Effect of ursodeoxycholic acid on hepatic LDL binding and
uptake in dietary hypercholesterolemic hamsters
Susan Ceryak
a, Bernard Bouscarel
a,b,*, Mauro Malavolti
c, Sander J. Robins
d,
Kathleen L. Caslow
a, Hans Fromm
aaDepartment of Medicine,Di6ision of Gastroenterology and Nutrition,The George Washington Uni6ersity Medical Center,2300I St,
N.W.523Ross Hall,Washington,DC20037,USA
bDepartment of Biochemistry and Molecular Biology,The George Washington Uni6ersity Medical Center,2300I St,N.W.523Ross Hall,
Washington,DC20037,USA
cIstituto di Clinica Medica e Gastroenterologica,Uni6ersity of Bologna,Bologna,Italy
dLipid Metabolism Laboratory and Department of Medicine,Veterans Administration Medical Center,Boston,MA, USA
Received 7 December 1998; received in revised form 26 December 1999; accepted 19 January 2000
Abstract
Administration of ursodeoxycholic acid (UDCA) has been shown to decrease serum total and low density lipoprotein (LDL) cholesterol in hypercholesterolemic patients with primary biliary cirrhosis. Results of previous studies prompted us to postulate that the cholesterol-lowering effect of UDCA may be due, at least in part, to a direct increment in hepatic LDL receptor binding [Bouscarel et al., Biochem J, 1991;280:589; Bouscarel et al., Lipids 1995;30:607]. The aim of the present investigation was to determine the ability of UDCA to enhance hepatocellular LDL receptor recruitment, as determined by its effect in vivo on LDL uptake, and its effect in vitro on LDL binding, under conditions of moderately elevated serum cholesterol. Study groups consisted of male golden Syrian hamsters fed either a standard chow diet (control), a 0.15% cholesterol-containing diet, or a 0.15% cholesterol-containing diet supplemented with either 0.1% UDCA, or 0.1% chenodeoxycholic acid (CDCA). Cholesterol feeding increased (PB0.01) total serum cholesterol by 44%, and was associated with a 10-fold accumulation of cholesteryl esters in the liver (PB0.01). In vivo, hepatic uptake of [U-14C]sucrose-labeled hamster LDL was increased (PB0.05) to a level of 4549101
ml in animals fed a cholesterol-containing diet supplemented with UDCA, compared to that either without UDCA (337956ml), or with CDCA (240949 ml). The hepatic uptake of [U-14C]sucrose-labeled methylated human LDL, a marker of LDL receptor-independent LDL uptake, was unaffected by bile acid feeding. In vitro, specific binding of [125I]hamster LDL to isolated
hepatocytes was determined at 4°C, in presence and absence of 700mmol/l UDCA. TheKDranged from 25 to 31mg/ml, and was
not affected by either cholesterol feeding or UDCA. In the presence of UDCA, theBmaxwas increased by 19% (PB0.05) in cells
isolated from control animals and by 29% (PB0.01) in cells isolated from hamsters fed a cholesterol-supplemented diet. In conclusion, in dietary hypercholesterolemic hamsters, both chronic in-vivo and acute in-vitro treatments with UDCA resulted in restoration of hepatic LDL binding and uptake to levels observed in control hamsters. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Cholesterol metabolism; Isolated hepatocytes; Bile acids
www.elsevier.com/locate/atherosclerosis
1. Introduction
The low density lipoprotein (LDL) receptor plays a central role in the regulation of cholesterol homeostasis,
while the liver is a key organ in the maintenance of whole body cholesterol balance [1,2]. In a number of species, including the hamster, most of receptor-medi-ated clearance of LDL occurs in the liver [3 – 5] by rapid internalization of the complex formed by the binding of the apolipoprotein-B100-containing particle to the LDL
receptor [6]. Once in the cell, the LDL particle dissoci-ates, and the receptor generally is cycled back to the cell surface membrane [7].
* Corresponding author. Tel.: +1-202-9942114; fax: + 1-202-9943435.
E-mail address:[email protected] (B. Bouscarel).
The activity of the LDL receptor is tightly regulated by changes in cholesterol supply and demand. Hepatic LDL receptor synthesis and cycling can be markedly suppressed by dietary cholesterol [8,9]. Conversely, ad-ministration of both bile acid binding resins, which augment hepatocellular bile acid synthesis, and cholesterol synthesis inhibitors, increase the number of LDL receptors, by increasing the cellular cholesterol demand [10,11]. The consequent changes in cellular cholesterol pools modulate the production of LDL receptor mRNA and the synthesis of the LDL receptor.
It has previously been shown in vivo, in the golden Syrian hamster, that chronic feeding of ursodeoxycholic acid (UDCA), in contrast to that of its 7a-hydroxyl epimer, chenodeoxycholic acid (CDCA), evoked a sig-nificant increment in hepatic LDL uptake. However, this occurred in spite of both a 55 – 71% enrichment of the bile acid pool with CDCA and a marked suppres-sion of bile acid synthesis [12]. This increased LDL receptor-dependent LDL uptake and degradation in vitro have also been documented, upon acute exposure to UDCA, in hepatocytes isolated from standard ro-dent chow-fed hamsters [13,14]. Furthermore, recent studies from our laboratory have demonstrated that UDCA interacts directly with the LDL receptor, in the absence of any effects on either the LDL particle or on the membrane lipid composition, and independently of any effects on LDL receptor synthesis or cycling [13,15]. Thus, the ability of UDCA to directly augment LDL receptor-dependent LDL uptake is independent of changes in cellular cholesterol pools.
Administration of UDCA has been shown to de-crease serum total and LDL cholesterol in hypercholes-terolemic patients with primary biliary cirrhosis [16]. In light of results of previous studies, it was postulated that the cholesterol-lowering effect of UDCA may be due, in part, to a direct increment in LDL receptor binding [13,15]. The present investigation was under-taken in order to determine the ability of UDCA to enhance hepatocellular LDL receptor recruitment, as determined by its effect both in vivo on LDL uptake, and in vitro on LDL binding, under conditions of moderately elevated serum cholesterol concentrations [17]. This was achieved by feeding male golden Syrian hamsters an excess of cholesterol in order to induce mild hypercholesterolemia. Under these conditions, the effects of UDCA on parameters of hepatic LDL metabolism were studied both in vivo and in vitro.
2. Materials and methods
2.1. Materials
Sodium 125I (specific activity 16 – 20 mCi
/mg) and
[U-14C]sucrose (specific activity 380
mCi/mmol) were purchased from Amersham Corporation (Arlington Heights, IL). Gelatin and bovine serum albumin (frac-tion V) were purchased from Sigma (St. Louis, MO). UDCA was supplied by Tokyo Tanabe (Tokyo, Japan), and CDCA by Dr Falk GmbH (Freiburg, Germany). Both UDCA and CDCA were 98 – 99% pure, as judged by gas – liquid chromatography. All other chemicals used were of analytical grade available from commer-cial sources.
2.2. Animals
Male golden Syrian hamsters (115 – 140 g body weight; Harlan Sprague Dawley, Indianapolis, IN), were divided into four groups, each of which consisted of 10 – 13 age-matched animals. The first group was fed a standard rodent chow diet (Ralston Purina, St. Louis, MO), containing 0.027% cholesterol (control, CONT). The second group received the stan-dard diet in which the cholesterol content had been increased to 0.15% (cholesterol, CHOL). In the other two groups, the 0.15% cholesterol-containing diet was supplemented with 0.1% UDCA (CHOL-UDCA) and 0.1% CDCA (CHOL-CDCA), respectively. The relative percentage of saturated, monounsaturated, and polyunsaturated fatty acids in the diet was 1.41, 1.44, and 1.65%, respectively, with the predominant species of 16:0 (66%), 18:1 (92%), and 18:2 (84%) (Purina Mills). Each group received the specific diet for 3 weeks. There were no differences among the treatment groups as far as food intake and weight gain were concerned. Mean food intake was 6.5 g/day, while mean body weight ranged from 120 – 140 g following treatment. The percentage of cholesterol in the cholesterol-supplemented diets corresponded to a daily intake of 0.075 g/day per kg body weight. Both bile acids were well tolerated by the animals, and livers appeared macroscopically normal after 3 weeks of the respective cholesterol and bile acid feeding. All animals received humane care in compli-ance with the George Washington University guidelines.
2.3. Serum lipid determinations
The total serum cholesterol concentration was deter-mined enzymatically using the cholesterol esterase – cholesterol oxidase/phenol-4-amine phenazone reaction (Boehringer Mannheim Diagnostics, Indianapolis, IN). Serum high density lipoprotein (HDL) cholesterol was measured as described for total serum cholesterol after precipitation of apo B-containing lipoproteins with dex-tran sulfate-MgCl2, using the method of Warnick et al.
2.4. Hepatocellular lipid determinations
Livers were removed from Nembutal-anesthetized hamsters 3 weeks after feeding a control diet, or a cholesterol-supplemented diet. Liver homogenates and enriched plasma membrane fractions were prepared by the method of Prpic’ et al. [19], as previously de-scribed [20]. Cellular and membrane protein content was assessed with a bicinchonic acid (BCA) protein assay kit (Pierce, Rockford, IL).
Total lipids were extracted from aliquots of liver homogenate and enriched plasma membrane fractions, respectively, according to the method of Bligh and Dyer [21]. Lipid extracts were kept under nitrogen in glass tubes to prevent oxidative degradation. Phos-pholipid and cholesterol amounts were determined in liver homogenate and plasma membrane lipid extracts using HPLC procedures previously described [22]. Quantitation was performed by integration of peak areas in conjunction with internal standards. Total phospholipid was quantitated by phosphorus mea-surement [23]. Lipid concentrations were expressed per mg of protein.
2.5. Biliary bile acid composition
In order to confirm the enrichment of the respec-tive bile acid pool by either UDCA or CDCA in the hamsters receiving the bile acid-supplemented diets, the relative bile acid composition was determined, by gas – liquid chromatography, in gallbladder bile as previously described [12,15].
2.6. Biliary lipid determinations
Total bile acid concentration was measured, using 3a-hydroxysteroid dehydrogenase (Worthington Bio-chemicals, Malvern, PA) [24]. Biliary phosphatidyl-choline concentration was determined by the enzymatic measurement of choline content using a choline oxidase/peroxidase reaction following treat-ment with phospholipase D (Nippon Shoji Kaisha, Higashi-Ko, Osaka, Japan) [25]. The biliary choles-terol content was analyzed enzymatically, using a cholesterol oxidase/catalase reaction (Boehringer Mannheim Biochemicals, Indianapolis, IN) [26].
2.7. Separation of plasma LDL
Blood was collected from normal human subjects, as well as from normocholesterolemic hamsters, into EDTA-containing vacuum collection tubes. The plasma lipoproteins were separated by density gradient ultracentrifugation, as previously reported [12 – 14,27]. The total protein concentration of the LDL fraction was determined by the method of
Brad-ford [28], using the Bio-Rad® protein assay (Bio-Rad
Laboratories, Richmond, CA). The purity of the LDL fraction was confirmed by SDS-page as de-scribed [12 – 14]. In some studies, human LDL was reductively methylated, as previously described [12 – 14].
2.8. In-6i6o hepatic uptake of[14C]sucrose-labeled LDL
Animals were maintained under diethyl ether anes-thesia for the duration of the surgical procedure as well as the subsequent infusion experiments. For in-vivo studies, both hamster LDL and methylated hu-man LDL were radiolabeled with [U-14
C]sucrose [12,27]. The final [14C]sucrose-labeled LDL had a
spe-cific activity of 11889719 dpm/mg protein. Either [14C]sucrose-labeled hamster LDL or methylated
hu-man LDL was administered via a jugular vein catheter (Silastic®, I.D., 0.012 in.; O.D., 0.025 in.,
Dow Corning, Midland, MI) in a bolus, containing 20 mg of LDL protein, followed by a constant infu-sion for 1 h at a rate of 1 mg/70 ml normal saline/min [12,27]. All LDL infusion experiments were carried out between 09:00 and 12:00 h. Each study on a given day was carried out in three animals, one of each group (CHOL, UDCA-CHOL, CDCA-CHOL), using the same LDL preparation. After completing the LDL infusion at 60 min, the abdomen was opened with a midline incision, and a 50 ml sample of blood was withdrawn from the inferior vena cava, the gallbladder bile was aspirated, the liver was removed, and the animals were exsanguinated. Livers were combusted in toto in a Packard Oxidizer (Packard Instrument, Downers Grove, IL) [29]. The radioactiv-ity in the combusted liver and blood was determined by scintillation counting.
The LDL tissue space in the liver was obtained by subtracting the [14C]albumin tissue space, which was
previously determined by the infusion of [14
C]sucrose-labeled hamster albumin (Research Plus, Bayonne, NJ) [12,27], from the [14
C]LDL tissue space. The up-take in the liver was normalized for an animal weigh-ing 100 g.
2.9. Hepatocyte isolation
2.10. In-6itro hepatocellular [125I]LDL binding
Native hamster LDL isolated from normocholes-terolemic animals was labeled with [125I]sodium iodide
to a specific radioactivity of 80 – 200 cpm/ng of protein, as previously described [13 – 15], using the iodine monochloride technique [30,31].
With hepatocytes isolated in tandem from 1 hamster of each group fed the 0.027 and the 0.15% cholesterol-containing diet, respectively, LDL binding was studied [13,14]. The cells (150 – 250 mg protein/ml) were incu-bated with increasing concentrations of 125I-labeled
hamster LDL (1 – 120 mg protein/ml) in the presence and absence of 700 mmol/l UDCA. Previous studies have shown that, at this concentration, UDCA is not toxic to the cell [32], and induces a maximum increase in hepatocellular LDL binding [13,14]. After 1 h incu-bation at 4°C, the cell-associated radioactivity was mea-sured in a Beckman model 4000 gamma-radiation counter. Nonspecific binding was determined by incu-bating the cells under the same conditions, but with an excess of native human LDL (2 – 2.5 mg/ml) [13,15]. Specific binding was determined by subtracting nonspe-cific binding from total binding. Saturation binding curves were derived, and the maximum number of binding sites (Bmax, ng/mg protein) and the dissociation
constant (KD, mg LDL protein/ml) were calculated
[13,15].
2.11. Statistical analysis
The paired t-test was used to compare the acute effect of the respective UDCA treatment and dietary cholesterol supplementation with that of the respective controls. An analysis of variance, and Student – Neu-man – Keuls test were used to compare the differences in the measured parameters as a result of bile acid feeding.
3. Results
3.1. Serum lipid concentrations
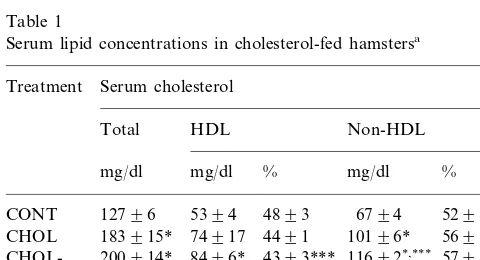
After 3 weeks of feeding a 0.15% cholesterol-supple-mented diet, total serum cholesterol concentrations in-creased by 44%, (Table 1). The percentage of serum cholesterol associated to HDL particles was signifi-cantly decreased from 48 to 44% of the total serum cholesterol in the hypercholesterolemic animals.
In those hamsters fed a 0.15% cholesterol-supple-mented diet, CDCA supplementation induced a signifi-cant decrease in the percentage of serum HDL cholesterol, and a consequent increase in the proportion of non-HDL cholesterol, when compared to both the UDCA-fed animals, and the bile acid-free diet group (CHOL).
3.2. Hepatocellular lipid concentrations
Both the unesterified cholesterol and total phospho-lipid concentrations were markedly higher, per mg of protein in the isolated liver membrane fraction, when compared to that in the total homogenate (data not shown). As a result of cholesterol feeding, there was 10-fold accumulation of cholesteryl esters observed in the liver homogenate (Table 2), and the absolute con-centrations of unesterified and esterified cholesterol were calculated to be 3.890.08 and 38.795.7 mmol/g tissue. However, there were no changes in the unes-terified cholesterol content, in either the homogenate or membrane fractions. Furthermore, there was no change in the phospholipid content or composition of either the liver homogenate or of the plasma membrane, as a result of cholesterol feeding (data not shown).
3.3. Biliary bile acid and lipid composition
The biliary bile acid composition in the bile acid-treated groups is shown in Table 3. In the animals fed the 0.15% cholesterol-containing diet, CDCA content in bile increased significantly during chronic treatment with CDCA, but was not increased by UDCA treat-ment, in contrast to what has previously been observed in normocholesterolemic hamsters [12]. Corresponding decreases in the relative percentages of biliary
deoxy-Table 1
Serum lipid concentrations in cholesterol-fed hamstersa
Serum cholesterol
aRespective groups of hamsters were fed either a standard chow
diet, containing 0.027% cholesterol (CONT, control); a 0.15% choles-terol-containing diet (CHOL); the 0.15% cholescholes-terol-containing diet supplemented with 0.1% ursodeoxycholic acid (CHOL-UDCA); or the same diet supplemented with 0.1% chenodeoxycholic acid (CHOL-CDCA) for 3 weeks. At the end of this period, serum cholesterol was determined photometrically, by standard enzymatic methods. High density lipoprotein (HDL) cholesterol was determined photometrically in serum following precipitation of the apoB-contain-ing lipoproteins with 2% dextran sulfate:1 M MgCl2. Non-HDL
cholesterol was determined by subtraction. Results are the means9
S.E.M. of determinations in 8–13 animals of each group. * Significantly different from CONT group,PB0.01. ** Significantly different from CHOL group,PB0.01.
Table 2
Lipid content of liver homogenates of cholesterol-fed hamstersa
Treatment Unesterified cholesterol Cholesteryl esters Phospholipids (nmol/mg protein) (nmol/mg protein) (nmol/mg protein)
CONT 2693 390.3 22916
300931*
3090.3 1792
CHOL
aRespective groups of hamsters were fed either a standard, 0.027% cholesterol-containing chow diet (CONT, control), or a 0.15%
cholesterol-containing diet (CHOL) for 3 weeks. At the end of this period, livers were removed, homogenized, and the lipids associated with the homogenate were extracted into chloroform/methanol, 1:2, v/v and measured as indicated in Section 2. Results are the means9S.E.M. of determinations in three animals of each dietary treatment group.
* Significantly different from respective control,PB0.01.
Table 3
Biliary bile acid composition in dietary hypercholesterolemic hamsters following chronic bile acid feedinga
Treatment LCA DCA CDCA UDCA CA Other
% of total
290.2
CHOL 1592 4593 n.d. 4194 190.7
894 4895
CHOL-UDCA 390.1 3093* 1293* 0.190.1
691* 7792* n.d.
590.5* 1091*
CHOL-CDCA 190.8
aRespective groups of hamsters were fed either a 0.15% cholesterol-containing diet (CHOL), the 0.15% cholesterol-containing diet supplemented
with 0.1% ursodeoxycholic acid (CHOL-UDCA), or the same diet supplemented with 0.1% chenodeoxycholic acid (CHOL-CDCA) for 3 weeks. At the end of this period, relative bile acid composition was determined by GLC in gallbladder bile. Results are the means9S.E.M. of determinations in four animals of each treatment group. ‘Other’ represents unidentified gas chromatographic peaks. Other abbreviations used: LCA, lithocholic acid; DCA, deoxycholic acid; CA, cholic acid.
* Significantly different from control,PB0.02.
cholic and cholic acids were observed following chronic treatment with both bile acids, due to the relative enrichment in the respective fed bile acid, and its metabolites.
No significant differences were observed in the re-spective biliary cholesterol, phospholipid and bile acid concentrations in the gallbladder bile obtained from animals fed a 0.15% cholesterol-supplemented diet when compared to those of the control animals, and the respective values were around 1.6, 3.2, and 62 mmol/l (data not shown). In a small number (2 – 3) of animals fed the bile acid-supplemented diets, there were also no significant differences in the biliary lipid composi-tion as a result of bile acid supplementacomposi-tion (data not shown).
3.4. In-6i6o hepatic LDL uptake
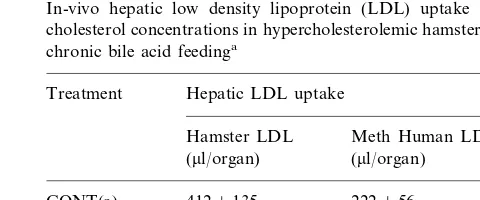
As reported in Table 4, in the animals fed a 0.15% cholesterol-containing diet supplemented with UDCA, the total hepatic uptake of [U-14C]sucrose-labeled
ham-ster LDL was significantly increased by 35 and 89% compared to the respective bile acid-free (CHOL) and CDCA-supplemented diets. In contrast, the receptor-in-dependent LDL uptake showed no significant differ-ences among the different study groups. Furthermore, as shown in Fig. 1, in hypercholesterolemic hamsters, stimulation of hepatic LDL uptake was positively
cor-related (r=0.6729, PB0.02) with an increase in the percentage of serum HDL cholesterol.
Table 4
In-vivo hepatic low density lipoprotein (LDL) uptake and serum cholesterol concentrations in hypercholesterolemic hamsters following chronic bile acid feedinga
Treatment Hepatic LDL uptake
Meth Human LDL Hamster LDL
(ml/organ)
(ml/organ)
4129135 222956 CONT(a)
337956 144961 CHOL
157959 4549101*,**
CHOL-UDCA
240949 153922 CHOL-CDCA
aFor experimental design and abbreviations, see legends of Table
1. Hepatic LDL uptake was determined in hamsters receiving the respective indicated diets, after 1 h intravenous infusion of either [U-14C]sucrose-labeled hamster LDL or methylated (meth) human
LDL. The tissue space of LDL (ml/g) was calculated, and liver uptake
was normalized for an animal weighing 100 g. Results are the means9S.E.M. of determinations of LDL uptake in five animals of each group. (a) Values obtained from a previously published study conducted with the same group of hamsters [12], in which animals were fed a standard chow diet, containing 0.027% cholesterol (CONT, control), results are the means9S.E.M. of determinations in five animals, and are not used in statistical analysis.
* Significantly different from CHOL group,PB0.05
Fig. 1. Correlation between in-vivo hepatic uptake of [U-14
C]sucrose-labeled hamster low density lipoprotein (LDL) and percent serum high density lipoprotein (HDL) cholesterol. Respective groups of hamsters were fed either a 0.15% cholesterol-containing diet (CHOL), the 0.15% cholesterol-containing diet supplemented with 0.1% ursodeoxycholic acid (CHOL-UDCA), or the same diet supplemented with 0.1% chenodeoxycholic acid (CHOL-CDCA). After 3 weeks of feeding the respective indicated diets, total liver uptake of LDL was determined, following 1 h intravenous infusion of [U-14
C]sucrose-la-beled hamster LDL. The tissue space of LDL (ml/g) was calculated, and the hepatic uptake of LDL was normalized for an animal weighing 100 g. Both total and HDL cholesterol concentrations in serum were determined photometrically. The correlation coefficient (r), P-value, and equation of the regression line are also reported.
4. Discussion
Results of the present study in dietary hypercholes-terolemic hamsters indicate that both chronic, in-vivo and acute, in-vitro treatments with UDCA were able to restore the respective hepatic LDL binding and uptake to the levels observed in normocholesterolemic ham-sters. Furthermore, the administration of UDCA in conjunction with a high cholesterol diet suppressed the biotransformation of UDCA to CDCA. This possibly removed some of the repressive effects of CDCA on hepatic LDL uptake, namely, an inhibition of bile acid synthesis [33], and the consequent decrease in LDL receptor activity [34], thus augmenting the stimulatory effects of UDCA.
Hamster serum cholesterol was significantly increased after 3 weeks of cholesterol supplementation, reflecting an increase in the total amount of cholesterol
associ-Fig. 2. Effect of ursodeoxycholic acid (UDCA) on the specific binding of [125I]hamster low density lipoprotein (LDL) to hepatocytes isolated
from cholesterol-fed hamsters. Hepatocytes were isolated in tandem from one animal of each group of hamsters receiving either the 0.027% cholesterol-containing control diet (A) or the diet containing 0.15% cholesterol (B). The binding of increasing concentrations (1 – 120 mg/ml) of [125I]hamster LDL to isolated hamster hepatocytes
(40 – 50 mg of cells) was measured after incubation for 60 min, at 4°C, in the absence (−UDCA, control, ) and in the presence of 700
mmol/l UDCA (+UDCA,). Nonspecific binding was determined by incubating the cells under the same conditions but in the presence of an excess of native human LDL (2.5 mg/ml). The saturation curves are representative of four experiments performed with hepatocytes isolated in tandem from animals of each treatment group.
3.5. Effect of UDCA on the specific binding of [125I]hamster LDL to hepatocytes isolated from hypercholesterolemic hamsters
With hepatocytes isolated in tandem from one ani-mal of each group of hamsters receiving either a control diet, or a 0.15% cholesterol-supplemented diet, LDL binding was studied at 4°C, using increasing concentra-tions (1 – 120mg/ml protein) of [125I]hamster LDL (Fig. 2). An excess of native human LDL (2.5 mg/ml) was used to measure nonspecific LDL binding. The nonspe-cific binding was not affected by cholesterol feeding in either the presence or absence of UDCA and repre-sented around 25% of the total binding. The Bmaxwas
103943 and 94938 ng/mg of cells, in the control and cholesterol-fed groups, respectively, and was not signifi-cantly different. In the presence of UDCA, the Bmax
was significantly increased by 19% in the hepatocytes isolated from the control-fed animals, and by 29% in the hepatocytes isolated from hamsters fed a 0.15% cholesterol-supplemented diet. Analysis of the satura-tion curves showed that the KD was not significantly
different from control following cholesterol feeding and furthermore, theKDwas not affected by UDCA (Table
Table 5
Effect of ursodeoxycholic acid (UDCA) in vitro on apparent low density lipoprotein (LDL) binding constants determined in hepato-cytes isolated from cholesterol-fed hamstersa
KD(mg/ml) Bmax(ng/mg of cells)
aFor experimental design and abbreviations, see legend of Table 1.
Hepatocytes were isolated in tandem from one animal of each group of hamsters receiving either a CONT or CHOL diet. The binding of increasing concentrations (1–120 mg/ml) of [125I]hamster LDL to
isolated hamster hepatocytes (40–50 mg of cells) was measured after incubation for 60 min, at 4°C in the absence (−UDCA, control) and in the presence of 700mmol/l UDCA (+UDCA). Nonspecific binding was determined by incubating the cells under the same conditions but in the presence of an excess of native human LDL (2.5 mg/ml). Results are the means9S.E.M. of four different experiments per-formed with hepatocytes isolated from one animal of each treatment group.
*PB0.05: significantly different in the presence of UDCA. **PB0.01.
Furthermore, in spite of the absence of an absolute serum LDL cholesterol-lowering effect of UDCA, the enhanced LDL uptake should result in a decreased residence time in the bloodstream. Walzem et al. [39] have demonstrated that the susceptibility to in-vitro oxidation of LDL particles was highly correlated with lipoprotein age in-vivo. Furthermore, there is consider-able evidence that the level of oxidation of the LDL particle may directly influence its atherogenicity [40]. Therefore, if the residence time of the LDL particle in the bloodstream is shorter, due to UDCA-induced en-hanced hepatic uptake, the potential for oxidative mod-ification of the LDL particle may be decreased. This could support a therapeutic role for UDCA in the treatment of mild hypercholesterolemia, however, this remains to be confirmed.
Increased cholesterol/phospholipid ratios in lipid bi-layers have been shown to increase the stability, and consequently, the activity of integral membrane span-ning proteins, such as the nicotinic acetylcholine recep-tor [41]. In addition, van de Heijning et al. [42] have shown that UDCA protects the cholesterol integrity of vesicles. This stabilizing effect of UDCA is enhanced with increasing vesicular cholesterol/phospholipid ratio [43,44]. In this study it has been shown that cholesterol feeding does not alter the hepatic membrane choles-terol/phospholipid ratio. In light of the present results, it was postulated that under the condition of hyperc-holesterolemia, domains of the plasma membrane may be selectively enriched in cholesterol, which, in turn, may affect the interaction of the LDL receptor protein with UDCA, as well as further facilitate its binding to the LDL particle.
The in-vivo 35% increment of LDL uptake associated with UDCA feeding was paralleled by the in-vitro 29% increase in LDL binding effected by UDCA in hepato-cytes isolated from hypercholesterolemic hamsters. Ad-ministration of UDCA has been shown to decrease serum total and LDL cholesterol in hypercholes-terolemic patients with primary biliary cirrhosis [16]. The putative cholesterol-lowering effect of UDCA was thought to occur due to its ability to decrease choles-terol absorption in the intestine [45]. A strong associa-tion between intestinal cholesterol absorpassocia-tion and total plasma cholesterol level through regulation of hepatic cholesterol pools has been well-documented [46]. In-deed, the addition of UDCA to a cholesterol-supple-mented diet significantly lowered cholesterol absorption and hepatic cholesterol concentration in hamsters [47]. While it can not be ruled out that UDCA may have additional regulatory effects on hepatocellular choles-terol pools, in light of the present results, it is clear that a direct, UDCA-induced augmentation of LDL recep-tor binding may be responsible, at least in part, for this effect in hypercholesterolemic patients.
ated predominately with apoB-containing lipoprotein fractions. However, the binding of LDL to the hepatic LDL receptor was not significantly changed, as a result of cholesterol feeding, when measured in-vitro. Numer-ous studies have shown that the regulation of LDL receptor expression may be more a function of dietary fat content and composition, rather than of serum cholesterol levels alone [27,35 – 37]. In the current study, the fat content was the same in all diets. Furthermore, the absolute hepatocellular concentrations of unes-terified and esunes-terified cholesterol, as measured after cholesterol feeding, were calculated to be 3.890.08 and 38.995.7mmol/g tissue, which is approximately equiv-alent to a total concentration of 16 mg/g. The data are supported by those of Horton et al. [38]. These authors have shown that, at this concentration of liver cholesterol, there is no change in either LDL receptor-dependent transport, or LDL receptor mRNA expres-sion.
Acknowledgements
The authors dedicate this work to Mauro Malavolti. His friendship and collaboration will be greatly missed. The authors are grateful to Joan Fasulo for assistance in the studies of hepatocellular lipid determinations. The studies were supported, in part, by grants from the American Heart Association Nation’s Capital Affiliate, Inc. (B.B.), and the General Medical Research Service of the Veterans Administration (S.J.R.). B.B. is sup-ported, in part, by NIH/NIDDK DK-46954. These studies were performed, in part, during the tenure of both a post-doctoral research fellowship from the American Heart Association Nation’s Capital Affiliate, as well as of a post-doctoral research fellowship from the American Institute for Cancer Research (S.C.). Portions of this manuscript work were derived from a dissertation presented by S.C. to the Columbian Col-lege and Graduate School of Arts and Sciences of The George Washington University. This paper was pre-sented, in part, at the annual meeting of the American Gastroenterological Association, San Francisco, CA, 1996.
References
[1] Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986;232:34 – 47.
[2] Vega GL, Grundy SM. Mechanisms of primary hypercholes-terolemia in humans. Am Heart J 1987;113:493 – 502.
[3] Turley SD, Dietschy JM. The metabolism and excretion of cholesterol by the liver. In: Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DA, editors. The Liver: Biology and Pathology. New York: Raven Press, 1988.
[4] Spady DK, Turley SD, Dietschy JM. Rates of low density lipoprotein uptake and cholesterol synthesis are regulated inde-pendently in the liver. J Lipid Res 1985;26:465 – 72.
[5] Brown MS, Goldstein JL. Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. J Clin Invest 1983;72:743 – 7.
[6] Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem 1977;46:897 – 930.
[7] Brown MS, Dana SE, Goldstein JL. Receptor-dependent hydrol-ysis of cholesteryl esters contained in plasma low density lipo-protein. Proc Natl Acad Sci USA 1975;72:2925 – 9.
[8] Goldstein JL, Brown MS. Regulation of the mevalonate path-way. Nature 1990;343:425 – 30.
[9] Briggs MR, Yokoyama C, Wang X, Brown MS, Goldstein JL. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. I. Identification of the protein and delineation of its target nucleotide sequence. J Biol Chem 1993;268:14490 – 6.
[10] Kovanen PT, Bilheimer DW, Goldstein JL, Jaramillo JJ, Brown MS. Regulatory role for hepatic low density lipoprotein recep-tors in vivo in the dog. Proc Natl Acad Sci USA 1981;78:1194 – 8.
[11] Gupta AK, Sexton RC, Rudney H. Differential regulation of low density lipoprotein suppression of HMG-CoA reductase activity in cultured cells by inhibitors of cholesterol biosynthesis. J Lipid Res 1990;31:203 – 15.
[12] Malavolti M, Fromm H, Ceryak S, Roberts IM. Modulation of low density lipoprotein receptor activity by bile acids: differential effects of chenodeoxycholic and ursodeoxycholic acids in the hamster. J Lipid Res 1987;28:1281 – 95.
[13] Bouscarel B, Fromm H, Ceryak S, Cassidy MM. Ursodeoxy-cholic acid increases low-density lipoprotein binding, uptake and degradation in isolated hamster hepatocytes. Biochem J 1991;280:589 – 98.
[14] Bouscarel B, Ceryak S, Fromm H. Comparative effect of ursodeoxycholic acid and calcium antagonists on the binding, uptake and degradation of LDL in isolated hamster hepatocytes. Biochim Biophys Acta 1996;1301:230 – 6.
[15] Bouscarel B, Ceryak S, Robins SJ, Fromm H. Studies on the mechanism of the ursodeoxycholic acid-induced increase in hep-atic low-density lipoprotein binding. Lipids 1995;30:607 – 17. [16] Poupon RE, Ouguerram K, Chretien Y, et al.
Cholesterol-lower-ing effect of ursodeoxycholic acid in patients with primary biliary cirrhosis. Hepatology 1993;17:577 – 82.
[17] Sessions VA, Salter AM. The effects of different dietary fats and cholesterol on serum lipoprotein concentrations in hamsters. Biochim Biophys Acta 1994;1211:207 – 14.
[18] Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+
precipitation procedure for quantitation of high-density-lipo-protein cholesterol. Clin Chem 1982;28:1379 – 88.
[19] Prpic V, Green KC, Blackmore PF, Exton JH. Vasopressin-, angiotensin II-, and alpha 1-adrenergic-induced inhibition of Ca2+ transport by rat liver plasma membrane vesicles. J Biol
Chem 1984;259:1382 – 5.
[20] Bouscarel B, Gettys TW, Fromm H, Dubner H. Ursodeoxy-cholic acid inhibits glucagon-induced cAMP formation in ham-ster hepatocytes: a role for PKC. Am J Physiol 1995;268:G300 – 10.
[21] Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911 – 7.
[22] Patton GM, Robins SJ. Extraction of phospholipids and analysis of phospholipid molecular species. Methods Enzymol 1990;187:195 – 215.
[23] Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem 1959;234:466 – 8.
[24] Admirand WH, Small DM. The physicochemical basis of choles-terol gallstone formation in man. J Clin Invest 1968;47:1043 – 52. [25] Gurantz D, Laker MF, Hofmann AF. Enzymatic measurement of choline-containing phospholipids in bile. J Lipid Res 1981;22:373 – 6.
[26] Fromm H, Amin P, Klein H, Kupke I. Use of a simple enzy-matic assay for cholesterol analysis in human bile. J Lipid Res 1980;21:259 – 61.
[27] Malavolti M, Fromm H, Ceryak S, Shehan KL. Cerebral low-density lipoprotein (LDL) uptake is stimulated by acute bile drainage. Biochim Biophys Acta 1991;1081:106 – 8.
[28] Bradford MM. A rapid and sensitive method for the quantita-tion of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248 – 54.
[29] Fromm H, Thomas PJ, Hofmann AF. Sensitivity and specificity in tests of distal ileal function: prospective comparison of bile acid and vitamin B 12 absorption in ileal resection patients. Gastroenterology 1973;64:1077 – 90.
[30] McFarlane AS. Efficient trace-labeling of proteins with iodine. Nature 1958;182:53 – 3.
[31] Bilheimer DW, Eisenberg S, Levy RI. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta 1972;260:212 – 21. [32] Bouscarel B, Fromm H, Nussbaum R. Ursodeoxycholate
mobi-lizes intracellular Ca2+and activates phosphorylase a in isolated
hepatocytes. Am J Physiol 1993;264:G243 – 51.
hydro-phobicity index and the activities of enzymes regulating choles-terol and bile acid synthesis in the rat. J Lipid Res 1989;30:1161 – 71.
[34] Angelin B, Raviola CA, Innerarity TL, Mahley RW. Regulation of hepatic lipoprotein receptors in the dog. Rapid regulation of apolipoprotein B,E receptors, but not of apolipoprotein E recep-tors, by intestinal lipoproteins and bile acids. J Clin Invest 1983;71:816 – 31.
[35] Ohtani H, Hayashi K, Hirata Y, et al. Effects of dietary choles-terol and fatty acids on plasma cholescholes-terol level and hepatic lipoprotein metabolism. J Lipid Res 1990;31:1413 – 22.
[36] Jackson B, Gee AN, Martinez Cayuela M, Suckling KE. The effects of feeding a saturated fat-rich diet on enzymes of choles-terol metabolism in the liver, intestine and aorta of the hamster. Biochim Biophys Acta 1990;1045:21 – 8.
[37] Sessions VA, Salter AM. Low density lipoprotein binding to monolayer cultures of hepatocytes isolated from hamsters fed different dietary fatty acids. Biochim Biophys Acta 1995;1258:61 – 9.
[38] Horton JD, Cuthbert JA, Spady DK. Regulation of hepatic 7 alpha-hydroxylase expression and response to dietary cholesterol in the rat and hamster. J Biol Chem 1995;270:5381 – 7. [39] Walzem RL, Watkins S, Frankel EN, Hansen RJ, German JB.
Older plasma lipoproteins are more susceptible to oxidation: a linking mechanism for the lipid and oxidation theories of atherosclerotic cardiovascular disease. Proc Natl Acad Sci USA 1995;92:7460 – 4.
[40] Jurgens G, Hoff HF, Chisolm GME, Esterbauer H.
Modifica-tion of human serum low density lipoprotein by oxidaModifica-tion — characterization and pathophysiological implications. Chem Phys Lipids 1987;45:315 – 36.
[41] Fernandez Ballester G, Castresana J, Fernandez AM, Arrondo JL, Ferragut JA, Gonzalez Ros JM. A role for cholesterol as a structural effector of the nicotinic acetylcholine receptor. Bio-chemistry 1994;33:4065 – 71.
[42] van de Heijning BJ, Stolk MF, van Erpecum KJ, Renooij W, Groen AK, van Berge Henegouwen GP. Bile salt-induced choles-terol crystal formation from model bile vesicles: a time course study. J Lipid Res 1994;35:1002 – 11.
[43] Heuman DM, Bajaj R. Ursodeoxycholate conjugates protect against disruption of cholesterol-rich membranes by bile salts. Gastroenterology 1994;106:1333 – 41.
[44] van de Heijning BJ, van de den Broek AM, van Berge Henegouwen GP. Membrane cholesterol content of cholesterol/ phospholipid vesicles determines the susceptibility to both dam-age and protection by bile salts: implications for bile physiology. Eur J Gastroenerol Hepatol 1997;9:473 – 9.
[45] Hardison WG, Grundy SM. Effect of ursodeoxycholate and its taurine conjugate on bile acid synthesis and cholesterol absorp-tion. Gastroenterology 1984;87:130 – 5.
[46] Dawson PA, Rudel LL. Intestinal cholesterol absorption [Re-view] [48 refs]. Curr Opin Lipidol 1999;10:315 – 20.
[47] Turley SD, Herndon MW, Dietschy JM. Reevaluation and application of the dual-isotope plasma ratio method for the measurement of intestinal cholesterol absorption in the hamster. J Lipid Res 1994;35:328 – 39.
![Fig. 1. Correlation between in-vivo hepatic uptake of [U-14C]sucrose-labeled hamster low density lipoprotein (LDL) and percent serumhigh density lipoprotein (HDL) cholesterol](https://thumb-ap.123doks.com/thumbv2/123dok/3148196.1384402/6.612.307.508.274.569/correlation-hepatic-lipoprotein-percent-serumhigh-density-lipoprotein-cholesterol.webp)