www.elsevier.nlrlocateraqua-online
Reduction of the bioavailability of 20
m
g
r
kg
aflatoxin in trout feed containing clay
R.W. Ellis
a,b,), M. Clements
a, A. Tibbetts
a, R. Winfree
ba
Chemistry Department, Boise State UniÕersity, 1910 UniÕersity DriÕe, Boise, ID, 83725 USA
b
US Fish and Wildlife SerÕice, USA
Accepted 10 August 1999
Abstract
Ž .
The absorption, metabolism, and excretion of dietary aflatoxin B1 AFB1 by trout given different diets were compared. Fish were fed one of two diets: an AFB contaminated basal diet or1 the contaminated basal diet with 2% sodium bentonite. Urine, feces, and gill excretions were collected and analyzed separately over a 7-day test period. On completion of the feeding trials, the fish were euthanized for chemical analysis of body fluids and major organs. Total organ loads as
Ž3 .
low as 0.05 ng AFB were quantified through the use of tritium1 H labeled AFB coupled with1 chloroformrmethanol extraction, oxygen bomb combustion and liquid scintillation counting of samples. Results demonstrated that 2% dietary bentonite supplementation blocked intestinal absorption of dietary aflatoxin, reducing liver and kidney aflatoxin loads by at least 80"10%, and increasing the amount of AFB found in the feces about 4701 "20%, compared to control fish not fed bentonite. AFB or metabolite concentrations in urine increased daily for 6 days for both1 groups, but were always significantly lower in the bentonite-fed group. On average, insoluble aflatoxin metabolites accounted for 40%–60% of the total aflatoxin load in tissues, indicating a
Ž .
high percentage conversion to an adduct that binds to protein or other materials in the tissues and is not extracted by solvents. All data indicate that 2% bentonite contained in trout diets contaminated with 20mgrkg AFB significantly reduces the amount of AFB absorbed from the1 1 digestive system following ingestion of contaminated diets. q2000 Elsevier Science B.V. All rights reserved.
Keywords: Trout; Aflatoxin; Bentonite; Diet
)Corresponding author. Chemistry Department, Boise State University, 1910 University Drive, Boise, ID, 83725 USA. Tel.:q1-208-426-3478; fax:q1-208-426-3027; e-mail: [email protected]
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
1. Introduction
Aflatoxins, a group of toxic mold metabolites, are significant to nutritionists because
Ž .
they affect human and animal health Bullerman, 1986 . Aflatoxin contamination occurs over large geographic regions and in many potential feedstuffs, such as cottonseed, peanut, corn, milo, rice, dried fish, shrimp, and meat meals. The principle aflatoxin, AFB , is one of the most toxic of all naturally occurring carcinogens. A major epidemic1
Ž .
of liver tumors hepatomas , which struck US trout hatcheries in the early 1960s, was
Ž .
traced to contaminated cottonseed meal in the feeds Wolf and Jackson, 1963 . Liver tumors are still a problem for many hatcheries producing 2–3-year-old trout for breeding
Ž .
purposes Charlie Smith, 1992, personal communication . Aflatoxin also continues to be a problem outside the United States, with noted outbreaks of fish aflatoxicosis reported
Ž . Ž .
in Germany Wunder and Korn, 1982 , Mexico RuizPerez et al., 1984 , Denmark ŽRasmussen et al., 1986 , and Chile Tim Phillips, 1990, personal communication .. Ž .
Ž
In farm animals, low level mycotoxicoses from aflatoxin and other mold metabo-.
lites are correlated with feed refusal, reduced feed conversion ratios, anemia,
reproduc-Ž .
tive failure, impaired immune response, and renal damage Hamilton, 1990 . Similar symptoms have been reported in hatchery reared fish. Under FDA regulations, most feed
Ž ingredients and nonruminant feeds are restricted to F20 mgrkg total aflatoxin FDA,
.
1989a,b . Heavily contaminated feeds are usually destroyed or are sold at a steep discount for non-feed uses. Farmers in Texas recently suffered several consecutive years of economic hardship resulting from contaminated corn crops, after which Texas relaxed quality controls by permitting heavily contaminated corn to be blended into cattle feeds ŽAnon, 1991 . When feed producers carry contaminated ingredients for use in livestock. feeds, the potential for cross-contamination of fish feeds is increased.
Ž Of all domesticated animals, trout are among the most sensitive to AFB1 CAST,
.
1989 . Tumors can take a year or more to develop, but 20 mgrkg is well above safe
Ž .
limits for trout, even for temporary feeding Lee et al., 1971 . Once ingested, AFB is1 metabolized to other forms. Carcinogenesis occurs when epoxide derivatives penetrate
Ž .
the nucleus of a cell and bind to genetic material DNA . However, aflatoxicosis is not limited to fish and farm animals. The same basic process is believed to occur both in
Ž . Ž .
trout Nakatsuru et al., 1990 and in humans Groopman et al., 1985 . New methods for Ž
controlling fish aflatoxicosis may therefore have human applications Anon, 1976; .
Bullerman, 1986 .
Aflatoxin extraction or detoxification has proven practical for only a few feed ingredients, e.g., ammoniation of cottonseed meal has been reported to effectively
Ž .
detoxify the aflatoxin contaminant Ahmad et al., 1996 , while most existing aflatoxin detoxification technologies are not applicable to most fish feed ingredients. A cost-effec-tive feed ingredient, capable of detoxifying low level aflatoxin contamination without deleterious side effects, would represent a significant advancement in animal and human
Ž .
health. Smith 1980 documented unexpected improvements in growth and feed conver-sion ratio among rainbow trout when clay minerals were added to their diet. Smith was unable to provide a suitable explanation until several years later, when researchers
Ž
.
al., 1988; Phillips et al., 1995; Lindemann et al., 1997 . Examination of Smith’s data supported his hypothesis, and initiated this study.
Ž .
Winfree and Allred 1992 developed a method to detect and measure clay–aflatoxin Žor substance–aflatoxin interactions, and calculated a numerical absorption coefficient. based on a substance’s capacity to remove aflatoxin from a mixture of feed and water. Sodium bentonite exhibited a high affinity for AFB , and preliminary feeding trials1 indicated that dietary bentonite could reduce the percentage of fish developing tumors
Ž .
after consuming AFB1 unpublished data . Bentonite is commonly employed as a feed
Ž . Ž .
binder, and previous work by Smith 1980 and Reinitz 1984 have established the mineral to be safe for trout at dietary inclusion levels up to 10%.
The current study was initiated to trace the absorption, metabolism, and elimination of dietary aflatoxin in fish consuming AFB contaminated diets, with or without 2%1 dietary bentonite added as an adsorbent. Our purpose was to determine whether
Ž .
bentonite’s demonstrated ability to adsorb AFB in vitro Winfree and Allred, 19921 would block absorption of the toxin in vivo.
2. Materials and methods
2.1. Diets
Two test diets were prepared. Each contained a casein-based diet, basal diet H440 ŽNRC, 1973 . Aflatoxin AFB. Ž 1.amounting to 20 mgrkg were added to each diet. The
Ž .
clay diet contained 20mgrkg AFB , 98% H440, and 2% sodium bentonite Volclay1 e. The non-clay diet contained 20 mgrkg AFB and 100% H440. The two diets prior to1 adding the AFB were vacuum-dried overnight at 601 8C. One gram of either diet was loaded into a hollow gelatin capsule supported upright in a drilled board. Exactly 0.100
Ž .
ml of a methanol solution containing 20 ng aflatoxin AFB1 radio-labeled with 0.2mCi
3 ŽŽ .
H Moravek Biochemicale was added to the 1.00-g diet in the gelatin capsule. The capsules were dried overnight in vacuo at 608C. The AFB1rmethanol solution and
Ž . 3
several finished pellets without bentonite were analyzed for AFB and H, respec-1 tively, to ensure specified concentration.
2.2. Feed trials and collection of excreta
Ž .
Male rainbow trout, Oncorhyncus mykiss, 266"12.6 g BW were conditioned to the H440 diets for 2 weeks before the experiment was started. The feeding trials were conducted in acrylic plastic metabolism chambers, one fish per chamber, using the
Ž .
system designed by Smith et al. 1980 . A total of 20 fish were used in the study. A group of five fish were fed the AFB diet without clay and five fish were fed the AFB1 1 diet with clay for 7 days. This procedure was repeated with 10 more fish the following week. The metabolism chambers were designed for separate collection of gill, fecal, and
Ž .
fitted, and sutured to the dorsal fin in each fish. Fish were maintained at 158C and a bottled-oxygen diffuser was installed in each head tank and oxygen levels were monitored daily with a YSI oxygen meter. Head water samples were changed daily. Only 1-day head water sample was saved for3H analysis. The fish were fed one capsule daily at the same time the head water was exchanged. Feeding was done by gently inserting a polished glass tube through the mouth and into the esophagus and ejecting a single feed capsule by means of a plunger.
Urine and fecal material were collected once daily. Urine was collected continuously with a flexible polyethylene catheter, inserted into the fish’s urinary bladder and draining to a sample bottle outside the chamber. Head and tail waters were concentrated by rotary evaporation at 508C, frozen in glass cylinders by means of a rotary shell, and freeze-dried to constant weight. Long-term storage of excreta, body fluids, and tissue samples, prior to analysis was aty608C in airtight polypropylene bottles or zipper-lock-ing polyethylene sample bags.
2.3. Euthanasia and tissue collection
At 7 days after the first feeding, the fish were removed and anaesthetized with MS-222. Blood samples were taken by direct cardiac puncture or through the caudal
Ž
artery, using a heparinized 18 gauge hypodermic needle and 60 ml syringe 10,000 units .
porcine sodium heparinrsyringe . The fish were then euthanized by severing the spinal cord and pithing neural material. The abdominal cavity was surgically opened and the contents of the urinary and gall bladders were withdrawn by hypodermic syringes and tuberculin needles. The liver and the intestinal tract from the esophagus to vent were removed. Kidneys were teased from the dorsal cavity by scalpel and a blunt probe. Blood was immediately centrifuged to separate plasma from the cellular material. The carcass, minus body fluids and tissue samples, was frozen for analysis.
2.4. Analysis of tissue and body fluids
3
H from the labeled AFB was counted in a Beckman LS 6800 liquid scintillation1 counter. The liver, kidney, lower GI tract, spleen and blood cells were extracted with chloroform–methanol. Minced tissue amounting to 0.5 g was combined with 1.8 ml of methanol and 0.9 ml of chloroform. After homogenization, the materials were cen-trifuged at 12,000=g for 15 min. The supernatant was transferred to a separatory funnel and 2.5 ml of a 4:2:1, methanolrchloroformrwater mixture was added. Follow-ing homogenization and centrifugation two more times, the supernatants were added to the separatory funnel and the tissue residue saved. A total of 10 ml of chloroform and 10 ml water were added to the separatory funnel and then left overnight. One milliliter of each fraction was then combined with 10 ml of Beckman Ready-Solv Hprb. This was set aside for a minimum of 3 days and then counted.
2.5. Combustion analysis of fecal and extracted tissue residues
Ž .
The method used was a modification by Sheppard and Rodegker 1962 . The dry sample was pressed into a pellet and placed in a Parre oxygen bomb, which was charged with 30 atm oxygen, submerged in a water bath and electrically combusted. The bomb was then cooled to condense the vapors, followed by slowly discharging the exhaust through a cold water trap. The bomb was then opened, flushed with absolute ethanol and the rinsate collected for3H analysis. The percentage recovery was approxi-mately 75"3.0%.
2.6. Statistical analysis
Ž .
The data expressed in counts per minute CPM recovered from the urine, bile, GI tract, and liver from the clay-fed and non-clay-fed fish were subjected to the Mann–
Ž .
Whitney U-test Siegel, 1956 . CPM data from the fecal matter obtained from these two
Ž .
groups were subjected to evaluation by the Student’s t-distribution McGhee, 1985 .
3. Results
Ž3 . Ž3 .
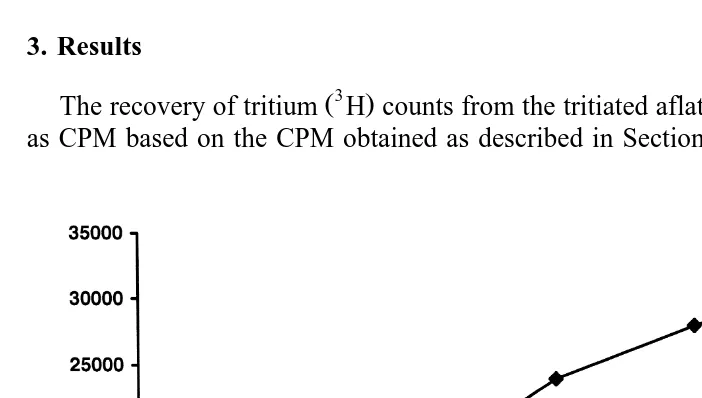
The recovery of tritium H counts from the tritiated aflatoxin H-AFB1 is reported as CPM based on the CPM obtained as described in Section 2. The CPM present in 20
Fig. 1. CPM recovered in the urine each day for a period of 7 days of feeding diets with 20mgrkg ofmgrkg
3 Ž
H-labeled AFB with and without 2% bentonite. n1 s3 for each point on each curve sampled randomly from
. Ž .
the 20 fish , except day 7 ns2 . The standard deviation range averages about 8% of the mean samples without clay and about 10% for samples with clay. Day 2 through day 6 showed a significant difference
ng of the3H-AFB , which was the amount fed in one capsule each day, was 1.041 =106 CPM.
The CPM recovered in the urine increased daily from day 1 to day 6, with the CPM
Ž .
at day 6 in the trout fed clay averaging about 85% less than for fish not fed clay Fig. 1 .
Ž .
Day 2 through day 6 showed a significant difference P)0.05, Mann–Whitney U-test . About 63% less label accumulated in the head waters from fish with clay compared to
Ž .
those not fed the clay. Only samples 4 for 1 day of head water collections were counted.
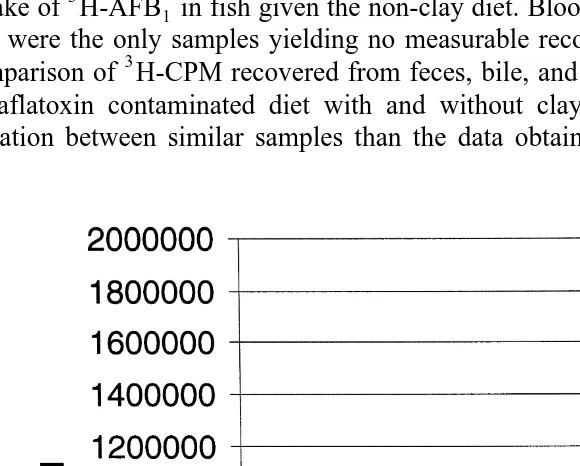
The fecal CPM recovered showed that those fish fed with clay averaged about 470%
Ž .
more label excreted in the feces than the fish not fed clay Fig. 2 . This was a significant
Ž .
difference P-0.001, Student’s t-test . The CPM recovered in the fecal material represents the only finding in which the trout fed with clay had values exceeding those not fed clay.
Results of tissue and body fluid analysis for the3H-label all indicate a much greater uptake of3H-AFB in fish given the non-clay diet. Blood samples from fish given either1 diet were the only samples yielding no measurable recovery of CPM. Fig. 2 shows the comparison of3H-CPM recovered from feces, bile, and the GI tract from fish given the 3
H-aflatoxin contaminated diet with and without clay. These data yielded a greater
Ž .
variation between similar samples than the data obtained from tissues Fig. 3 . There
Ž .
Fig. 2. CPM recovered in the bile, GI tract, and feces including contents . Trout were fed diets with 20
mgrkg3H-AFB with and without 2% bentonite. Fecal samples from eight different fish fed with clay were1
Ž .
significantly different from eight different fish fed without clay P-0.001, Student’s t-distribution . The bile
Ž . Ž
and GI tract values from the two groups three in each group were also significantly different P-0.05,
.
was some difficulty in collecting all the bile present in each fish for3H counting. Even with the large standard deviation among samples, there was a clear difference between average values obtained from fish fed the clay and non-clay diets. The bile and GI tracts from fish given the non-clay diet yielded about a 7.4 and 3.5, respectively, greater CPM
Ž
recovery than that from fish fed the clay diet significant difference P-0.05, Mann–
. 3
Whitney U-test . The amount of H in the GI tract was probably mostly due to bile
Ž 3 .
products transformed from H-AFB1 since about 90% of this activity could be rinsed away in samples from fish with or without clay. Also about 88% and 96% of the total CPM obtained from the unrinsed GI tract from the clay and non-clay diet-fed fish, respectively, appeared in the methanol fraction and the remainder in the chloroform fraction. The bile derivatives of AFB tended to be more methanol soluble, while AFB1 1
Ž .
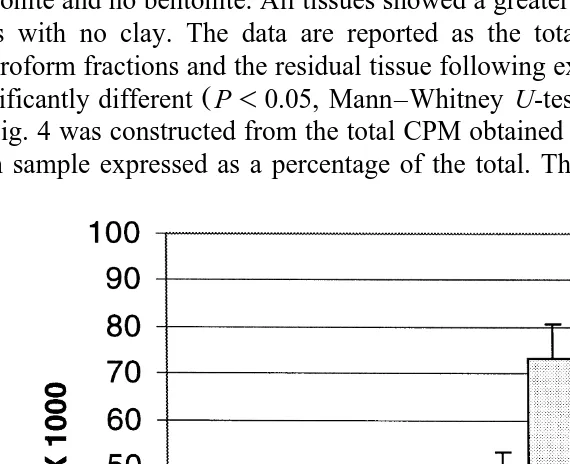
itself partitioned more extensively into the chloroform phase Wong and Hsieh, 1980 . Fig. 3 shows the comparison of label from3H-AFB accumulated in the kidney, liver,1 and carcass after 7 days of feeding fish 3H-AFB contaminated diets containing 2%1 bentonite and no bentonite. All tissues showed a greater accumulation of label in fish fed diets with no clay. The data are reported as the total CPM from the methanol and chloroform fractions and the residual tissue following extraction. The liver samples were
Ž .
significantly different P-0.05, Mann–Whitney U-test .
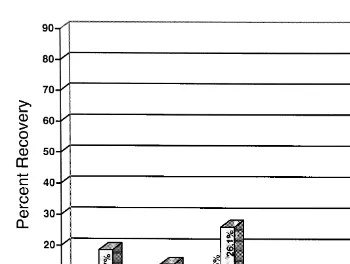
Fig. 4 was constructed from the total CPM obtained from all the materials listed with each sample expressed as a percentage of the total. The only sample from the clay-fed
Fig. 3. CPM recovered in the kidney, carcass, and liver after sacrificing fish following the 7-day feeding period. Trout were fed diets with 20mgrkg3H-AFB with and without 2% bentonite. n1 s2 for each kidney and carcass value for each dietary treatment and ns3 for liver samples from each treatment. The liver
Ž
samples from fish fed diets with and without clay were significantly different P-0.05, Mann–Whitney
.
Ž .
Fig. 4. Percentage recovery of CPM for bile, urine, GI tract including contents , kidney, carcass, liver, and feces based on the total CPM recovered from these tissues and excreta, from fish fed non-clay and 2% clay containing diets contaminated with 20mgrkg H-AFB .1
Ž .
trout containing a higher percentage and also more CPM than that from the non-clay-fed
Ž .
trout was the fecal sample 85% vs. 23% .
4. Discussion
Ž .
An in vitro study by Winfree and Allred 1992 showed that the amount of AFB1 recovered from methanol–water extracts declined by an average of 70% within 1 h of adding 10% bentonite to moistened trout feed, suggesting that the aflatoxin readily adsorbed to the surface of bentonite. Several in vivo studies suggest that this adsorption process reduces the bioavailability of aflatoxin to animals ingesting aflatoxin
contami-Ž . Ž
nated diets containing clay. Huff et al. 1992 showed that HSCAS hydrated aluminosil-.
icate reduced the toxicity of aflatoxin in broilers but did not affect the toxicity of Ž .
another mycotoxin, Ochratoxin A. Phillips et al. 1995 found that phyllosilicate clay decreased the bioavailability of aflatoxin in young rats, chickens, turkeys, lambs and pigs. They found that the b-dicarbonyl system of aflatoxin was essential for tight
Ž .
Ž .
aflatoxin. Sodium bentonite 0.5% amendment to this diet resulted in total growth recovery.
The three observations of urine, fecal and head water CPM recoveries support the Ž
idea that the aflatoxin molecule is adsorbed on the clay surface 2% bentonite in the .
diet and its uptake into the trout was greatly reduced from diets containing the bentonite. The CPM recovered in the fecal material represents the only finding in which the trout fed the clay had values exceeding those not fed clay suggesting that a large percentage of the dietary aflatoxin was held to the clay surface and passed unabsorbed
3 Ž .
through the GI tract. In our experiments, trout fed the H-AFB1 20 mgrkg -con-taminated diets without bentonite showed a relatively high level of label accumulating in the liver and bile. The liver microsomal mixed function oxidase system is known to produce hydroxylated derivatives of AFB1 that may also be conjugated to form glucouronides. These metabolic derivatives are water soluble and accumulate in the bile
Ž . 3
Wong and Hsieh, 1980 . H-AFB1 is taken into the liver and transformed a the 2,3-epoxide derivative. This derivative may form water soluble compounds and also be
Ž .
conjugated to glucuronic acid Loveland et al., 1984; Avery et al., 1992 . Liver and kidney tissues had significant methanol extract CPMs reflecting this metabolism. The 2,3-epoxide may also react with DNA forming an insoluble adduct. The epoxide could also react with protein side chains forming an insoluble adduct. This metabolic partition-ing is reflected in the extensive 3H activity found in the residual tissue of liver and kidney following methanol and chloroform extraction. The same methanolrchloroform partitioning pattern was observed in samples of bile, kidney, liver and unrinsed GI tract from trout fed the clay-containing diet.
The percentage CPM reduction of tissue or fluid accumulation from3H-AFB in the1 trout fed the contaminated diet with clay ranged from 34% in the carcass to an 83% reduction in the liver and an 87% reduction in the bile.
All tissues and excreta examined supported the contention that the presence of 2% bentonite in the diet greatly decreased the bioavailability of AFB to the trout fed 201
mgrkg AFB1 in the diet. Since 20 mgrkg is an allowable level of aflatoxin in commercial diets, the strategy of adding 2% bentonite to AFB -contaminated diets1 would be very beneficial.
References
Ahmad, M.A., Shamsuddin, Z.A., Khan, B.A., 1996. Elimination of naturally occurring aflatoxins in cottonseed meal. Pak. J. Sci. Ind. Res. 39, 183–185.
Anon, 1976. Epidemic of hepatitis in man due to aflatoxicosis. Nutr. Rev. 34, 45–46. Anon, 1991. Texas ammoniation, blending plan takes effect. Feedstuffs 63, 3.
Avery, E.H., Lee, B.L., Freedland, R.A., Cornelius, C.E., 1992. Bile pigments in gallbladder and freshly-secreted hepatic duct bile from fed and fasted rainbow trout, Oncorhynchus mykiss. Comp. Biochem. Physiol. 101A, 857–861.
Bullerman, L.B., 1986. Mycotoxins and food safety. Food Technology 40, 59–66.
Ž .
CAST Council for Agricultural Science and Technology . 1989. Mycotoxins: Economic and health risks. CAST. Ames, IA, 91 pp.
Ž .
Ž .
FDA Food and Drug Administration , 1989b. Aflatoxin in animal feed: regulatory review. FDA Veterinarian IV, 7–8.
Groopman, J.E., Donahue, P.R., Zhe’, J., Chen, J.S., Wogan, G.N., 1985. Aflatoxin metabolism in humans: detection of metabolites and nucleic acid adducts in urine by affinity chromatography. Proc. Natl. Acad. Sci. U.S.A. 82, 6492.
Hamilton, P., 1990. Problems with mycotoxins persist, but can be lived with. Feedstuffs 62, 22–23. Harvey, R.B., Kubena, L.F., Phillips, T.D., Huff, W.E., Corrier, D.E., 1988. Approaches to the prevention of
aflatoxicosis explored. Feedstuffs 60, 11–12.
Huff, W.E., Kubena, L.F., Harvey, R.B., Phillips, T.D., 1992. Efficacy of hydrated sodium calcium aluminosilicate to reduce the individual and combined toxicity of aflatoxin and ochratoxin A1. Poult. Sci. 71, 64–69.
Lee, D.J., Wales, J.H., Sinnhuber, R.O., 1971. Promotion of aflatoxin-induced hepatoma growth in trout by methyl malvalate and sterculate. Cancer Res. 31, 960–963.
Lindemann, M.D., Blodgett, D.J., Harper, A.F., Korneyay, E.T., Doerr, J.A., 1997. Appraisal of the value of selected clays and minerals in diets with and without aflatoxin-contaminated maize fed to young pigs. J. Anim. Feed Sci. 6, 507–519.
Ž .
Loveland, P.M., Nixon, J.E., Bailey, G.S., 1984. Glucuronides in bile of rainbow trout Salmo gairdneri
w x Ž .
injected with 3H -aflatoxin B1 and the effects of dietary -napthoflavone . Comp. Biochem. Physiol. 78C, 13–19.
McGhee, J.W., 1985. Introductory Statistics. West Publishing, St. Paul, 249 pp.
Nakatsuru, Y., Xiusheng, Q., Mashahito, P., Ishikawa, T., 1990. Immunological detection of in vivo aflatoxin B1-DNA adduct formation in rats, rainbow trout, and coho salmon. Carcinogenesis 11, 1523–1526.
Ž .
NRC National Research Council , 1973. Nutrient Requirements of Trout, Salmon, and Catfish. National Academy of Sciences, Washington, DC, USA.
Phillips, T.D., Sarr, A.B., Grant, P.F., 1995. Selective chemisorption and detoxification of aflatoxins by phyllosilicate clay. Natural Toxins 3, 204–213.
Rasmussen, H.B., Larsen, K., Hald, B., Moeller, B., Elling, F., 1986. Outbreak of liver-cell carcinoma among saltwater-reared rainbow trout Salmo gairdneri in Denmark. Dis. Aquat. Org. 1, 191–196.
Reinitz, G., 1984. The effect of nutrient dilution with sodium bentonite in practical diets for rainbow trout. Prog. Fish-Cult. 46, 249–253.
RuizPerez, A., PaaschMartinez, L., AdamedePaasch, P., RosilesMartinez, R., 1984. Hepatic neoplasia in the
Ž . Ž .
rainbow trout Salmo gairdneri bred in El Zarco Fish Hatchery, Federal District. Veterinaria Mex. 15, 255–261.
Sheppard, H., Rodegker, W., 1962. Determination of H3 and C14 in biological materials using oxygen bomb combustion. Anal. Biochem. 4, 246–251.
Siegel, S., 1956. Non-parametric Statistics for the Behavioral Sciences. McGraw-Hill, New York. 116 pp. Smith, R.R., 1980. Recent advances in nutrition: clay in trout diets. Salmonid, pp. 16–18.
Smith, R.R., Peterson, M.C., Allred, A.C., 1980. Effect of leaching on apparent digestion coefficients of feedstuffs for salmonids. Prog. Fish-Cult. 42, 195–199.
Winfree, R.A., Allred, A., 1992. Bentonite reduces measurable aflatoxin B1 in fish feed. Prog. Fish-Cult. 54, 157–162.
Wolf, H., Jackson, E.W., 1963. Heptomas in rainbow trout: descriptive and experimental epidemiology. Science 142, 676–678.
Wong, A.A., Hsieh, D.P.H., 1980. The comparative metabolism of toxicokinetics of aflatoxin B1 in the monkey, rat, and mouse. Toxicol. Appl. Pharmacol. 55, 115–125.
Ž . Ž .