Efficacy and safety of a combination of fluvastatin and bezafibrate
in patients with mixed hyperlipidaemia (FACT study)
Paolo Pauciullo
a,*, Carlo Borgnino
b, Rodolfo Paoletti
c, Mario Mariani
d,
Mario Mancini
aaDepartment of Clinical and Experimental Medicine,Medical School of the Uni6ersity‘Federico II’,Via S.Pansini5,80131Naples,Italy bMedical Department,No6artis Farma S.p.A.,Origgio,Italy
cInstitute of Pharmacological Sciences,Uni6ersity of Milan,Milan,Italy dDepartment of Cardiology,Angiology and Pneumology,Uni6ersity of Pisa,Pisa,Italy Received 26 July 1999; received in revised form 22 November 1999; accepted 7 January 2000
Abstract
Preliminary data suggest that fluvastatin may be safely combined with fibrates. The Fluvastatin Alone and in Combination Treatment Study examined the effects on plasma lipids and safety of a combination of fluvastatin and bezafibrate in patients with coronary artery disease and mixed hyperlipidaemia. A total of 333 patients were randomly allocated in this multicentre double-blind trial to receive 40 mg fluvastatin alone (n=80), 400 mg bezafibrate (n=86), 20 mg fluvastatin+400 mg bezafibrate (n=85) or 40 mg fluvastatin+400 mg bezafibrate (n=82) for 24 weeks. Low-density lipoprotein (LDL)-cholesterol decreased
\20% in all fluvastatin-containing regimens, with significantly greater decreases compared with bezafibrate alone (PB0.001). Bezafibrate alone and fluvastatin+bezafibrate combinations resulted in greater increases in high-density lipoprotein (HDL)-cholesterol and decreases in triglycerides compared with fluvastatin alone (PB0.001). Fluvastatin (40 mg)+bezafibrate was the most effective for all lipid parameters with a decrease from baseline at endpoint in LDL-cholesterol of 24%, a decrease in triglycerides of 38% and an increase in HDL-cholesterol of 22%. All treatments were well tolerated with no increase in adverse events for combination therapy versus monotherapy, or between combination regimens. No clinically relevant liver (aspartate aminotransferase or alanine aminotransferase (ASAT or ALAT) greater than three times the upper limit of normal) or muscular (creatine phosphokinase (CPK) greater than four times the upper limit of normal) laboratory abnormalities were reported. This large study shows 40 mg fluvastatin in combination with 400 mg bezafibrate to be highly effective and superior to either drug given as monotherapy in mixed hyperlipidaemia, and to be safe and well tolerated. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Hyperlipidaemia; LDL cholesterol; Triglyceride; Fluvastatin; Bezafibrate; Combination therapy
www.elsevier.com/locate/atherosclerosis
1. Introduction
Hypercholesterolaemia is well established as a major risk factor in the development of atherosclerosis and coronary artery disease [1,2]. Treatment of hypercholes-terolaemia with 3-hydroxy-3-methlyglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) reduces the incidence of fatal and non-fatal myocardial
infarc-tion by 30 – 35% with fewer coronary revascularisainfarc-tion procedures and fewer strokes [3 – 8]. Statins are cur-rently the most potent and best-tolerated agents for lowering low-density lipoprotein (LDL)-cholesterol.
Considerable evidence is now accumulating showing triglycerides to be an independent risk factor for coro-nary artery disease [9,10]. Triglyceride reduction by bezafibrate has been shown to slow progression of coronary atherosclerosis independently from LDL-cholesterol lowering in dyslipidaemic patients after my-ocardial infarction [11].
In patients with mixed hyperlipidaemia, treatment should therefore aim at normalising both LDL-choles-terol and triglyceride levels. In these patients, a
This work has been carried out on behalf of the FACT Study Centers.
* Corresponding author. Tel.: +39-81-7462011/2303; fax: + 39-81-5466152.
nation of a statin and a fibrate would be a rational approach, as fibrates are more effective than statins in reducing triglycerides and also in increasing high-den-sity lipoprotein (HDL)-cholesterol [12]. However, combining statins and fibrates has not been recom-mended because episodes of rhabdomyolysis have fol-lowed concomitant use of lovastatin or simvastatin and gemfibrozil [13 – 16].
Small studies to date suggest that a combination of fluvastatin and bezafibrate [17,18] or gemfibrozil, [19,20] is well tolerated with no evidence of any clini-cally significant interactions. The Fluvastatin Alone and in Combination Treatment (FACT) Study was performed to examine the effects on plasma lipids and safety of a combination of fluvastatin and bezafibrate in patients with coronary artery disease (CAD) and mixed hyperlipidaemia.
2. Patients and methods
2.1. Patients
Male and female patients aged between 40 and 70 years with CAD and mixed hyperlipidaemia were eligi-ble to participate in the study. CAD was defined by stable angina for at least 4 months, and previous my-ocardial infarction or previous coronary vascularisation procedure. Patients were required to have a serum LDL-cholesterol between 135 and 250 mg/dl and serum triglycerides between 180 and 400 mg/dl after 7 weeks of placebo and dietary run-in. In addition, patients had to show at least 85% compliance with treatment during the run-in period.
Important exclusion criteria were type I, III, IV or V hyperlipidaemia; conditions associated with secondary hyperlipidaemia, including diabetes mellitus, nephrotic syndrome, hepatobiliary disease, alcoholism, chronic pancreatitis, autoimmune disease or hyperthyroidism; congestive heart failure; unstable angina, myocardial infarction, stroke or coronary revascularisation within the preceding 4 months; uncontrolled hypertension; or body mass index \30 kg/m2.
2.2. Study design
The study was a double-blind, randomised, parallel group, multicentre (45 centres) trial carried out in Italy. Eligible patients were instructed to adhere to a hypolip-idaemic isocaloric diet (according to guidelines of the European Atherosclerosis Society) throughout the study. Patients underwent an 8-week placebo run-in period, after which they were randomly allocated to one of four treatment groups. Patients received daily doses of either 40 mg fluvastatin, 400 mg bezafibrate, 20 mg fluvastatin+400 mg bezafibrate, or 40 mg
fluvas-tatin+400 mg bezafibrate for 24 weeks. Fluvastatin was administered once daily (o.a.d.) in the evening and bezafibrate (retard formulation; Boehringer Mannheim) was given o.a.d. in the morning. To maintain blinding, a double-dummy technique was used with patients as-signed to monotherapy receiving a placebo matching either fluvastatin or bezafibrate.
Any other medication with the potential to interfere with the evaluation of efficacy, safety and tolerability of trial medication was prohibited during the study; par-ticularly, drugs with known effects on plasma lipids, steroids, unless administered topically or for post-menopausal hormone replacement therapy, anticoagu-lants, cyclosporine A, erythromycin, ketoconazole or cytotoxic agents.
Patients were assessed at baseline and then at 4-week intervals. At each visit, serum lipids were measured including total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides. All biochemical analyses were carried out by a central laboratory (Exacta, Verona, Italy). Lipids were analysed using standard techniques [21 – 23], with LDL calculated according to the Friedewald formula [24] when triglycerides were
5400 mg/dl, or directly determined by means of a homogeneous method (N-geneousTM LDL-C; Gen-zyme Diagnostics, West Malling, UK) [25] when triglycerides were \400 mg/dl. All assays had an intra-assay coefficient of variation of less than 2% and inter-assay variation of less than 5%. Liver function tests (ASAT, ALAT, bilirubin, alkaline phosphatase) and creatine-kinase were measured, and the occurrence of any adverse events recorded at 4-week intervals. Pulse rate and blood pressure were measured at 8-week inter-vals with an electrocardiogram performed at baseline and after 24 weeks. Routine haematology and a wider set of safety blood chemistry were carried out at base-line and at the end of the study.
2.3. Statistical methodology
Efficacy was primarily assessed by determining the mean percentage reduction from baseline in LDL-cholesterol measured at the end of the study.
A sample size of 80 subjects for the treatment group was estimated to be required to detect a difference in mean LDL-cholesterol reduction of 10% or more, with S.D. of 18, power of 80% and a significance level of 0.008 (significance levels of 0.05 adjusted for multiple treatment comparison).
The comparability of the four treatment groups with respect to demographics and baseline characteristics was assessed in a descriptive way considering all ran-domized patients.
ana-lyzed. In case of missing data, the final observation carried forward approach was used.
The statistical analysis was carried out on the per-centage change from baseline applying the analysis of covariance (ANCOVA, PROC GLM of SAS). The statistical model included centre and treatment as fac-tors, and baseline values as covariate. A statistical test was provided for all six pairwise treatment compari-sons. The 95% confidence interval of the estimated difference between treatments in percentage change from baseline was provided for each of these pairwise treatment comparisons.
Standard safety laboratory parameters were assessed by evaluating the incidences of worsening during treat-ment and of the worst newly occurring events (labora-tory value falling outside normal range) with respect to baseline on all randomised patients. The incidence of patients having an adverse experience, related and not related to trial treatment, was summarised by treatment.
3. Results
3.1. Patients
A total of 333 patients were randomised to receive fluvastatin alone (n=80), bezafibrate alone (n=86), 20
mg fluvastatin+bezafibrate (n=85), 40 mg fluvas-tatin+bezafibrate (n=82).
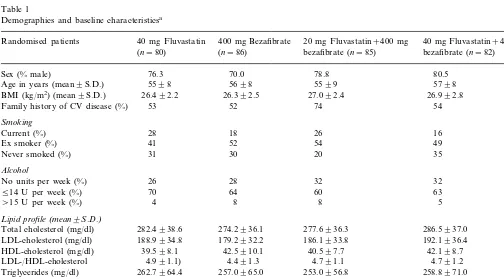
Table 1 shows that there were no important differ-ences between the groups with respect to demographics and baseline measurements. The groups were compara-ble with respect to CAD, with approximately one-half the patients (55%) having angina pectoris, 51% having a history of myocardial infarction, and 30% having previ-ous coronary revascularisation. There was also no dif-ference between the groups with respect to previous management of hyperlipidaemia, with 60% of patients having used a lipid-lowering diet and 54% having taken lipid-lowering drugs prior to the study. The most com-mon previous lipid-lowering drugs were statins (38% of all patients), and fibrates (28%). Resins, fish oils, or probucol were each used in B5% of all patients.
A total of 293 (88%) of patients completed the 24-week treatment period with fourteen, eight, eight and ten patients discontinuing prematurely in the fluvastatin (fluva), bezafibrate (beza), 20 mg fluva+beza and 40 mg fluva+beza groups, respectively (Table 2).
3.2. Efficacy
Table 3 shows the percentage decrease from baseline in lipid parameters. LDL-Cholesterol decreased in all groups from baseline value within 4 weeks of starting therapy. However, decreases of \20% were only
ob-Table 1
Demographics and baseline characteristicsa
Randomised patients 40 mg Fluvastatin 400 mg Bezafibrate 20 mg Fluvastatin+400 mg 40 mg Fluvastatin+400 mg bezafibrate (n=85)
(n=80) (n=86) bezafibrate (n=82)
80.5
Sex (% male) 76.3 70.0 78.8
5598 5698
Age in years (mean9S.D.) 5599 5798
BMI (kg/m2) (mean9S.D.) 26.492.2 26.392.5 27.092.4 26.992.8 52
53
Family history of CV disease (%) 74 54
Smoking
26 16
Current (%) 28 18
54 49
Ex smoker (%) 41 52
35
31 20
Never smoked (%) 30
Alcohol
26 28
No units per week (%) 32 32
514 U per week (%) 70 64 60 63
\15 U per week (%) 4 8 8 5
Lipid profile(mean9S.D.)
286.5937.0 282.4938.6 274.2936.1 277.6936.3
Total cholesterol (mg/dl)
188.9934.8 179.2932.2
LDL-cholesterol (mg/dl) 186.1933.8 192.1936.4
39.598.1 42.5910.1
HDL-cholesterol (mg/dl) 40.597.7 42.198.7
4.991.1) 4.491.3
LDL-/HDL-cholesterol 4.791.1 4.791.2
253.0956.8 257.0965.0
262.7964.4
Triglycerides (mg/dl) 258.8971.0
Blood pressure(mmHg)
133.2911.7 136914.6
Systolic BP (mean9S.D.) 135.5915.2 135.8914.2
80.497.3
Diastolic BP (mean9S.D.) 82.898.2 83.598.6 81.997.7
69.998.0 69.798.5
Heart rate (b.p.m.) (mean9S.D.) 69.298.4 70.699.6
Table 2
Premature discontinuations
40 mg Fluvastatin 400 mg Bezafibrate 20 mg Fluvastatin+400 mg 40 mg Fluvastatin+400 mg (n=86)
(n=80) bezafibrate (n=85) bezafibrate (n=82)
Total number of patients 14 (17.5%) 8 (9.3%) 8 (9.4%) 10 (12.2%)
Reason for discontinuation
3 3
6 5
Adverse events
1 –
Concomitant diseases – –
– –
3 1
Withdrawal consent
– 1
Dose violation 1 –
3 2
3 2
Lost to follow up
1
Other 1 2 2
Table 3
Percentage change in lipid parameters from baseline to 24-week endpoint
40 mg Fluvastatin 20 mg Fluvastatin+400 mg
Least-squares means 400 mg Bezafibrate 40 mg Fluvastatin+400 mg
bezafibrate (n=85) bezafibrate (n=82) (95% confidence interval) (n=80) (n=86)
−22.5a(−26.2,−18.9)
LDL-cholesterol −9.6 (−13.2,−6.1) −23.3a(−26.9,−19.8) −23.6a(−27.4,−19.9) −9.8 (−12.4,−7.2) −19.9a(−22.6,−17.3)
−17.3a(−20.0,−14.6) −20.4a(−23.1,−17.6)
Total cholesterol
0.3 (−3.8, 4.5)
HDL-cholesterol 17.4b(13.4, 21.4) 20.9b(16.9, 24.9) 21.9b(17.8, 26.1) Triglycerides −6.0 (−14.2, 2.3) −25.1b(−33.0,−17.3) −35.7b(−43.6,−27.7) −37.7b,c(−45.9,−29.4)
aPB0.001 versus 400 mg bezafibrate. bPB0.001 versus 40 mg fluvastatin. cP=0.031 versus 400 mg bezafibrate.
served in fluvastatin monotherapy and combination with fluvastatin, 20 mg fluva+beza, and 40 mg fluva+ beza all showing significantly greater decreases than beza alone (PB0.001). There was no significant differ-ence in LDL-cholesterol reduction between fluvastatin monotherapy and either fluva+beza combination therapies.
Total cholesterol also decreased from baseline within 4 weeks of therapy in all groups. All regimens contain-ing fluvastatin were significantly better than bezafibrate alone (PB0.001). Mean decreases of \20% were seen in the two fluva+beza groups, although there was no statistically significant difference between fluvastatin monotherapy and the combination regimens (P=0.171 versus 20 mg fluva+beza; P=0.119 versus 40 mg fluva+beza) or between the combination regimens themselves (P=0.824). The greatest decrease was ob-served in the 40 mg fluva+beza group (least-squares mean decrease from baseline to 24-week endpoint of 20.4%).
HDL-Cholesterol increased from baseline in all groups, although the increase in the fluvastatin monotherapy group was relatively small. Both bez-afibrate alone and fluva+beza resulted in significantly greater increases in HDL-cholesterol compared with fluvastatin alone (PB0.001). The largest increase was observed in the 40 mg fluva+beza group (least-squares mean increase from baseline to 24-week endpoint of 21.9%).
Triglycerides decreased in all groups from baseline within 4 weeks. Falls in the fluvastatin alone group were relatively small (least-squares mean decrease from baseline to 24-week endpoint of −6.0%). Although bezafibrate alone resulted in a significant decrease from baseline (25.1%), greater reductions were observed with the combinations (35.7% for 20 mg fluva+beza, 37.7% for 40 mg fluva+beza). The combination of 40 mg fluva+beza was significantly superior to bezafibrate alone in lowering triglycerides at 24-week endpoint (P=0.031).
3.3. Safety and tolerability
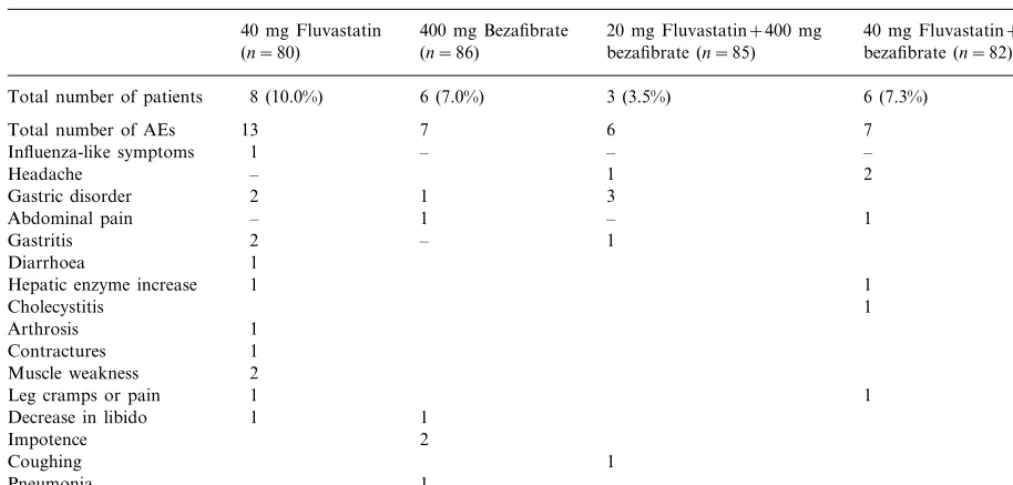
All treatments were well tolerated with no important differences between monotherapy and combination therapy. Adverse events were reported by 28.8, 23.3, 24.7 and 26.8% of patients in the fluvastatin, bez-afibrate, 20 mg fluva+beza, and 40 mg fluva+beza, respectively. A total of eight patients (10%) on fluvas-tatin, six patients (7%) on bezafibrate, three patients (3.5%) on 20 mg fluva+beza and six patients (7%) on 40 mg fluva+beza reported adverse events assessed by the investigator as being at least possibly related to trial medication (Table 4).
Table 4
Adverse events (AEs) assessed by the investigator to be at least possibly related to study medication
40 mg Fluvastatin 400 mg Bezafibrate 20 mg Fluvastatin+400 mg 40 mg Fluvastatin+400 mg bezafibrate (n=82) bezafibrate (n=85)
(n=86) (n=80)
8 (10.0%)
Total number of patients 6 (7.0%) 3 (3.5%) 6 (7.3%)
7
Total number of AEs 13 6 7
–
Influenza-like symptoms 1 – –
1
– 2
Headache
1
Gastric disorder 2 3
1 –
four times the upper limit of normal) laboratory abnor-malities were reported.
A total of 16 patients (4.8%) discontinued medication due to adverse events, of which six, three, three and five were in the fluvastatin, bezafibrate, 20 mg fluva+beza, 40 mg fluva+beza, respectively. Of these, five patients prematurely interrupted treatment due to cardiovascu-lar adverse events, such as angina worsening (one pa-tient on 20 mg fluva+beza, one on 40 mg fluva+beza), blood pressure increase (one patient on fluvastatin, one on 40 mg fluva+beza) or syncope (one patient on 40 mg fluva+beza). No deaths, myocardial infarction, or coronary revascularisation procedures oc-curred during the study.
4. Discussion
So far, this trial represents the largest study carried out on a combination of a statin and fibrate in CAD patients with mixed hyperlipidaemia.
Fluvastatin is a fully synthetic statin and is struc-turally distinct from other members of this class of agents. Fluvastatin effectively reduces LDL-cholesterol, retards the progression of coronary atherosclerosis [26] and reduces mortality and incidence of myocardial infarction in patients undergoing balloon angioplasty [27]. Fluvastatin compares favourably, from a drug-in-teraction perspective, with most other statins, in part due to its high selectivity for HMG-CoA without affinity towards other enzymes or receptor systems [28], and also because of its favourable pharmacokinetic
profile. Its half-life in plasma of 1.2 h and its ]99% plasma protein binding [29] indicate a low level of systemic exposure to the drug, and make it unlikely that, when combined with a fibrate, fluvastatin could exert an additive toxic effect on muscular tissue, as was suggested by some animal and in vitro studies [30,31]. Fluvastatin has not been reported to interact signifi-cantly with cyclosporine A, a serious interaction with some other statins leading to myopathy and rhabdomy-olysis. Due to its low drug interaction potential, fluvas-tatin was considered the statin of choice for combination treatment.
Bezafibrate was chosen as a triglyceride lowering agent since it has been shown to slow progression of coronary atherosclerosis independently from LDL-cholesterol lowering in dyslipidaemic patients after my-ocardial infarction [11].
The results of the FACT study confirm the predomi-nant LDL-cholesterol lowering of fluvastatin and triglyceride lowering of bezafibrate, and show the two agents to be effective and safe in combination. The 40 mg fluvastatin+bezafibrate combination was the most effective regimen for all parameters, with a decrease from baseline in LDL-cholesterol of 24%, a reduction in triglycerides of 38%, and an increase in HDL-choles-terol of 22%. The 40 mg fluvastatin+bezafibrate com-bination was significantly more effective than bezafibrate monotherapy in reducing LDL-cholesterol, and significantly more effective in increasing HDL-cholesterol compared with fluvastatin monotherapy.
efficacious than bezafibrate alone in reducing triglyce-rides. This result is probably explainable with the abil-ity of statins to increase in the liver the expression of B – E receptors. These receptors recognise as ligands not only LDL apoliprotein (apo) B, but also the apo E molecules of triglyceride-rich particles like very low-density lipoprotein (VLDL). Monotherapy with statins, particularly with atorvastatin, can be effective as a triglyceride lowering regimen [32]. It should be consid-ered, however, that a 20% increase of HDL-cholesterol — as was obtained in the present study with the combination treatment — is an unlikely effect of monotherapy with statins.
An interesting finding of this study is the quite small additive effect on LDL-cholesterol obtained by dou-bling the fluvastatin dosage from 20 to 40 mg in combination treatment. In the study by Bakker-Arkema et al. [32] in hypertriglyceridaemic patients, a fourfold increase of atorvastatin dosage (from 20 to 80 mg) achieved only an 8% further decrease of LDL-cholesterol, and a 13% further decrease of plasma triglycerides. In the present study, 40 mg fluvastatin in combination with bezafibrate was significantly more effective on plasma triglycerides than bezafibrate alone, while this was not true for the combination of 20 mg fluvastatin+bezafibrate compared with bezafibrate alone. This additive effect on plasma triglycerides in combination treatment can be explained by the in-creased uptake of VLDL particles due to the inin-creased expression of the B – E receptors in the liver. Apo E molecules — particularly well represented in VLDL particles — are more effective ligands of B – E receptors than the unique apo B molecule, which is the ligand of LDL particles [33]. As a consequence, the increased expression of B – E receptors in the liver, caused by the increased statin dose, that should lead to a reduction of LDL particles, is probably partially counteracted by the competitive occupation of the receptors by the VLDL particles, thus explaining the small further reduction of LDL-cholesterol observed with double statin dose. However, recently published intervention trials with fluvastatin in coronary patients [27,34] indicate that monotherapy with 40 – 80 mg achieves significant results on hard clinical endpoints. Therefore, the anti-atherosclerotic potential of using only 20 mg fluvastatin in combination treatments with bezafibrate in coronary patients with mixed hyperlipidaemia should be evaluated.
There was no increase in adverse events for the combination compared with monotherapy and no evi-dence of any increase in myalgia or muscle damage with the two agents given together. The safety profile of the two combination regimens was comparable.
The good safety and tolerability profile of the combi-nation observed in this study is in keeping with data from small studies of fluvastatin in combination with
bezafibrate or gemfibrozil [17 – 20], and confirms the low drug interaction potential of fluvastatin. The data are in contrast to several studies with other statins and fibrates, which have shown interactions resulting in safety issues [13 – 16,35]. Although some publications have claimed efficacy and tolerability of other statins in combination with fibrates, these studies have either been relatively small [35,36] or performed in patients who were stable on the combination prior to the study, resulting in a selected population in whom the combi-nation was already well tolerated [37].
The findings of this large study show 40 mg fluvas-tatin in combination with 400 mg bezafibrate to be highly effective in decreasing LDL-cholesterol and triglycerides, and increasing HDL-cholesterol with no increase in side effects compared with monotherapy. The results demonstrate that fluvastatin is an effica-cious and safe choice in combination with fibrates for patients with mixed hyperlipidaemia.
Appendix A. Trial committees and participating clinical investigators
Figures in parentheses are the number of randomised patients.
A.1.Committees
A.1.1. Steering committee
Prof. M. Mancini, Trial Chairman, Napoli; Prof. M. Mariani, Pisa; Prof. R. Paoletti, Milano.
A.1.2. Safety Committee
Prof. G. Baggio, Padova; Prof. A. Corsini, Milano; Prof. M. Cortellaro, Milano; Prof. E. Marubini, Milano.
A.2.Trial in6estigators
Clemenza, Dr V. Bucca, Palermo (8); Prof. M. Montag-nani, Dr M. Mulinari, Siena (7); Dr G. Perani, Dr A. Frattoni, Pavia (7); Dr D. Mazzoleni, Dr M. Toccagni, Bergamo (6); Dr E. Boni, Dr L. Corda, Brescia (6); Dr A. Deorsola, Dr V. Ferrero, Cuneo (6);Prof. P. Pintus, Dr S. Pintus, Cagliari (6); Dr P. Marnini, Dr I. Franzetti, Varese (6); Prof. F. Rengo, Dr V. Canonico, Napoli (6); Prof. G. Licata, Prof. S. Paterna, Palermo (6); Prof. P. Zardini, Dr L. Franceschini,Verona (5); Prof. M. Mariani, Dr O. Biadi, Pisa (5); Dr G. Zuliani, Dr S. Volpato, Ferrara (5); Prof. I. Richichi, Dr B. Magnani, Pavia (4); Prof. S. Chierchia, Dr L. Filippo, Milano (4); Prof. G.P. Carboni, Dr L. Fiorani, Roma (4); Dr P.A. Ravazzi, Dr G. Varosio, Alessandria (4); Dr M. Tardio, Dr M. Vernazza, Parma (3); Prof. S. Bertolini, Dr S. De Mattei, Genova (3); Prof. A.L. Catapano, Dr A. Zoppo, Milano (3); Dr L. Denti, Dr G. Ceresini, Parma (2); Prof. E. Feraco, Dr T. De Vuono, Cosenza (2); Prof. F. Bacca, Dr A. Fazio, Lecce (2); Prof. V. Ceci, Dr F. Lumia, Roma (2);Dr G. Colombo, Dr P. Quorso, Melegnano (2); Prof. A. Arco-raci, Dr G. Turiano, Messina (1); Prof. D. Sommariva, Dr A.M. Fiorenza, Bollate (1).
References
[1] LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation 1990;81:1721 – 33.
[2] Neaton JD, Wentworth DF. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Over-all findings and differences by age for 316 099 white men: Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med 1992;152:56 – 64.
[3] Mulddon MF, Criqui MH. The emerging role of statins in the prevention of coronary heart disease. Br Med J 1997;315:1554 – 5.
[4] Plehn JF, Davis BR, Sacks FM, et al. Reduction of stroke incidence after myocardial infarction with pravastatin: the cholesterol and recurrent events (CARE) study. Circulation 1999;99(2):216 – 23.
[5] The Scandinavian Simvastatin Survival Study (4S). Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study Group. Lancet 1994;344:1383 – 9.
[6] Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995;333:1301 – 7.
[7] Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravas-tatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001 – 9. [8] Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. J Am Med Assoc 1998;279:1615 – 22.
[9] Sprecher DL. Triglycerides as a risk factor for coronary artery disease. Am J Cardiol 1998;82(12A):49U – 56U.
[10] Durrington PN. Triglycerides are more important in atheroscle-rosis than epidemiology has suggested. Atherosclerosis 1998;141:S57 – 62.
[11] de Faire U, Ericsson CG, Grip L, Nilsson J, Svane B, Hamsten A. Secondary preventive potential of lipid-lowering drugs. The Bezafibrate Coronary Atherosclerosis Intervention Trial (BE-CAIT). Eur Heart J 1996;17(Suppl F):37 – 42.
[12] Farmer JA, Gotto AM Jr. Currently available hypolipidaemic drugs and future therapeutic developments. Baillieres Clin En-docrinol Metab 1995;9(4):825 – 47.
[13] Knoll RW, Ciafone R, Galen M. Rhabdomyolysis and renal failure secondary to combination therapy of hyperlipidemia with lovastatin and gemfibozil. Conn Med 1993;57(9):593 – 4. [14] Pierce LR, Wysowski DK, Gross TP. Myopathy and
rhabdomy-olysis associated with lovastatin-gemfibrozil combination ther-apy. J Am Med Assoc 1990;264(1):71 – 5.
[15] van Puijenbroek EP, Du Buf-Vereijken PW, Spooren PF, van Doormaal JJ. Possible increased risk of rhabdomyolysis during concomitant use of simvastatin and gemfibrozil. J Intern Med 1996;240(6):403 – 4.
[16] Tal A, Rajeshawari M, Isley W. Rhabdomyolysis associated with simvastatin-gemfibrozil therapy. South Med J 1997;90(5):546 – 7. [17] Leitersdorf E, Muratti EN, Eliav O, et al. Efficacy and safety of a combination of fluvastatin – bezafibrate treatment for familial hypercholesterolaemia: comparative efficacy with a fluvastatin – cholestyramine combination. Am J Med 1994;96:401 – 7. [18] Eliav O, Schurr D, Pfister P, Friedlander Y, Leitersdorf E.
High-dose fluvastatin and bezafibrate combination treatment for heterozygous familial hypercholesterolaemia. Am J Cardiol 1995;76:76A – 9A.
[19] Spence JD, Munoz CE, Hendricks L, Latchinian L, Khouri HE. Pharmacokinetics of the combination of fluvastatin and gemfi-brozil. Am J Cardiol 1995;76:80A – 3A.
[20] Smit JWA, Jansen GH, De Bruin JWA, Erkelens DW. Treat-ment of combined hyperlidaemia with fluvastatin and gemfi-brozil alone and in combination does not induce muscle damage. Am J Cardiol 1995;76:126A – 8A.
[21] Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20(4):470 – 5.
[22] Fossati P, Prencipe L. Serum triglycerides determined colorimet-rically with an enzyme that produces hydrogen peroxide. Clin Chem 1982;28(10):2077 – 80.
[23] Vikari J. Precipitation of plasma lipoproteins by PEG-6000 and its evaluation with electrophoresis and ultracentrifugation. Scand J Clin Lab Invest 1976;36(3):265 – 8.
[24] Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499 – 502.
[25] Bonetti G, Bosio C, Pagani F, Panteghini M. A new homoge-neous assay for the direct measurement of LDL cholesterol in serum. Biochim Clin 1998;22:273.
[26] Herd JA, Ballantyne CM, Farmer JA, et al. Effects of fluvastatin on coronary atherosclerosis in patients with mild to moderate cholesterol elevations (Lipoprotein and Coronary Atherosclero-sis Study [LCAS]). Am J Cardiol 1997;80:278 – 86.
[27] Serruys PW, Foley DP, Jackson G, et al. A randomized placebo controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty: final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J 1999;20(1):58 – 69.
[28] Appel S, Dingemanse J. Clinical pharmacokinetics of fluvastatin. Drugs Today 1996;32(Suppl. A):37 – 55.
[30] Willis RA, Folkers K, Tucker JL, Ye CQ, Xia LJ, Tamagawa H. Lovastatin decreases coenzyme Q levels in rats. Proc Natl Acad Sci USA 1988;87:8928 – 30.
[31] Mastaglia FL. Adverse effects of drugs on muscle. Drugs 1982;24:304 – 21.
[32] Bakker-Arkema RG, Davidson MH, Goldstein RJ, Davignon J, Isaacsohn JL, Weiss SR, Keison LM, Brown WV, Miller VT, Shurzinske LJ, Black DM. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hyper-triglyceridemia. J Am Med Assoc 1996;275:128 – 33.
[33] Bradley WA, Gianturco SH. ApoE is necessary and sufficient for the binding of large triglyceride-rich lipoproteins to the LDL receptor; apoB is unnecessary. J Lipid Res 1986;27: 40 – 8.
[34] Riegger G, Abletshauser C, Ludwig M, Schwandt P, Widimsky J, Weidinger G, Welzel D. The effect of fluvastatin on cardiac events in patients with symptomatic coronary artery disease during one year of treatment. Atherosclerosis 1999;144:263 – 70. [35] Hutchesson AC, Moran A, Jones AF. Dual bezafibrate – simvas-tatin therapy for combined hyperlipidaemia. J Clin Pharm Ther 1994;19(6):387 – 9.
[36] Iliadis EA, Rosenson RS. Long-term safety of pravastatin – gemfibrozil therapy in mixed hyperlipidemia. Clin Cardiol 1999;22:25 – 8.
[37] Feher MD, Foxton J, Banks D, Lant AF, Wray R. Long-term safety of statin – fibrate combination treatment in the manage-ment of hypercholesterolaemia in patients with coronary artery disease. Br Heart J 1995;74(1):14 – 7.
.