are called lateral veins. Small veins with small vessels and no protoxylem lacuna are called either in-termediate or small (Kuo, O’Brien and Canny, 1974). Maize leaves, as used here, have lateral veins and both intermediate and small veins (Fig. 1). Lateral veins are far apart, with many intermediate and small veins between them.
MATERIALS AND METHODS
Plants of Zea mays L. (cv. 41–47, Pick Seeds Ltd., Lindsay, Ontario, Can-ada) were grown in the field for 2 mo, watered and fertilized as necessary, and were starting to tassel. Leaves selected for sampling were undamaged and in the middle to upper part of the shoot.
Samples of leaves were taken to measure embolisms from five leaves at each of the following times: 0600, 1000, 1400, 1800, and 2200 (Eastern Sum-mer Time). Each sample was frozen on the plant by clamping a piece of the lamina between the heavy copper jaws of a specially constructed pair of pliers that had been cooled to the temperature of liquid nitrogen (LN2) (McCully,
Huang, and Ling, 1998). The piece was cut from the leaf while still held in the pliers, then released into LN2, trimmed parallel to the veins to strips;5
mm wide, and placed in a labelled cryo-vial. The vials were stored at LN2
temperature. At each time of sampling measurements of leaf water potential were made on five other (unbagged) leaves with a pressure chamber (Plant water status console model 3000, Soilmoisture Corp., Santa Barbara, Califor-nia, USA), and the air temperature was recorded.
Embolisms in the vessels of the leaf veins were observed by the method outlined in Canny (1997a). Small lengths were cut from the center of the leaf strips under LN2and mounted in a slot in an aluminium stub with Tissue Tek
Figs. 1–6. 1. Cross section of fresh maize leaf lamina showing one lateral and one small vein. The lateral vein has two large metaxylem vessels (v), with a band of small tracheary elements between them. Above them is the protoxylem lacuna with remnants of the helical thickening of a protoxylem vessel within. The phloem is at the bottom. In this and all the micrographs, the upper surface of the leaf is uppermost. Hand section, toluidine blue stain, bright-field optics. Bar5100mm. Figs. 2–6. Planed transverse faces of snap-frozen maize leaf laminas, viewed still frozen and fully hydrated in the cryo-scanning electron microscope. 2. Overall view of a leaf cross section showing four veins (arrowheads, 3 small and 1 intermediate). Note paucity of gas spaces. Leaf frozen at 1400. Bar5100mm. 3. Lateral vein with all xylem elements filled with sap. The protoxylem lacuna contains one intact vessel as well as the sap-filled space. Leaf frozen at 0600. Bar550mm. 4. Lateral vein with both metaxylem vessels embolized. The small tracheary elements and protoxylem are still sap-filled. Leaf frozen at 1800. Bar550mm. 5. Lateral vein with two of three metaxylem vessels and the protoxylem lacuna embolized. The small tracheary elements are still sap-filled. Leaf frozen at 1400. Bar550mm. 6. Intermediate vein with all four xylem elements (arrowheads) sap-filled. Leaf frozen at 1400. Bar550mm.
separately. At each sampling time the counts of vessels and embolisms for all five leaves were pooled to give single totals for each vein class. There are thus no estimates of variance. The extent of embolism at each sampling time was expressed as the percentage of gas-containing vessels observed in each vein class. Because the values for intermediate and small veins were not distinguishable, these two classes were combined.
RESULTS
Fig. 9. Time course of percentage of embolized vessels in maize leaves during 24 h, in lateral veins (open circles) and small and intermediate veins (closed circles), together with the balance pressure measured by pressure chamber (dashed, with SD). Hatched zones indicate hours of darkness.
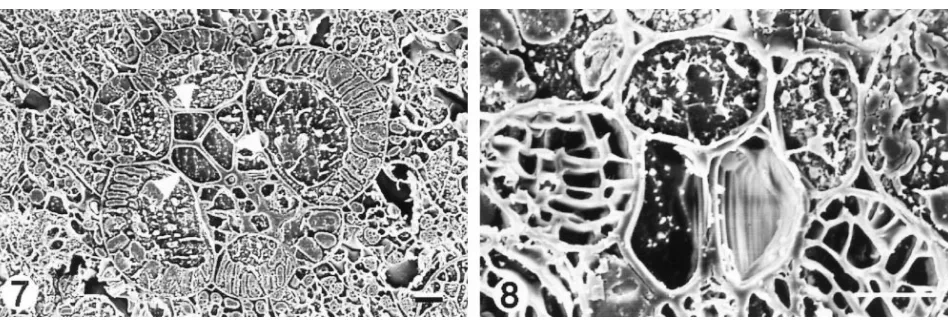
Figs. 7–8. More planed transverse faces of snap-frozen maize leaf laminas, viewed still frozen and fully hydrated in the cryo-scanning electron microscope. 7. Small vein with three sap-filled vessels (arrowheads). Leaf frozen at 1000. Bar510mm. 8. Part of a small vein with two vessels, one of which is fully embolized. Sap has retreated slightly from their common wall in the other vessel. Leaf frozen at 1400; same leaf piece as in Fig. 5. Bar510mm.
strands of fibers that extend to the epidermises. Two large metaxylem vessels stand one on either side of the vein, joined by a band of small tracheary elements, interpreted by some to be tracheids (e.g., Hayward, 1938; Sharman, 1942) and by others to be vessels (e.g., Cheadle, 1942; Esau, 1943). Below them is a strand of phloem. Above them is the protoxylem lacuna, sometimes containing remnants of protoxylem vessels. Small and intermediate veins have a single sheath of large, thin-walled bundle sheath cells containing chloroplasts. Their xylem vessels are few and small. Below the vessels lies the phloem.
The frozen hydrated leaf specimens showed a surprisingly low proportion of gas space. The surface of a transverse face was mostly frozen liquid (Fig. 2). The proportion of large veins was small, on average one to every 37 of the small and intermediate veins.
At 0600 and 2200 nearly all the lateral veins had all their tracheary elements filled with liquid, including the protoxylem lacuna (Figs. 3, 9). In Fig. 3, two bands of thick-walled fibers lie above and below the vein, of which only the upper one is visible. The living cells of the mesophyll and vein are filled with white (electron emissive) lines of solute crystallized by freezing. The sap in the tracheary elements has fewer solutes and is less emissive. It can be seen filling the two large
meta-xylem vessels and, between them, the band of angular small tracheary elements. Above these is a protoxylem element, and beyond that, the protoxylem lacuna, both sap-filled.
At other sampling times one or both of the large vessels usually contained gas (Fig. 4) and sometimes some debris from the planing. Occasionally, in the 1400 samples, the pro-toxylem lacuna also contained gas (Fig. 5). The small trache-ary elements between the vessels were never seen to contain gas.
The vessels of the intermediate (Fig. 6) and small (Fig. 7) veins almost never contained gas. Of 4021 vessels examined only 19 contained gas (Fig. 8), and 17 of these were at the 1000 and 1400 sampling times.
A summary of the changes in embolism during 24 h is shown in Fig. 9, along with the measured balance pressures. As has been said, embolism of vessels in the smaller veins was negligible. The peak of embolisms in the large vessels (.70%) was found at 1400. At this time the leaf balance pres-sure was 0.6 MPa. The highest value of balance prespres-sure (;1.2 MPa) was recorded late in the afternoon, at which time large vessel embolisms had fallen to ;20%. The embolism values for the protoxylem lacuna have not been included on the graph. They were zero for all times except 1400, when 25% of the cavities were embolized. Air temperature ranged from 218to 278C.
DISCUSSION
The typical pattern of embolization and refilling during the day and night already shown in roots and petioles of all plants studied by the present method was found also in the lateral veins of maize leaf laminas. A feature of this pattern, that the proportion of embolized large tracheary elements is greater than that of small elements, is even more pronounced in the leaves, where both the xylem of intermediate and small veins, and the small tracheary elements of the lateral veins were al-most never embolized. This contradicts the intuitive prediction stated at the outset that, because the tension in the xylem sap of small veins must be greater than that in the large veins, they might show more embolism. The mere existence of a higher tension in the water did not produce more observed embo-lisms.
sim-pler techniques. Strasburger (1891) reviewed this literature and concluded: ‘‘I myself have never found air bubbles in the ul-timate branches of vessels of leaves of many woody plants, which I investigated during daylight hours in summer. How-ever, in the larger veins of the lamina, I confirmed (similar) published statements of Elfving ‘that in the outermost tra-cheids, air bubbles never occur’. And so also, Scheit has found in the transfusion tissue of conifer leaves, no air, but only water or water vapor.’’ The absence of embolisms in the fine veins of leaf laminas and their concurrent appearance in large vessels of larger veins appear to be very general phenomena. A similar (and possibly related) distinction between large and small veins was reported by Lo Gullo et al. (1997) for the acoustic emissions (often considered as evidence of cavitation) from drying holly leaves. Most of the emissions were detected near the midrib. Few emissions were detected from parts of leaves where there were only small veins.
The traditional explanation of the difference in embolisms between large and small vessels would be that vulnerability to cavitation is proportional to diameter (Zimmermann, 1983). This is less an explanation than a restatement of the fact. A causative link has been surmised: for example, that there are more likely to be nuclei to initiate cavitation in a larger volume or surface or that air seeding would be more likely in a large vessel because the pores in its pit membranes might be larger (Jarbeau, Ewers, and Davis, 1995). The claimed correlation between vulnerability to cavitation and vessel diameter is con-trary to the observations presented here, that the protoxylem lacuna is much less embolized than the large vessels.
The fact that the protoxylem lacuna, whether in Equisetum or in the monocotyledon vascular bundles, is filled with liquid and is a pathway for water transport parallel with the vessels of the bundle has been known since the investigation of Wes-termaier (1884). Strasburger (1891) ascribes water conduction as the main function of the lacuna. The conduction of water by the lacuna was confirmed with dye tracers by Buchholz (1921) for a wide range of monocotyledons, Equisetum and several water plants, by Bierhorst (1958) for Equisetum, and by Dong, McCully, and Canny (1997) for sugarcane.
The conducting protoxylem lacuna does not conform to the traditional view (confirmed here for vessels of maize leaf veins) that large vessels are more vulnerable to cavitation than small ones. The discovery that the lacuna is much less subject to embolism than the two vessels flanking it, although it is of comparable diameter, requires explanation. It seems most un-likely that the lacuna would be less susceptible to air seeding than the vessels, with their stout walls and small pits.
Possible explanations of both the relation of embolism to vessel diameter, and of the anomalous behavior of the proto-xylem lacuna, follow from the observed refilling of embolized vessels as outlined by Canny (1998b). He proposes a small flux of water (supplementary water) into vessels from the sur-rounding tissues, driven by tissue pressure, and expressing wa-ter by reverse osmosis from reservoir cells at lower pressure. In Appendix 1 of that paper some calculations were attempted about the flux of water and the necessary pressure difference. The time to refill is proportional to vessel diameter, and this could explain why small vessels are found to have less em-bolism than large vessels. Even with a common rate of cavi-tation, small vessels would spend less time empty than large ones. The objections to such a mechanism put forward by Tyree et al. (1999) show that he has not understood the
pro-posal. He does not refer to the statement of it in Canny (1998b).
To explain the low embolism of the wide protoxylem lacuna requires a further step. In the calculations of Appendix 1 of Canny (1998b) the whole surface of the vessel was assumed to be permeable to water. That this is an oversimplification is shown by the published images of water entering embolized vessels through pits (e.g., fig. 2 of Canny, 1998b; fig. 10 of Canny, 1998a; fig. 4 j, l of McCully, Huang, and Ling, 1998). The calculations should include a proportionality factor for the pit area as a fraction of the total wall area. With this revision, the special property of the protoxylem lacuna is that it has no impermeable walls, but is surrounded by thin-walled paren-chyma cells. If the pits occupy say 10% of the walls of the large vessels, then the protoxylem lacuna would fill ten times faster than the vessels and would spend a tenth of the time that they do empty. Looked at in this way, the protoxylem lacuna would form part of that water continuum that maintains minimum embolism (along with the small tracheary elements of the lateral veins and the small vessels of the intermediate and small veins). It would be a vital part of the water supply network at times of high water demand. In justice, it must be stated that Westermaier (1884) was fully aware of the special permeability of the walls around the lacuna to water.
The existence of sufficient reservoirs of water to supply the refilling process is less easy to imagine in leaves than in other organs. The large proportion of ice in the frozen transverse leaf face in Fig. 2 is in contrast to the comparable views of frozen hydrated dicotyledon leaves, where air spaces make up a large part of the surface (e.g., Jeffree et al., 1987). Maize leaves do have a low volume fraction of gas space. Byott (1976) showed that the air space volume of C4plants was less
than that of C3plants, and for C4monocotyledons ranged from
10.5% in Setaria to 2.8% in Saccharum. Zea was at the higher end of the range, with 10%. Dicotyledon leaves could have percentages of air space ranging up to 52% in Nicotiana. For maize leaves, the volume of water in the lamina available for refilling appears substantial. It seems possible that the higher water use efficiency of C4 leaves may derive, in part, from
their having a larger volume of reservoir water for refilling embolisms.
LITERATURE CITED
BIERHORST, D. W. 1958. Vessels in Equisetum. American Journal of Botany 45: 534–537.
BYOTT, G. S. 1976. Leaf air space systems in C3 and C4 species. New Phytologist 76: 295–299.
BUCHARD, C., M. MCCULLY,ANDM. CANNY. 1999. Daily embolism and refilling of root xylem vessels in three dicotyledonous crop plants. Agronomie 19: 97–106.
BUCHHOLZ, M. 1921. U¨ ber die Wasserleitungsbahnen in den interkalaren Wachstumszonen monokotyler Sprosse. Flora 114: 119–186.
CANNY, M. J. 1990. What becomes of the transpiration stream? New Phy-tologist 114: 341–368.
———. 1993. The transpiration stream in the leaf apoplast: water and sol-utes. Philosophical Transactions of the Royal Society (London) B 341: 87–100.
———. 1997a. Vessel contents after excision—a test of Scholander’s as-sumption. American Journal of Botany 84: 1217–1222.
———. 1997b. Vessel contents during transpiration—embolisms and refill-ing. American Journal of Botany 84: 1223–1230.
———. 1998a. Transporting water in plants. American Scientist 86: 152– 159.
CHEADLE, V. I. 1942. The occurrence and types of vessels in the various organs of the plant in the Monocotyledonae. American Journal of Botany 29: 441–450.
DONG, Z., M. E. MCCULLY, ANDM. J. CANNY. 1997. Does Acetobacter diazotrophicus live and move in the xylem of sugarcane stems? Some anatomical and physiological data. Annals of Botany 80: 147–158. ESAU, K. 1943. Ontogeny of the vascular bundle in Zea mays. Hilgardia 15:
327–356.
HAYWARD, E. H. 1938. The Structure of Economic Plants. Macmillan, New York.
JARBEAU, J. A., F. W. EWERS,ANDS. D. DAVIS. 1995. The mechanism of water-stress induced embolism in two species of chapparal shrubs. Plant Cell and Environment 18: 189–196.
JEFFREE, C. E., N. D. READ, J. A. C. SMITH,ANDJ. E. DALE. 1987. Water droplets and ice deposits in leaf intercellular spaces: redistribution of water during cryofixation for scanning electron microscopy. Planta 172: 20–37.
KUO, J., T. P. O’BRIEN ANDM. J. CANNY. 1974. Pit-field distribution, plas-modesmatal frequency and assimilate flux in the mestome sheath cells of wheat leaves. Planta 121: 97–118.
LOGULLO, M. A., A. NARDINI, H. RICHTER,ANDS. SALLEO. 1997. Ultra-sound acoustic emissions from dehydrating leaves of deciduous ever-green leaves. Plant, Cell and Environment 20: 1381–1390.
MCCULLY, M. E. 1999. Root xylem embolisms and refilling. Relation to water potentials of soil, roots, and leaves and osmotic potentials of root xylem sap. Plant Physiology 119: 1001–1008
———, C. X. HUANG,ANDL. E. C. LING. 1998. Daily embolism and re-filling of xylem vessels in the roots of field-grown maize. New Phytol-ogist 138: 327–342.
PATE, J. S.,ANDM. J. CANNY. 1999. Quantification of embolisms by direct observation: a comparison of two methods. New Phytologist 141: 33– 44.
SHANE, M.,ANDM. E. MCCULLY. 1999. Root xylem embolisms: implica-tions for water flow to the shoot in large, field-grown maize plants with only one root. Australian Journal of Plant Physiology 26: 107–114. SHARMAN, B. C. 1942. Developmental anatomy of the shoot of Zea mays L.
Annals of Botany NS 6: 245–262.
STRASBURGER, E. 1891. U¨ ber den Bau und die Verrichtungen der Leitungs-bahnen in den Pflanzen. Histologischer Beitra¨ge 3. Jena.
TYREE, M. T., S. SALLEO, A. NARDINI, M. A. LOGULLO,ANDR. MOSCA. 1999. Refilling embolized vessels in young stems of laurel. Do we need a new paradigm? Plant Physiology 120: 11–21.
WESTERMAIER, M. 1884. Untersuchungen u¨ber die Bedeutung todter Ro¨hren und lebender Zellen fu¨r die Wasserbewegung in der Pflanze. Sitzungs-berichte der deutschen Akademie der Wissenschaft zu Berlin 1105–1117. ZIMMERMANN, M. H. 1983. Xylem structure and the ascent of sap.