www.elsevier.nlrlocateraqua-online
Interaction between dietary levels of iron and
vitamin C on growth, hematology, immune
ž
response and resistance of channel catfish Ictalurus
/
punctatus to Edwardsiella ictaluri challenge
Chhorn Lim
a,), Phillip H. Klesius
a, Meng H. Li
b,
Edwin H. Robinson
ba
Aquatic Animal Health Research Laboratory, USDA-ARS, MSA, P.O. Box 952, Auburn, AL 36830 USA
b
Thad Cochran National Warmwater Aquaculture Center, Mississippi State UniÕersity, StoneÕille, MS 38776 USA
Accepted 21 October 1999
Abstract
Nine egg-white-based diets supplemented with three levels of vitamin C from L
-ascorbyl-2-Ž .
polyphosphate 0, 50 and 3000 mgrkg for each of the three levels of iron from iron methionine
Ž0, 30 and 300 mgrkg were each fed to juvenile channel catfish in triplicate aquaria twice daily.
to apparent satiation for 14 weeks. Fish fed with iron-deficient diets had decreased weight gain, feed conversion and survival. Supplementation of ascorbic acid to the iron-deficient diets further decreased weight gain and survival. Feed conversion was not significantly affected by dietary level of vitamin C or iron and vitamin C interaction. No gross signs of vitamin C deficiency were
Ž . Ž . Ž .
observed. Total cell count TCC , red blood cell count RBC , hematocrit HCT and hemoglobin
ŽHb were significantly lower for fish fed the iron deficient diet. TCC and RBC significantly.
increased when 3000 mg of vitamin Crkg was added. However, in the absence of dietary iron, supplementation of ascorbic acid resulted in significant decrease in HCT and Hb values. Hepatic iron concentration increased with increasing dietary level of iron. Supplementation of high level of ascorbic acid to the diet containing 300 mgrkg of supplemental iron significantly increased the liver iron content. Liver ascorbate increased with increasing dietary level of ascorbic acid. Dietary level of iron and the interaction between iron and vitamin C had no effect on liver content of vitamin C. Mean macrophage migration in the absence or presence of Edwardsiella ictaluri exoantigen was significantly higher for fish fed the iron supplemented diets. The significant effect
)Corresponding author. Tel.:q1-334-887-3741; fax:q1-334-887-2983.
Ž .
E-mail address: [email protected] C. Lim .
0044-8486r00r$ - see front matterq2000 Published by Elsevie Science B.V.
Ž .
Ž .
of vitamin C was obtained only when high level 3000 mgrkg was used. Neither dietary levels of iron nor vitamin C or their interaction influenced survival of juvenile channel catfish against E.
ictaluri 14-day post challenge. However, the onset of mortality was earlier for fish fed the
iron-deficient diet.q2000 Published by Elsevier Science B.V.
Keywords: Iron; Ascorbic acid; Hematology; Immune response; Disease resistance
1. Introduction
Iron is an essential element for the functioning of organs and tissues of higher animals, including fish, because of its important role in oxygen transport and cellular respiration. Iron is also one of the most important micronutrients in terms of its effect on
Ž
immune system functions and host defense against infections Beisel, 1982; Bhaskaram, .
1988 . Fish can absorb soluble iron from the water across the gill membrane and
Ž .
intestinal mucosa Roedar and Roedar, 1968 . However, feed is considered the major Ž source of iron for fish due to low concentrations of soluble iron in natural waters NRC,
.
1993 . The total dietary iron requirement for optimum growth, feed efficiency, hemato-logical values and immune response of juvenile channel catfish has been determined to
Ž .
be about 30 mgrkg diet Gatlin and Wilson, 1986; Lim et al., 1996; Sealey et al., 1997 . Chemotactic response of peritoneal macrophages to Edwardsiella ictaluri exoantigen
Ž
was depressed in iron-deficient channel catfish Lim and Klesius, 1997; Sealey et al., .
1997 but this abnormality was remedied by feeding an iron-replete diet for 4 weeks ŽLim and Klesius, 1997 . The effect of dietary iron on resistance of channel catfish to.
Ž .
bacterial pathogens is unclear, although Sealey et al. 1997 suggested that high level of
Ž .
dietary iron 180 mgrkg may lead to increased susceptibility.
Ascorbic acid or vitamin C is essential for most fishes, including channel catfish ŽNRC, 1993 . The requirement varies depending on the metabolic function and form of. vitamin C used. Using ascorbic acid as the dietary source, a level of 30 mgrdiet was adequate for optimum growth, but 60 mgrkg was needed for prevention of all
Ž
deficiency signs Andrews and Murai, 1975; Lim and Lovell, 1978; Li and Lovell,
. Ž .
1985 . When a stable form of vitamin C L-ascorbyl-2-polyphosphate was used, 11 to
Ž
45 mgrkg diet was adequate for growth and prevention of deficiency signs El Naggar .
and Lovell, 1991; Robinson, 1992 . Evidence of the role of ascorbic acid in fish immunity and disease resistance is not consistent, although numerous studies have shown that feeding fish ascorbic acid at levels higher than those required for growth and prevention of deficiency signs enhanced their immune response and resistance to bacterial challenges.
Ascorbic acid is known to be involved in the metabolism of iron in animals including
Ž .
fish Harper, 1975; Monsen, 1982; Hilton, 1989; NRC, 1993 . Ascorbic acid enhances the absorption of iron from the intestine by reducing ferric iron to the ferrous state, a
Ž
more soluble form that is readily absorbed El-Alwary et al., 1975; Harper, 1975;
. Ž .
Monsen, 1982 . Ascorbic acid is also involved with adenosine triphosphate ATP in the release and reduction of the ferric iron from ferritin and its subsequent incorporation
Ž
with iron-binding protein, apoferritin and transferrin, into tissue ferritin Mazur et al., .
No studies have been conducted on the combined influence of dietary levels of iron and ascorbic acid on the response of channel catfish. Thus, this study was conducted to evaluate the interaction between dietary levels of iron and ascorbic acid on growth, hematology, immune response and resistance of channel catfish to E. ictaluri challenge.
2. Materials and methods
2.1. Experimental diets
Ž .
The egg-white-based diet used in this study Table 1 was modified from Gatlin and
Ž . Ž
Wilson 1986 . The basal diet was supplemented with three levels of iron 0, 30 and 300
. Ž . Ž
mgrkg from iron methionine Zinpro, Chaska, MI and three levels of vitamin C 0, 50
. Ž .
and 3000 mgrkg fromL-ascorbyl-2-polyphosphate Hoffmann-La Roche, Paramus, NJ
Ž .
at each iron level at the expense of cellulose 3=3 factorial experiment . The intermediate levels of iron and vitamin C represented optimum concentrations necessary
Ž
for good growth and prevention of deficiency signs Lim and Lovell, 1978; Lim et al., .
1996; Gatlin and Wilson, 1986; Robinson, 1992 . The diets were formulated to contain approximately 34% crude protein and 3.1 kcal of digestible energyrg based on feedstuff
Ž .
values reported in by NRC 1993 . Diets were prepared as 3-mm diameter, semi-moist Žapproximately 25% moisture pellets as described by Lim et al. 1996 . Pellets were. Ž . broken into small pieces and stored aty188C until needed. Iron content of the basal diet without iron supplementation was determined to be 9.2 mg ironrkg by an
inductively-Ž .
coupled argon plasma ICAP spectrophotometer according to the method of Campbell Ž .
and Plank 1992 .
Table 1
Composition of basal diet
Ingredient grkg Diet
Ždry matter basis.
Egg white 399.0
Corn starch 475.8
Cellulose 5.0
Cod liver oil 32.5
Corn oil 32.5
a
Iron-free mineral mix 40.0
b
Vitamin C-free vitamin mix 15.0
Ethoxyquin 0.2
a Ž .
Contains as grkg of premix : calcium carbonate, 300.0; potassium phosphate, dibasic, anhydrous, 319.0; sodium phosphate, monobasic, 200.34; magnesium sulfate heptahydrate, 132.0; zinc sulfate heptahydrate, 3.00; sodium chloride, 43.50; cobalt chloride, 1.00; manganous sulfate monohydrate, 0.80; cuprous chloride, 0.20; potassium iodide, 0.15; sodium selenite, 0.011.
b Ž .
2.2. Fish and feeding
Ž .
USDA-ARS strain 103 channel catfish Ictalurus punctatus fingerlings from a single spawn which had been maintained at the USDA Aquatic Animal Health Research Laboratory on commercial diets to an average weight of 10.6"0.1 g were randomly stocked into twenty-seven 110-l aquaria at a density of 100 fish per aquarium. Aquaria were supplied with flow-through dechlorinated tap water at a rate of 0.6–1.2 lrmin. Water flow rates were checked and adjusted daily to insure proper water exchange rate. Water temperature was maintained by a centralized heater at 26"28C. Water was continuously aerated and photoperiod was maintained at 12:12 h light:dark schedule. The water contained less than 0.5 mg ironrl.
Fish in triplicate aquaria were randomly assigned to each of the nine experimental
Ž .
diets and fed their respective diets twice daily between 0730–0800 and 1430–1500 h to satiation for a period of 14 weeks. During each feeding, feed was offered by hand three to four times until satiation was reached. The quantity of feed consumed was recorded daily by calculating the differences in weights of feeds prior to the first and after the last feeding. All aquaria were cleaned once weekly by scrubbing and siphoning accumulated wastes. On cleaning days fish were fed only once in the afternoon. Fish in each aquarium were counted and weighed collectively at biweekly intervals. No feeding was done on sampling days.
2.3. Measurement of liÕer contents of iron and ascorbic acid
At the end of 12 weeks, five fish from each of the triplicate tanks were randomly taken and sacrificed for measurement of liver concentrations of iron and ascorbic acid. Livers from each of the five fish from the same aquarium were pooled to obtain one composite sample and stored aty808C. Iron content of the liver was determined by an
Ž .
ICAP spectrophotometer according to the method of Campbell and Plank 1992 . Liver levels of total vitamin C were analyzed using reverse-phase high performance liquid chromatography with electrochemical detection following the procedures described by
Ž .
Wang et al. 1988 .
2.4. Hematological assay
Blood samples were obtained from fish at the end of week 14. Two to five fishrtank for the treatment fed the diet containing 0 mg iron and 3000 mg vitamin Crkg, and five fish from each tank of the other treatments were randomly chosen and anesthetized with
Ž .
tricaine methanesulfonate MS-222; Argent Chemical, Redmond, WA at 125 mgrl. Blood samples were collected from the caudal vein with heparinized 27-gauge needles
Ž . Ž .
and tuberculin syringe 20 unitsrml for determination of hematocrit HCT , total cell ŽTCC and red blood cell counts RBC , and hemoglobin Hb . HTC was determined by. Ž . Ž .
Ž .
the microhematocrit method described by Brown 1988 . TCC cell and RBC were determined by diluting whole blood and enumeration in a hemacytometer. Hemoglobin
Ž .
by the cyanomethemoglobin correction factor for channel catfish described by Larsen Ž1964 ..
2.5. Collection of peritoneal exudate cells
Collection and isolation of peritoneal exudate cells followed the procedure of Klesius
Ž .
and Sealey 1996 . At the end of week 14, three fishrtank for fish fed the diet containing 0 mg iron and 3000 mg vitamin Crkg, and five fish from each tank of other
Ž .
treatment were randomly chosen, injected intraperitoneally i.p. with 0.25 ml of
Ž .
squalene Sigma and transferred into 57-l aquaria where they continued to be fed the various experimental diets. Five to seven days later, fish were anesthetized with MS-222
Ž . Ž .
and injected i.p. with 15-ml sterile, cold phosphate-buffered saline PBS . Then, PBS was removed along with the squalene-elicited exudate cells using a 20-gauge needle attached to a three-way valve into a 50-ml centrifuge tube. The peritoneal fluids of five fish from the same tank were combined and centrifuged at 300=g for 10 min. The supernatant was discarded, and the cells suspended in calcium- and magnesium-free
Ž . Ž .
Hank’s Balance Salt Solution HBSS without phenol red Gibco, Grand Island, NY for chemotaxis assay. Cell counts and viability were established following enumeration with a hemocytometer in 5% Trypan blue counting solution.
2.6. Chemotaxis assay
Chemotaxis was determined by a modification of the lower-surface method of
Ž . Ž .
Boyden 1962 as described by Klesius and Sealey 1996 . Assays were performed in
Ž .
triplicate using blind well chemotactic chambers Corning CoStar, Cambridge, MA and
Ž .
8-mm pore diameter polycarbonate membrane filters Nucleopore, Pleasonton, CA
Ž .
pre-soaked for 5 min in RPMI-1640 Gibco containing 1% horse serum. In the bottom
Ž . Ž
of the chambers 20 ml of either E. ictaluri exoantigen 6.10 mg proteinrml Klesius, .
1993 was added together with 180ml RPMI-1640q1% horse serum. In the bottom of the control chamber only RPMIq1% horse serum was added. Peritoneal macrophages
Ž
were added to the upper compartment of the chamber separated from the bottom
. 5
chamber by a filter at a concentration of approximately 5=10 cellsrchamber. The
Ž .
chambers were incubated on a horizontal platform shaker 100 rpm for 100 min at 258C. Following incubation, filters were removed, inverted, placed on a slide, attached with clear finger nail polish and stained with Leukostat. The number of macrophages on the surface of the filter were counted in five fields of triplicate filters at 100=.
2.7. E. ictaluri challenge
Ž .
E. ictaluri AL-75-94 from a virulent outbreak of ESC was grown in brain–heart
Ž . Ž .
infusion BHI broth for 24 h and used for bacterial challenge Klesius, 1992 . At the end of week 14, 20 fish from each of three replicate tanks per diet were randomly selected, placed in perforated 5-gallon plastic buckets and immersed for 1 h in static, aerated aquaria containing 1.7–2.0=107 cellsrml of E. ictaluri. Each group of fish
lrmin. Water flow and feeding were discontinued for the first 24 h after challenge. Mortality was monitored and recorded twice daily before feeding for 14 days.
2.8. Statistical analysis
Data were analyzed by a two-way analysis of variance using the general linear model
Ž .
procedure SAS Institute, Carey, NC, 1993 . Duncan’s multiple-range test was used to determine significant differences due to dietary iron, vitamin C, and iron and vitamin C interactions. When a main effect was found significant but without interaction effect, the differences between treatment means were determine by Duncan’s multiple range test. If a significant interaction was observed, the differences between simple effects were determined by Student’s t-test. Differences were considered significant at the 0.05 probability level.
3. Results
Ž .
Mean final weight gain, feed conversion ratio FCR and survival are given in Table 2. Fish fed diets without iron supplementation had significantly lower overall weight gain. There were no significant differences among the weight gain of fish fed the 30 or 300 mg supplemental ironrkg diet. Weight gain was also significantly affected by dietary levels of vitamin C. Fish fed diets without vitamin C supplementation and diets supplemented with 50 mg vitamin Crkg had similar mean weight gain. When vitamin C
Table 2
Mean final weight gain per fish1, survival and feed conversion ratio of channel catfish fed diets containing
various levels of iron and vitamin C2
Iron added Vitamin C Weight gain Feed conversion Survival
3
Iron=vitamin C NS NS 0.0008
1
Initial weights10.6"0.1 g.
2 Ž . Ž .
Column means "S.E. having the same superscript are not significantly different P)0.05 .
3 Ž . Ž .
Feed conversionsDry feed fed grwet weight gain g .
4
Ž .
was added at the excessive level 3000 mgrkg , a significant reduction of weight gain was observed. There were no interactions between dietary levels of iron and vitamin C for weight gain. Mean FCR was poorest for iron-deficient diets but significantly improved when the diets were supplemented with iron. The addition of vitamin C had no effect on FCR. Likewise, there were no interactions between dietary levels of iron and vitamin C for FCR. Survival was significantly affected by dietary levels of iron, vitamin C and their interaction. Fish fed the iron-deficient diets had significantly lower survival than those fed the 30 mg and 300 mg iron diets. There were no significant differences among the survival of fish fed the 0 and 50 mg vitamin C diets. However, increasing vitamin C to 3000 mgrkg diet significantly decreased survival. Survival was lowest for fish fed the iron-deficient diet supplemented with 3000 mg of vitamin C. No gross signs of vitamin C deficiency were observed in catfish fed vitamin C-deficient diets with or without iron supplementation.
Mean hematological values of channel catfish fed diets containing various levels of dietary iron and vitamin C are presented in Table 3. The treatment without iron supplementation had the lowest TCC and RBC. These values significantly increased at each incremental level of iron. The effect of vitamin C on these parameters was significant only when an excessive level was added. When fish were fed iron-deficient diets, TCC and RBC significantly increased as dietary vitamin C level increased. However, in the presence of dietary iron, significant increases of TCC and RBC were obtained only when 3000 mg vitamin Crkg was used. Vitamin C level had no effect on HTC and Hb values. These values were lowest for fish fed iron-deficient diets but significantly increased when dietary iron was added. In the absence of dietary iron, supplementation of vitamin C significantly reduced HTC and Hb. When dietary iron was added, Hb and HTC values were not affected by the addition of vitamin C.
Table 3
Mean total cell count, red blood cell count, hematocrit and hemoglobin of channel catfish fed diets containing various levels of iron and vitamin C for 14 weeks1
Iron added Vitamin C Total cell count Red blood cell Hematocrit Hemoglobin
6 6
Vitamin C 0.0001 0.0001 NS NS
Iron=vitamin C 0.0001 0.0001 0.0001 0.03
1 Ž .
Values represent the means of ns9–15 determinationsrtreatment. Column means "S.E. having the
Ž .
Table 4
Mean iron and vitamin C content of livers of channel catfish after 12 weeks of feeding diets containing various levels of iron and vitamin C1
Iron added Vitamin C Iron content Vitamin C content
Žmgrkg. added mgŽ rkg. Žmgrg. Žmgrg.
3000 492.4"31.2 167.8"22.5
Ž .
Statistical analysis P level
Iron 0.0001 NS
Vitamin C 0.002 0.0001
Iron=vitamin C 0.006 NS
1
Values represent means of ns2–3 determinationsrtreatment. Column means having the same
super-Ž .
script are not significantly different P)0.05 .
Liver iron content was significantly affected by dietary levels of iron, vitamin C and
Ž .
their interactions Table 4 . Fish fed iron-deficient diets had the lowest liver iron content but was not significantly different from the group fed diets containing 30 mg of ironrkg.
Table 5
Mean macrophage migration and chemotaxis ratio of channel catfish fed diets containing various levels of iron and vitamin C for 14 weeks1
Iron added Vitamin C Mean macrophage migration Macrophage
2
Žmgrkg. added mgŽ rkg. 0 ug Exoantigen 50 ug Exoantigen chemotaxis ratio
cd d
Iron=vitamin C 0.0001 0.0001 NS
1
Values represent means of ns6–9 determinationsrtreatments. Column means having the same
super-Ž .
script are not significantly different P)0.05 .
2
Macrophage chemotactic ratio represents the number of migrating cells with E. ictaluri exoantigen
Ž .
Table 6
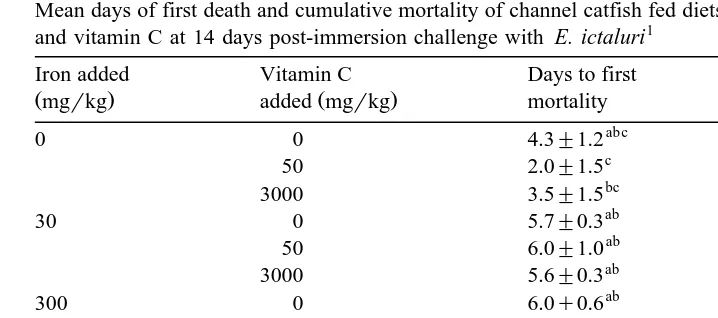
Mean days of first death and cumulative mortality of channel catfish fed diets containing various levels of iron and vitamin C at 14 days post-immersion challenge with E. ictaluri1
Iron added Vitamin C Days to first Cumulative
Žmgrkg. added mgŽ rkg. mortality mortality %Ž .
Values represent means of ns2–3 determinationsrtreatment. Column means having the same
super-Ž .
script are not significantly different P)0.05 .
These values, however, were significantly lower than those of fish fed diets with 300 mg ironrkg. Also, for the group of fish fed the 300 mg-iron diets, increasing dietary vitamin C level increased the liver iron content but the effect became significant only when 3000 mgrkg was used. Liver vitamin C content was not affected by dietary iron level or the interaction between dietary levels of iron and vitamin C. However, increasing dietary level of vitamin C significantly increased liver content of vitamin C.
Mean macrophage migration in the absence or presence of E. ictaluri exoantigen was Ž significantly affected by dietary levels of iron, vitamin C and their interactions Table
.
5 . Significantly higher mean macrophage migration without or with the presence of exoantigen was obtained in fish fed the 30-mg iron diets. The significant effect of vitamin C on these parameters was observed only when the vitamin was added at 3000 mgrkg. However, the effect of dietary iron, vitamin C or their interaction was not significant when the values were expressed in terms of chemotaxis ratio.
The average number of days at which the first mortality occurred after E. ictaluri exposure was significantly earlier for fish fed the iron-deficient diets but did not differ
Ž .
for fish fed diets containing supplemental iron Table 6 . This parameter was not affected by vitamin C or the interaction between vitamin C and iron. Cumulative mortality of fish at day 14 following E. ictaluri challenge was not affected by either
Ž .
dietary iron, vitamin C or their interaction Table 6 .
4. Discussion
Fish fed the diets without iron supplementation had significantly lower weight gain, Ž
poorer feed conversion, and reduced hematological values total blood cell count, RBC,
. Ž .
Ž . Ž . Ž .
Lim et al. 1996 and Lim and Klesius 1997 . Lim et al. 1996 , however, did not detect the adverse effect of iron deficiency on feed conversion. The significant decrease in
Ž .
survival observed in this study has also been reported by Lim and Klesius 1997 when catfish were fed the iron-deficient diet for more than 13 weeks. Gatlin and Wilson Ž1986 and Lim et al. 1996 , however, indicated that dietary iron had no effect on the. Ž . mortality of channel catfish.
Ascorbic acid has been reported to be involved in the metabolism of iron in fish ŽHilton, 1989; Maage et al., 1990; Andersen et al., 1998 . In our study, however, there. was no interaction between dietary levels of vitamin C and iron on weight gain and feed conversion, indicating that the growth response of channel catfish to varying levels of iron from iron methionine was not affected by the presence of ascorbic acid. No gross signs of vitamin C deficiency were observed in catfish fed the vitamin C-deficient diets for 14 weeks. This is in contrast to earlier studies which showed that channel catfish fed
Ž
vitamin C-deficient diets exhibited growth retardation, poor feed conversion Li et al.,
. Ž
1993 and developed characteristic signs of scurvy Lovell, 1973; Wilson and Poe, 1973; Andrews and Murai, 1975; Lim and Lovell, 1978; Lovell and Lim, 1978; Robinson,
.
1992 . Significant interactions between dietary levels of ascorbic acid and iron were observed for the survival and hematological values. At each level of iron supplementa-tion, TCC and RBC significantly increased when vitamin C was added at 3000 mgrkg diet. This increase in the number of red blood cells was probably the direct effect of
Ž .
ascorbic acid on erythropoiesis as has been suggested by Dinning 1962 and Cox Ž1968 ..
Ž The development of macrocytic anemia has been reported for channel catfish Lim
. Ž . Ž
and Lovell, 1978 , rainbow trout Hilton et al., 1978 , snakehead Agrawal and Mahajan,
. Ž .
1980 and hybrid tilapia Shiau and Jan, 1992 fed ascorbic acid deficient diets. In contrast, we observed that supplementation of ascorbic acid to iron-deficient diets resulted in decreased HTC, Hb and survival. Anemic conditions were not observed in fish fed the iron supplemented diets with or without addition of vitamin C. Thus, high levels of dietary vitamin C in the absence of dietary iron may accelerate iron deficiency and increase the severity of microcytic anemia in channel catfish. Depletion of stored iron in fish may occur sooner for fish fed the iron-deficient diets supplemented with vitamin C since this vitamin plays an important role in the release of ferritin-bound iron from the livers as well as in the transport of plasma iron to the liver and its subsequent
Ž .
incorporation into tissue ferritin Mazur et al., 1960 . A hepatic iron concentration of 30–40mgrg found in fish fed the iron-deficient diets may be unavailable for utilization and probably be indicative of microcytic anemia in channel catfish. Andersen et al. Ž1996 showed that a hepatic iron concentration of 30. mgrg was insufficient to maintain erythropoiesis and prevent the reduction of blood Hb concentration in Atlantic salmon.
Ž .
Andersen et al. 1998 reported that addition of iron exceeding the requirement did
Ž .
not increase hepatic iron in Atlantic salmon. Baker et al. 1997 showed that liver iron as well as muscle and serum iron concentrations of African catfish were unaffected by feeding diets containing 663.5 or 6354.4 mg ironrkg, even though fish fed the higher iron diet had significantly reduced growth. In the present study, hepatic iron concentra-tions increased with increasing dietary iron, although a significant increase was noted
Ž
.
level . The values for fish fed the 30 mg iron diets, although not statistically different from those of fish fed the iron unsupplemented diets, were 53% to more than 100% higher. Liver iron levels were also significantly affected by increasing dietary levels of ascorbic acid. However, significant increase in hepatic iron levels was observed only in fish fed the diet supplemented with 300 mg of iron and 3000 mg of ascorbic acid. With
Ž .
rainbow trout, Hilton et al. 1978 found an increase in hepatic iron but a decrease in spleen iron levels with increasing dietary levels of vitamin C. For the same species,
Ž .
Dabrowski and Kock 1989 reported that ascorbic acid increased the absorption of
Ž .
ferrous iron, while Lanno et al. 1985 found that vitamin C did not appear to enhance the absorption of this form of iron. The lack of a positive effect of increased level of ascorbic acid on the uptake of the ferrous iron by salmonids was because the reduced
Ž q2. Ž
form of iron Fe is readily available for salmonids Hilton, 1989; Andersen et al., .
1998 . In the present study, even though iron methionine has been shown to have bioavailability equal to or higher than the ferrous iron from iron sulfate for channel
Ž .
catfish Lim et al., 1996; Paripatananont and Lovell, 1997 , there was a significant interaction between dietary levels of iron and ascorbic acid. Thus, further studies to evaluate the effect of ascorbic acid on iron metabolism are needed.
Hepatic ascorbic acid concentrations increased as dietary ascorbic acid levels in-Ž
creased as have been shown in previous studies with channel catfish Lim and Lovell, 1978; Durve and Lovell, 1982; Robinson, 1992; Li and Robinson, 1994; Li et al., 1993,
.
1998 . A liver vitamin C level below 30mgrg has been suggested as an indication of
Ž .
vitamin C deficiency Lim and Lovell, 1978 . In the present study, although no ascorbic acid deficiency signs were observed, fish fed the diets without vitamin C
supplementa-Ž .
tion had liver vitamin C content as low as 3.2–2.0 mgrg. Robinson 1992 indicated that liver ascorbate levels may be indicative of vitamin C status of channel catfish but the exact concentration is difficult to recommend. He found that catfish fed diets containing 45–60 mg of vitamin Crkg which is adequate to prevent deficiency signs had liver ascorbate levels of 16.5 and 18.2 mgrg, respectively.
Dietary iron levels had no effect on hepatic vitamin C concentration which is in
Ž .
agreement with previous studies in Atlantic salmon Andersen et al., 1997, 1998 . In
Ž .
rainbow trout, Desjardins 1985 observed a decrease in hepatic ascorbic acid concentra-tion with increasing dietary iron levels. However, it was suggested that this apparent interaction was probably due to the effect of iron supplementation on diet rancidity and destruction of ascorbic acid during feed processing and storage. In the present study, diet rancidity and destruction of ascorbic acid were minimal because ethoxyquin was added, the diets were cold-pelleted and stored frozen until fed, and the stable form of vitamin C,L-ascorbyl-2-polyphosphate, was used.
Iron deficiency or excess suppressed macrophage chemotactic response, although the effect was more pronounced in fish fed the iron-deficient diets. This is in agreement
Ž .
with Berger 1996 who reported that either a deficiency or an excess of iron could compromise the immune system. It has also been reported that iron-deficiency had no
Ž .
affect on the specific antibody response Sealey et al., 1997 but decreased macrophage
Ž .
chemotaxis in channel catfish Lim and Klesius, 1997; Sealey et al., 1997 . The suppression of macrophage chemotaxis, however, was reversed when the iron-deficient
Ž .
The mean number of macrophage migration decreased for fish fed the vitamin C-deficient or replete diets but significantly increased when a high level of vitamin C Ž3000 mgrkg diet was added. The stimulating effect of high dietary level of ascorbic. acid on macrophage chemotaxis was observed at all levels of dietary iron
supplementa-Ž . Ž .
tion. Li and Lovell 1985 found that megadose feeding of ascorbic acid 3000 mgrkg had no effect on phagocytosis but significantly enhanced antibody production and serum
Ž .
complement activity. Li et al. 1998 , however, found no difference in post-infection antibody levels among catfish fed diets containing 3–257 mg vitamin Crkg. Likewise,
Ž .
Johnson and Ainsworth 1991 found no difference in percentage phagocytosis, phago-cytic index of anterior kidney neutrophils of channel catfish fed diets containing 100 or
Ž .
1000 mg ascorbic acidrkg. In guinea pigs, Ganguley et al. 1976 observed that macrophages from vitamin C-deficient animals had both normal phagocytic and bacteri-cidal capabilities. However, the migratory ability of the peritoneal exudate cells from deficient animals on a glass surface was significantly impaired as compared to controls. Dietary levels of iron had no effect on cumulative mortality of channel catfish 14 days after exposure to E. ictaluri but iron-deficient fish were more susceptible to infection than those with adequate iron as can be seen by the low number of day to first
Ž .
death. This agrees with the results of a recent study by Lim and Klesius 1997 , which showed that dietary iron did not protect against mortality of channel catfish from E. ictaluri, but the onset of mortality of iron-deficient fish was earlier than those fed the
Ž .
iron-replete diet. Sealey et al. 1997 , however, observed increased mortality of iron-de-ficient channel catfish due to ESC. With Atlantic salmon, it has been reported that fish
Ž
fed a low-iron diet had some protection against Vibrio anguillarum S.P. Lall, Institute .
of Marine Biosciences, NRC, Halifax, Canada, personal communication . Ravndal et al. Ž1994 observed a significant association between high concentrations of serum iron and.
Ž .
mortality of Atlantic salmon infected with V. anguillarum. Sherman 1992 suggested that a delicate balance exists between the need for iron for host defense mechanisms and the need for iron to sustain microbial growth.
The results of previous studies on the effect of ascorbic acid on disease resistance in channel catfish are not consistent. Earlier studies with channel catfish showed that high
Ž
levels of ascorbic acid increased their resistance against E. ictaluri Li and Lovell, 1985;
. Ž . Ž .
Liu et al., 1989 and E. tarda Durve and Lovell, 1982 . Li et al. 1993 found higher mortality for channel catfish fed vitamin C-free diet but increasing the vitamin C concentration higher than the level required for growth did not improve disease
Ž .
resistance. In a more recent study, however, Li et al. 1998 showed that cumulative mortality of catfish 21 days post-challenge with E. ictaluri was lower in fish fed the
Ž .
basal diet 3 mg vitamin Crkg than those fed diets containing supplemental vitamin C. In salmonids, information on the effects of ascorbic acid on disease resistance is also contradictory. High levels of vitamin C have been shown to improve resistance of
Ž .
rainbow trout against V. anguillarum Navarre and Halver, 1989 and Atlantic salmon
Ž .
against Aeromonas salmonicida Hardie et al., 1991; Waagbo et al., 1993 . Other studies, however, found no significant effect of high dietary vitamin C level on the
Ž resistance of Atlantic salmon infected with A. salmonicida or V. anguillarum Lall et
.
differences between the results of these studies may be related to differences in species, strain, size and nutritional status of the fish used, feeding management and duration, pathogenicity of the bacteria, and methods and dose of challenge.
Results of the present study indicate that iron deficiency resulted in microcytic anemia as has been previously reported. In the absence of dietary iron, supplementation of ascorbic acid may accelerate iron deficiency as can be seen by the significant decrease in weight gain, survival, HTC and Hb values. Hepatic iron content was directly
Ž .
related to the dietary level of iron. At high level of dietary iron 300 mgrkg ,
Ž .
supplementation of high level of vitamin C 3000 mgrkg further increased liver iron content. Ascorbic acid at 3000 mgrkg were needed to stimulate macrophage chemo-taxis. However, neither dietary iron nor ascorbic acid protected juvenile channel catfish from E. ictaluri infection, although iron deficiency accelerate the onset of mortality. In the absence of clear cut information and because nutrient deficiency predisposes the animals to infection due to synergistic effects of nutrient deficiency and infection, adequate levels of iron and vitamin C to meet the requirements for growth and prevent deficiency signs should be used. However, more studies are needed to better understand the interrelationships between dietary levels of iron and ascorbic acid on iron metabolism, immune response and disease resistance.
References
Agrawal, N.K., Mahajan, C.L., 1980. Hematological changes due to vitamin C deficiency in Channa punctatus bloch. J. Nutr. 110, 2172–2182.
Andersen, F., Lorentzen, M., Waagbo, R., Maage, A., 1997. Bioavailability and interactions with other micronutrients of three dietary iron sources in Atlantic salmon, Salmo salar, smolts. Aquacult. Nutr. 3, 239–246.
Andersen, F., Lysen, B., Maage, A., Waagbo, R., 1998. Interaction between two dietary levels of iron and two forms of ascorbic acid and the effect on growth, antioxidant status and some non-specific immune response
Ž .
parameters in Atlantic salmon Salmo salar smolts. Aquaculture 161, 437–451.
Andersen, F., Maage, A., Julshamn, K., 1996. An estimation of dietary iron requirement of Atlantic salmon, Salmo salar L., parr. Aquacult. Nutr. 2, 41–47.
Andrews, J.W., Murai, T., 1975. Studies on vitamin C requirements of channel catfish. J. Nutr. 105, 557–561. Baker, R.T.M., Martin, P., Davies, S.J., 1997. Ingestion of sub-lethal levels of iron sulphate by African catfish
affects growth and tissue lipid peroxidation. Aquat. Toxicol. 40, 51–61. Beisel, W.R., 1982. Single nutrient and immunity. Am. J. Clin. Nutr. 35, 417–468. Berger, L.L., 1996. Trace minerals: key to immunity. Salt Trace Miner. 28, 1–4.
Ž .
Bhaskaram, P., 1988. Immunology of iron-deficient subjects. In: Chandra, R.K. Ed. , Nutrition and Immunol-ogy. Alan R. Liss, New York, pp. 149–168.
Boyden, S., 1962. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J. Exp. Med. 115, 453–466.
Ž .
Brown, B.A., 1988. Routine hematology procedures. In: Brown, B.A. Ed. , Hematology: Principles and Procedures. Leo and Febiger, Philadelphia, PA, pp. 79–122.
Ž .
Campbell, C.R., Plank, C.O., 1992. Organic matter destruction-dry ashing. In: Plank, C.O. Ed. , Plant Analysis Reference Procedures for the Southern Region of the United States. The Georgia Agriculture Experiment Stations, Southern Coop. Ser. Bull. 368 The University of Georgia, Athens, pp. 5–6.
Ž .
Dabrowski, K., Kock, G., 1989. Absorption of ascorbic acid and ascorbic acid sulphate and their interaction
Ž .
with mineral in the digestive tract of rainbow trout Oncorhynchus mykiss . Can. J. Fish. Aquat. Sci. 46, 1952–1957.
Desjardins, L.M., 1985. The effect of iron supplementation on diet rancidity and on the growth and physiological response of rainbow trout. MSc thesis, University of Guelph, Ontario, Canada.
Dinning, J.S., 1962. Nutritional requirements for blood cell formation in experimental animals. Physiol. Rev. 42, 169–180.
Ž .
Durve, V.S., Lovell, R.T., 1982. Vitamin C and disease resistance in channel catfish Ictalurus punctatus . Can. J. Fish. Aquat. Sci. 39, 948–951.
El-Alwary, M.F.S., El-Shobaki, F.A., Kholeif, T., Sakr, R., El-Bassoussy, M., 1975. The absorption of iron, with or without supplements of single amino acids and ascorbic acid, in healthy and Fe-deficient children. J. Nutr. 33, 351–355.
El Naggar, G.O., Lovell, R.T., 1991.L-ascorbyl-2-monophosphate has equal antiscorbutic activity asL-ascorbic acid butL-ascorbyl-2-sulfate is inferior toL-ascorbic acid for channel catfish. J. Nutr. 121, 1622–1626. Erdal, J.I., Evensen, O., Kaurstad, O.K., Lillehaug, A., Solbakken, R., Thorud, K., 1991. Relationship between
Ž .
diet and immune response in Atlantic salmon Salmo salar L. after feeding various levels of ascorbic acid and omega-3 fatty acids. Aquaculture 93, 361–379.
Ganguley, R., Durieux, M.F., Waldman, R.H., 1976. Macrophage function in vitamin C-deficient guinea pigs. Am. J. Clin. Nutr. 29, 762–765.
Gatlin, D.M. III, Wilson, R.P., 1986. Characterization of iron deficiency and the dietary iron requirement of fingerling channel catfish. Aquaculture 52, 191–198.
Hardie, L.J., Fletcher, T.C., Secombes, C.J., 1991. The effect of dietary vitamin C on the immune response of
Ž .
the Atlantic salmon Salmo salar L. . Aquaculture 95, 201–214.
Harper, H.A., 1975. Review of Physiological Chemistry. 15th edn. Lange Medical Publications, Los Altos, CA.
Hilton, J.W., 1989. The interaction of vitamins, minerals and diet composition in the diet of fish. Aquaculture 79, 223–244.
Hilton, J.W., Cho, C.Y., Slinger, S.J., 1978. Effect of graded levels of ascorbic acid in practical trout diets fed
Ž .
to rainbow trout Salmo gairdneri . J. Fish. Res. Board Can. 35, 431–436.
Johnson, M.R., Ainsworth, A.J., 1991. An elevated dietary level of ascorbic acid fails to influence the response of anterior kidney neutrophils to Edwardsiella ictaluri in channel catfish. J. Aquat. Anim. Health 3, 266–273.
Klesius, P.H., 1992. Carrier state of channel catfish infected with Edwardsiella ictaluri. J. Aquat. Anim. Health 4, 227–230.
Klesius, P.H., 1993. Rapid enzyme-linked immunosorbent tests for detecting antibody to Edwardsiella ictaluri in channel catfish, Ictalurus punctatus, using exoantigen. Vet. Immunol. Immunopathol. 36, 359–368. Klesius, P.H., Sealey, W.M., 1996. Chemotactic and chemokinetic responses of channel catfish macrophages
to exoantigen from Edwardsiella ictaluri. J. Aquat. Anim. Health 8, 314–318.
Lall, S.P, Oliver, G., Weerakoon, D.E.M., Hines, J.A., 1989. The effect of vitamin C deficiency and excess on
Ž .
immune response in Atlantic salmon Salmo salar L. . In: Proceedings of 3rd International Symposium on Feeding and Nutrition in Fish, Toba, Japan 28, August–1 September. pp. 427–441.
Lanno, R.P., Slinger, S.J., Hilton, J.V., 1985. Effect of ascorbic acid on dietary copper toxicity in rainbow
Ž .
trout Salmo gairdneri Richardson . Aquaculture 49, 269–287.
Larsen, H.N., 1964. Comparison of various methods of hemoglobin determination of catfish blood. Prog. Fish-Cult. 25, 11–15.
Li, M.H., Johnson, M.R., Robinson, E.H., 1993. Elevated dietary vitamin C concentration did not improve resistance of channel catfish, Ictalurus punctatus, against Edwardsiella ictaluri infection. Aquaculture 117, 303–312.
Li, M.H., Robinson, E.H., 1994. Effect of dietary vitamin C on vitamin C concentration in channel catfish, Ictalurus punctatus, and clearance rate at two temperatures — A preliminary investigation. J. Appl. Aquacult. 4, 59–71.
Li, Y., Lovell, R.T., 1985. Elevated levels of dietary ascorbic acid increase immune response in channel catfish. J. Nutr. 115, 123–131.
Ž .
Lim, C., Klesius, P.H., 1997. Responses of channel catfish Ictalurus punctatus fed iron-deficient and replete diets to Edwardsiella ictaluri challenge. Aquaculture 157, 83–93.
Lim, C., Lovell, R.T., 1978. Pathology of vitamin C deficiency syndrome in channel catfish. J. Nutr. 108, 1137–1141.
Lim, C., Sealey, W.M., Klesius, P.H., 1996. Iron methionine and iron sulfate as sources of dietary iron for channel catfish Ictalurus punctatus. J. World Aquacult. Soc. 27, 290–296.
Liu, P.R., Plumb, J.A., Guerin, M., Lovell, R.T., 1989. Effect of megalevels of dietary vitamin C on the immune response of channel catfish Ictalurus punctatus in ponds. Dis. Aquat. Org. 7, 191–194. Lovell, R.T., 1973. Essentiality of vitamin C in feeds for intensively fed caged channel catfish. J. Nutr. 103,
134–139.
Lovell, R.T., Lim, C., 1978. Vitamin C in pond diets for channel catfish. Trans. Am. Fish. Soc. 107, 134–139. Maage, A., Waagbo, R., Olsson, P.E., Julshamm, K., Sandnes, K., 1990. Ascorbate-2-sulfate as a dietary
Ž .
vitamin C source for Atlantic salmon Salmo salar : 2. Effects of dietary levels and immunization on the metabolism of trace elements. Fish Physiol. Biochem. 8, 229–236.
Mazur, A., Green, S., Carleton, A., 1960. Mechanism of plasma iron incorporation into hepatic ferritin. J. Biol. Chem. 235, 595–603.
Ž .
Monsen, E.R., 1982. Ascorbic acid: an enhancing factor in iron absorption. In: Kies, C. Ed. , Nutritional Bioavailability of Iron. American Chemical Society, Washington, DC.
Navarre, O., Halver, J.E., 1989. Disease resistance and humoral antibody production in rainbow trout fed high levels of vitamin C. Aquaculture 79, 207–221.
Ž .
NRC National Research Council , Nutrient Requirements of Fish, National Academy Press, Washington, DC, 1993.
Paripatananont, T., Lovell, R.T., 1997. Comparative net absorption of chelated and inorganic trace mineral in channel catfish Ictalurus punctatus diets. J. World Aquacult. Soc. 28, 62–67.
Ravndal, J., Lovold, T., Bentsen, H.B., Roed, K.H., Gjedrem, T., Rorvik, K.A., 1994. Serum iron levels in farmed Atlantic salmon: family variation and associations with disease resistance. Aquaculture 125, 37–45. Robinson, E.H., 1992. Vitamin C studies with catfish: Requirement, bioavailability and stability. Technical
Bulletin 182, Mississippi Agricultural and Forestry Experiment Station, Mississippi State, MS. Roedar, M., Roedar, R.H., 1968. Effect of iron on the growth rate of fish. J. Nutr. 90, 86–90. SAS Institute, 1993. SAS System for Elementary Statistical Analysis. Carey, NC.
Sealey, W.M., Lim, C., Klesius, P.H., 1997. Influence of dietary level of iron from iron methionine and iron sulfate on immune response and resistance of channel catfish to Edwardsiella ictaluri. J. World Aquacult. Soc. 28, 142–149.
Sherman, A.R., 1992. Zinc, copper and iron nutriture and immunity. J. Nutr. 122, 604–609.
Shiau, S.Y., Jan, F.L., 1992. Dietary ascorbic acid requirement of juvenile tilapia Oreochromis niloticus=O aureus. Nippon Suisan Gakkaishi 58, 671–675.
Waagbo, R., Oines, S., Raa-Nilsen, E., Sandnes, K., 1993. Dietary vitamin C, immunity and disease resistance
Ž .
in Atlantic salmon Salmo salar . Fish Physiol. Biochem. 2, 61–73.
Wang, X., Liao, M., Hung, T., Seib, P.A., 1988. Liquid chromatographic determination ofL -ascorbic-2-poly-phosphate in fish feeds by enzymatic release ofL-ascorbate. J.A.O.A.C. 71, 1158–1161.