www.elsevier.com / locate / bres
Research report
Pituitary adenylate cyclase-activating polypeptide innervation of the
mudpuppy cardiac ganglion
b a a ,
*
Linda K. Schoenfeld , Jennifer A. Souder , Jean C. Hardwick
a
Biology Department, Ithaca College, Ithaca, NY 14850, USA b
Neuroscience Department, Case Western Reserve University, Cleveland, OH 44106, USA Accepted 22 August 2000
Abstract
The presence and potential origin of the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) was determined in cardiac ganglia of the mudpuppy, Necturus maculosus. Although PACAP has been implicated in the regulation of cardiac function in several mammalian species, the presence of this peptide in the autonomic nervous system (ANS) of other species is unclear. Thus, this study is the first to characterize this highly conserved peptide in the ANS of a non-mammalian species. PACAP-immunoreactivity was observed in nerve fibers throughout the mudpuppy cardiac ganglia and often was co-localized with the sensory neuropeptides substance P and calcitonin gene-related peptide. Removal of all extrinsic inputs to the ganglia by organ culture eliminated PACAP-immunoreactivity in the cardiac ganglia, whereas bilateral vagotomies only partially reduced PACAP-labeling. PACAP-immunoreactive neurons were observed in both high thoracic dorsal root ganglia and in vagal sensory ganglia. While no PACAP-positive neurons were observed in caudal medulla brainstem regions, PACAP-containing nerve fibers were found in the region of the nucleus solitarius. These results suggest that, in the mudpuppy, PACAP is found primarily in visceral afferent fibers, originating from cells in either the dorsal root ganglia or vagal sensory ganglia. Based on their anatomic localization, these afferent fibers may function to transmit important sensory information to cardiovascular centers in the brain as well as serving as local reflex inputs to modulate postganglionic parasympathetic output within the cardiac ganglion itself. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Peptides: anatomy and physiology
Keywords: PACAP; Parasympathetic; Afferent; Brainstem; Immunohistochemistry
1. Introduction the superior cervical ganglion [9], and the ciliary ganglion
[4] as well as parasympathetic ganglia such as the otic and Pituitary adenylate cyclase-activating polypeptide sphenopalatine ganglia [17] and in cardiac ganglia [1].
(PACAP), originally isolated from ovine hypothalamus PACAP-containing cell bodies have also been found in
[8,16], is a member of the VIP/ secretin / glucagon family of neurons of the dorsal root ganglia and the nodose ganglia peptides. Following its initial discovery in the brain, in rats [3,17], implying a role for the peptide in visceral PACAP has subsequently been localized in fibers of both afferent systems. PACAP has also been found in the central the sympathetic and parasympathetic branches of the nervous system of several non-mammalian species, includ-autonomic nervous system [1,3,4,9,10,17]. PACAP is ing amphibians [11,24] and teleosts [12]. In these studies, found in two forms, a 38 amino acid peptide (PACAP-38) PACAP peptides and / or receptors were found in a variety or the C-terminally truncated 27 amino acid form of brain regions, suggesting multiple functional roles for
(PACAP-27). In mammals, PACAP peptides have been this neuropeptide [11,12,24].
found in fibers and neurons of sympathetic ganglia, such as The physiological function of PACAP in the autonomic nervous system (ANS) in mammals is still largely un-known, although investigators have found evidence that
*Corresponding author. Tel.: 11-607-274-3213; fax: 1
1-607-274-PACAP can alter cardiovascular functions such as changes
1131.
E-mail address: [email protected] (J.C. Hardwick). in systemic blood pressure and cardiac contractility [2].
PACAP has also been shown to have a negative chronot- peptide (CGRP) were examined with rabbit polyclonal ropic action on the heart via the activation of the intracar- antibodies (Peninsula Laboratories) at concentrations of diac parasympathetic postganglionic neurons [6,23]. Re- 1:500. Tyrosine hydroxylase (TH) was examined with a
cent studies have provided evidence that PACAP acts mouse monoclonal antibody (1:50), developed by
directly on guinea pig intracardiac parasympathetic neu- LeDourain and Ziller and obtained from the Developmen-rons to produce an increase in neuronal excitability [1]. tal Studies Hybridoma Bank at the University of Iowa.
Although evidence for the involvement of PACAP in Tissue was incubated in primary antibody solutions for
autonomic regulation in mammals is increasing, the origin 12–20 h at 48C. Following incubation in the primary and function of PACAP-containing fibers in the ANS of antibodies, the tissue was washed in PBS and incubated for other species is less well studied. The present study was 90 min at 20–228C with fluorescently-tagged species undertaken to explore the origins of PACAP in the ANS, specific secondary antibodies (donkey anti-guinea pig-in-specifically the parasympathetic cardiac ganglion, of an docarbocyanine (Cy3), donkey anti-rabbit-fluorescein
iso-amphibian system. thiocyanate (FITC), or donkey anti-mouse-FITC, all at
The mudpuppy (Necturus maculosus) cardiac ganglion 1:400, Jackson ImmunoResearch Laboratories). The tissue is well characterized, both anatomically and physiological- was then washed and mounted on gelatin-coated slides and
ly [15,21]. The source and actions of many of the coverslipped with Citifluor (Marivac Ltd., Halifax NS,
neuropeptides affecting parasympathetic postganglionic Canada). For preabsorption studies, the primary antibodies neuron activity have been well described [21], and com- were incubated with 5mM of either 38 or PACAP-plimentary mammalian studies suggest that many of these 27 peptide (Peninsula Laboratories) for 12–20 h at 48C peptidergic modulators are conserved across species. Thus, prior to the addition of the tissue.
additional information concerning the localization of For immunohistochemical analysis of tissue sections, the PACAP within the ANS of these amphibians will add to animals were initially anesthetized in 0.1% MS222 (Sigma our understanding of neuropeptide modulation of cardiac Chemical Co., St. Louis, MO) and then perfused through
function across species. the conus arteriosus with 150 ml of ice cold calcium-free
saline solution, followed by 150 ml of 4% paraformal-dehyde solution. Brain, dorsal root ganglia from the 2. Materials and methods cervical and high thoracic (T1–T3) regions, and ganglia of the X cranial nerve (nodose ganglia) were removed and
2.1. General methods postfixed in 4% paraformaldehyde for 2–12 h at 48C. The
tissue was cryoprotected in 30% sucrose, embedded in All experiments were performed on tissue isolated from OCT (Tissue Tek), and either used immediately or stored mudpuppies in accordance with procedures reviewed and at 2808C until sectioning. Tissue sections (16 mm) were approved by the Institutional Animal Care and Use Com- mounted onto gelatin-coated slides, washed with PBS, and mittee. Animals were obtained from commercial suppliers incubated in PBS with 0.03% Triton X-100 and 1% goat (Lembergers, Oshkosh, WI) and maintained in aquaria at serum at room temperature. Sections were then incubated 10–158C. For most experiments, animals were euthanized with primary antibodies (1:800–1:1000) for 12–20 h at by brainstem transection and pithing. The parasympathetic 48C. Sections were washed and incubated with fluorescent-cardiac ganglion, which lies in a connective tissue sheath ly labeled species-specific secondary antibodies (1:500) for adjacent to the heart, was removed as previously described 60–90 min at room temperature, washed, and coverslipped [15] and pinned in a Sylgard-lined petri dish containing a with Citifluor. Immunofluorescence was viewed with an
HEPES-buffered saline solution (120 mM NaCl, 2.5 mM Olympus BX50 fluorescent microscope. Slides of images
KCl, 3.6 mM CaCl , 5.0 mM HEPES, pH 7.3). The tissue2 were scanned into Adobe Photoshop and minimally cor-was dissected to remove overlying connective tissue and rected for brightness and contrast.
fixed in 4% paraformaldehyde (in 0.1 M Millonigs buffer,
pH 7.2) overnight at 48C. 2.3. Organ culture and vagotomies
2.2. Immunohistochemistry For organ explants, whole mount preparations
contain-ing the cardiac ganglion were removed from the animal For whole mount preparations, following fixation, the and pinned in sterile saline solution. The saline was
tissue was washed in 0.1 M phosphate buffered saline replaced with L-15 growth medium (Sigma Chemical Co.)
solution (PBS) and then incubated in PBS containing 0.5% containing 7% fetal bovine serum and penicillin / strep-Triton X-100 and 4% goat serum. PACAP immunoreactivi- tomycin (200 units / ml, Sigma Chemical Co.). The ex-ty was examined with a guinea pig polyclonal antibody to plants were maintained at 18–208C for 14 days, and the
either PACAP-38 or PACAP-27 (Peninsula Laboratories) at medium changed daily. Following culture, the whole
For vagotomies, animals were anesthetized in 0.1% tions (n55), substance P-like immunoreactivity (SP-ir, MS222. Bilateral, dorsal incisions were made to expose the Fig. 2). In the mudpuppy, both SP and CGRP are thought
vagus nerve, and a 5–10 mm segment was removed on to be derived from sensory afferent ganglia [19,21]. In
each side [22]. For sham surgeries, the vagus nerve was addition, SP-ir is also found in a subpopulation of small visualized but not disturbed, and the wound was sutured. intensely fluorescent (SIF) cells within the cardiac ganglia
All animals (both experimental and control) were main- [18] (Fig. 2D, arrow). However, no PACAP-ir was
ob-tained in aquaria at room temperature for 14–21 days served in any SIF cells. The co-localization of PACAP
following the surgery. The animals were then euthanized with SP in nerve fibers was not absolute. In addition to and the tissue removed for immunohistochemistry. In fibers which showed immunoreactivity to both antibodies, addition, the surgical site was examined to insure that the there were also a population of fibers which stained
vagus nerve remained lesioned. positively only for SP. There were also sporadic examples
of fibers which demonstrated immunoreactivity only for
2.4. Colchicine treatments PACAP (Fig. 2C,D). The same patterns were observed for
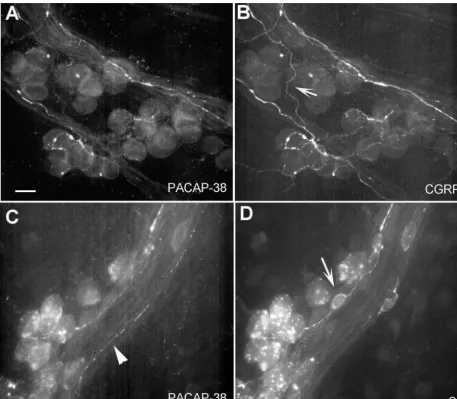
PACAP and CGRP, with some fibers showing evidence of To insure that sufficient peptide for immunohistochemi- co-localization, some fibers labeling only with CGRP (Fig. cal detection was present in cell somas within the brain, 2A,B), and a very small number labeling only with three animals were injected with colchicine (0.5 mg in 10 PACAP (data not shown).
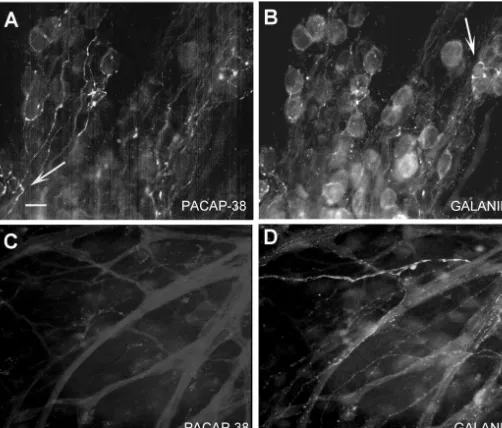
ml of saline solution) intraventricularly as previously Galanin has been found in the cell somas of both the described [13]. Prior to the colchicine injection, animals parasympathetic postganglionic neurons of the mudpuppy were first anesthetized in 0.1% MS222, injected with cardiac ganglion, as well as a subpopulation of SIF cells colchicine solution, and then maintained in 10–158C [14,20]. In four preparations labeled with antibodies to aquaria for 3 days prior to euthanasia and tissue collection both galanin and PACAP, there was very little evidence of
as described above. co-localization of the two peptides (Fig. 3). GAL-ir fibers
could be observed coursing through areas outside the ganglia itself, presumably innervating muscle fibers in
3. Results these regions (Fig. 3), however, there was no evidence of
PACAP-ir fibers in these regions. In addition, galanin 3.1. PACAP immunoreactivity in the mudpuppy cardiac labeled both postganglionic neurons and SIF cells, with no ganglion evidence for PACAP-ir in these cells. A few, sporadic examples of fibers that appeared to label for galanin and
PACAP-immunoreactivity (PACAP-ir) was examined PACAP were observed (Fig. 3). However, the majority of
using antibodies to either the long form of PACAP, galanin- and PACAP-immunoreactivity did not show
co-PACAP-38, or to the truncated form, PACAP-27. PACAP- localization.
ir was observed with the PACAP-38-specific antibody in Previous studies found PACAP in mammalian
sympa-nerve fibers coursing throughout the ganglia, as well is in thetic preganglionic fibers and postganglionic cells bodies pericellular complexes surrounding postganglionic neurons within sympathetic ganglia [4,9]. To determine if PACAP (Fig. 1). The PACAP-27 antibody showed no specific was found in sympathetic postganglionic fibers within the staining in three separate preparations, even at higher cardiac ganglia of the mudpuppy, we examined the degree concentrations (Fig. 1). No evidence of cell bodies with of co-localization of PACAP with tyrosine hydroxylase PACAP-ir to either antibody was observed in any of the (TH), a marker for catecholaminergic fibers. In three preparations. The specificity of the PACAP-38 antibody in different preparations, there was no evidence of co-locali-the mudpuppy was examined by pre-absorption with eico-locali-ther zation of PACAP-ir fibers with TH-immunoreactivity (data
5 mM PACAP-38 peptide or 5 mM PACAP-27 peptide not shown). TH-immunoreactive fibers could be seen
(n53 for each condition). Pre-absorption with PACAP-38 primarily in close proximity to contractile fibers in the peptide eliminated all specific labeling, whereas pre-ab- tissue adjacent to the ganglion, with little TH-immuno-sorption with PACAP-27 peptide did not alter the immuno- reactivity in or around the parasympathetic postganglionic
reactivity to the PACAP-38 antibody (data not shown). neurons.
3.2. Co-localization of PACAP with SP, CGRP, Galanin, 3.3. Effects of total or selective axon lesions on and Tyrosine Hydroxylase PACAP-ir
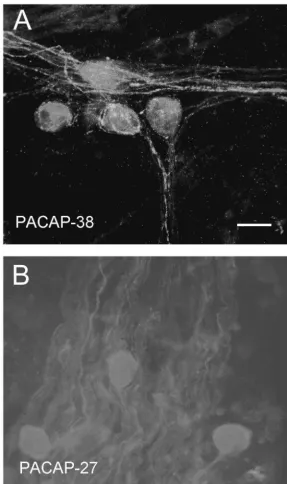
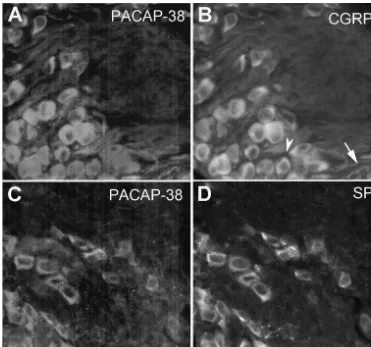
Fig. 1. PACAP immunoreactivity in the mudpuppy cardiac ganglion. Whole mount preparations containing the cardiac ganglion were labeled with antibodies to either PACAP-38 (at a concentration of 1:500) or to PACAP-27 (at a concentration of 1:100). PACAP-38-immunoreactivity was observed in all preparations tested (A), whereas no evidence for specific labeling with the PACAP-27 antibody was observed in three additional preparations (B). PACAP-38-ir was observed in nerve fibers and pericellular complexes, but not in cells within the preparations. Calibration bar550mm.
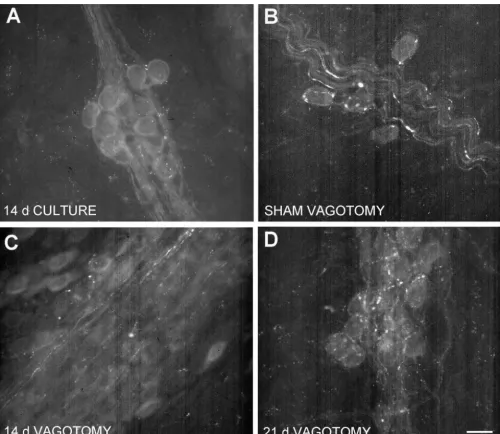
for 14 days at room temperature, and then examined for sources and further experiments were designed to examine PACAP-38 and CGRP immunoreactivity. PACAP-ir in the one input to the cardiac ganglia, the vagus nerve. cardiac ganglion was greatly reduced following prolonged Studies in mammals found evidence for PACAP in the culture (Fig. 4A), and the few PACAP-positive fibers that nodose ganglia as well as the dorsal motor nucleus of the were observed in one preparation showed signs of degene- vagus [3,10,17]. Thus, one source of the PACAP-con-ration. A similar pattern of reduced immunoreactivity and taining nerve fibers in the cardiac ganglia could be the
degeneration was observed in CGRP staining in these vagus nerve. This possibility was explored in the
Fig. 2. Co-localization of PACAP with CGRP and SP. Cardiac ganglia were labeled with PACAP-38 antibody (1:500) and either anti-CGRP (1:500) or anti-SP (1:500). Co-localization of immunoreactivity was observed in numerous nerve fibers for both CGRP (A and B) and SP (C and D). In some cases, fibers stained for only one of the antibodies. Panel B (arrow) shows a CGRP-positive nerve fiber which did not stain for PACAP, whereas Panel C (arrowhead) shows a PACAP-positive fiber which did not stain with SP antibody. SP-immunoreactive SIF cells can also be observed in Panel D (arrow) which do not label with the PACAP antibody. The intense staining within the neurons in panels C and D is due to the autofluorescence of pigmented granules commonly observed in these preparations. Calibration bar550mm.
vagus nerve was removed bilaterally from the animals, the preparations, there was also evidence of non-degenerated
wound was sutured, and the animals were allowed to PACAP-ir nerve fibers to some regions of the cardiac
recover for 14–21 days. Previously, Roper [22] showed ganglion in these animals (Fig. 4D), suggesting that not all that a time period of 14 days was sufficient to destroy all PACAP-ir fibers were affected by the surgery.
physiological responses to stimulation of the vagal trunk in
the mudpuppy cardiac ganglia. 3.4. PACAP-ir in dorsal root ganglia, vagal sensory
PACAP-ir showed a marked reduction in staining at 14 ganglia, and brainstem days post-surgery (n53) compared to the control (sham
Fig. 3. Immunoreactivity for PACAP-38 and galanin in the cardiac ganglion. Preparations were labeled with the antibody to PACAP-38 (1:500) and an antibody to galanin (1:500). Galanin immunoreactivity could be observed in fibers innervating contractile tissue outside the ganglia itself (D) and within the postganglionic neurons and SIF cells (B, arrow). Conversely, no PACAP immunoreactivity was observed in the fibers innervating the contractile tissue (C) or in the cells (A). An occasional fiber was seen that appeared to show positive staining for both galanin and PACAP (A, arrow). However, there was relatively little evidence of co-localization of galanin and PACAP-38 in the majority of fibers in 4 different preparations. Calibration bar550mm.
sections (16 mm) were then labeled with PACAP-38 centrations within neuronal cell somas for immunohistoch-antibody and either SP or CGRP immunohistoch-antibody for immuno- emical detection. Sections from the vagal sensory ganglia, histochemistry. PACAP-ir cell somas and nerve fibers were which contain cell bodies for vagal afferent neurons, observed in the DRG (Fig. 5). Virtually all of the PACAP- showed PACAP-ir cell somas and nerve fibers (Fig. 6) in ir positive neurons were also immunoreactive for either SP both untreated and colchicine-treated tissue. No evidence or CGRP (Fig. 5). However, not all SP or CGRP-ir cells of either CGRP or SP-ir was observed in these same tissue were also positive for PACAP. In addition, PACAP-ir nerve sections (data not shown). Caudal sections (16mm) of the fibers were observed entering the dorsal horn in sections of brainstem ranging from the most caudal medulla to the
the cervical spinal cord (Fig. 7). level of the VII cranial nerve root were also examined for
Fig. 4. Explant and vagotomy reduces PACAP immunoreactivity. Cardiac ganglia were maintained in organ culture for 14 days at room temperature prior to staining for PACAP-38 immunoreactivity (Panel A). Ganglion explants showed a dramatic reduction in PACAP-ir. Specific lesioning of the vagus nerve also reduced the amount of PACAP-ir in the cardiac ganglion (Panel C) as compared to sham-operated controls (Panels B). Increasing the post-operative recovery time to 21 days showed an overall decrease in PACAP-ir, but selective regions still showed intact fibers and pericellular complexes (Panel D). Calibration bar550mm.
brainstem sections, including the region originally iden- sequence [7]. Such evolutionary conservation would sug-tified by Herrick [5] as the dorsal motor nucleus, in either gest that this peptide may perform a vital function(s) across the control or the colchicine-treated tissue (Fig. 7). species. The exact nature of this function(s), though, still remains largely unknown. However, increasing evidence from other investigators and the present study suggests that
4. Discussion one of the roles of PACAP may be in the regulation of
autonomic function across species.
Pituitary adenylate cyclase-activating polypeptide is a In mammals, PACAP has been found in autonomic
highly conserved peptide across species [7], with the ganglia in both the periphery and in regions of the
Fig. 5. Co-localization of PACAP with SP and CGRP in dorsal root ganglion neurons. Sections (16mm) of dorsal root ganglia from high thoracic regions were stained with the antibody to PACAP-38 (1:800) and either SP (1:800) or CGRP (1:800). PACAP-ir cell somas were observed in the ganglia which were also immunoreactive for either CGRP (panel A and B) or SP (panel C and D). A CGRP-ir nerve fiber is also present (B, arrow) which is also immunoreactive for PACAP. Occasional neurons were observed that were immunoreactive for CGRP alone (B, arrowhead). Calibration bar550mm.
[1,6,23] as well as by direct stimulatory actions on the PACAP innervation of the cardiac ganglion in the mud-cardiac muscle fibers [2]. This study provides anatomical puppy appears to derive solely from extrinsic sources, with
evidence that PACAP may serve as a visceral afferent no evidence of endogenous PACAP production within the
neuromodulator of cardiac function in amphibians as well. ganglia. This differs from results found in the guinea pig, In the mudpuppy, PACAP can be found in afferent fibers in which PACAP-positive cell bodies and PACAP mRNA and neurons innervating the parasympathetic postgan- could be detected within the ganglia itself [1].
glionic neurons of the cardiac ganglion. These PACAP-ir Previous studies in mammals had found PACAP in both fibers were found as pericellular complexes surrounding parasympathetic preganglionic efferent fibers and afferent parasympathetic postganglionic neurons and in varicosities fibers of the vagus nerve [3,10,17]. Thus, one potential
throughout the ganglion. This finding would imply that extrinsic source of PACAP to the mudpuppy cardiac
PACAP can be released in close proximity to the neurons, ganglion is via the vagus nerve. This possibility was tested
presumably to act as modulators of their activity. The by lesioning the vagus nerve and examining PACAP
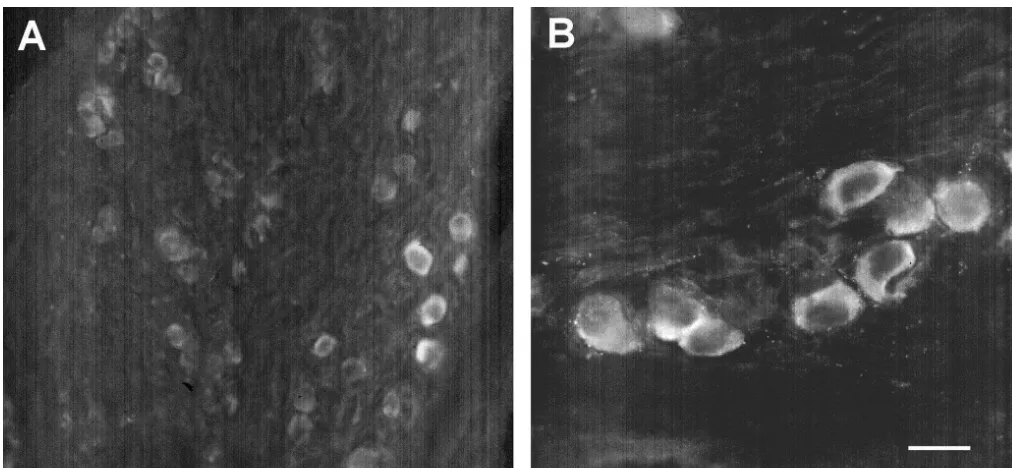
Fig. 6. PACAP immunoreactive neurons in the vagal sensory ganglia. Sections (16mm) of the ganglia of the X cranial nerve (vagal sensory ganglia) were stained with the antibody to PACAP-38 (1:800). Immunoreactive cell somas were observed throughout the ganglia, although not all neurons stained positively for PACAP. Calibration bar550mm in Panel B, and 100mm in Panel A.
there is a small, but significant amount of PACAP which PACAP-ir fibers innervating regions of the nucleus
was unaffected by the lesions, and therefore, must origi- solitarius, a major afferent input center for control of
nate from other sources. cardiovascular function. No PACAP-ir cell somas were
The co-localization of the PACAP with SP and CGRP in observed in the nucleus solitarius. Nor were PACAP-ir cell the mudpuppy cardiac ganglion suggest that one alternative bodies observed in the sections from the caudal medulla, source of PACAP is from fibers originating in the dorsal which would include the regions of the dorsal motor root ganglia. This hypothesis was supported by the co- nucleus and the nucleus ambiguous, where the majority of localization of PACAP-ir cells within sections of the dorsal vagal motor fibers to the heart originate. These results were root ganglia with either SP or CGRP. In addition, PACAP- observed in both untreated and colchicine-treated animals ir fibers could be observed entering the dorsal horn of the to determine if the lack of staining in control animals was
spinal cord. due to low cytoplasmic concentrations of the peptide in the
The lack of co-localization of PACAP with tyrosine cell somas. Colchicine treatment did not result in any hydroxylase in the cardiac ganglion suggests that PACAP difference in peptide levels. This does not provide defini-is not found in sympathetic postganglionic fibers innervat- tive proof that PACAP is not found in vagal efferent ing the cardiac ganglion. However, since no other studies nuclei, but does suggest that PACAP is localized primarily have yet looked at the potential localization of PACAP in in visceral afferent fibers of the vagus in the mudpuppy. other sympathetic fibers or autonomic neurons in am- Further studies are necessary to insure that no PACAP-phibians, it is not possible to conclude that PACAP is not containing cells are present in the vagal motor nucleus. found in the sympathetic nervous system in these animals. This result does appear to differ from that observed in
The PACAP-containing nerve fibers carried in the vagus mammals, where PACAP was found in both sensory
nerve could be of either motor or sensory origins. The neurons and in a small population of efferent vagal
exact nature of the input was explored by examining the neurons [3,10].
sources of these two different vagal fiber populations. The In summary, the results of this study demonstrate that vagal afferent fibers originate primarily from the nodose the evolutionarily-conserved neuropeptide, PACAP, may
(vagal sensory) ganglia. Sections of this ganglia were play an important role in autonomic function across
examined for PACAP staining and both PACAP-ir cell species, and in particular, appears to play a role in the bodies and fibers were observed. The vagal motor outputs control of cardiac function in both mammals and am-originate from nuclei in the caudal brainstem. Sagittal and phibians. Although the physiological effects of PACAP in
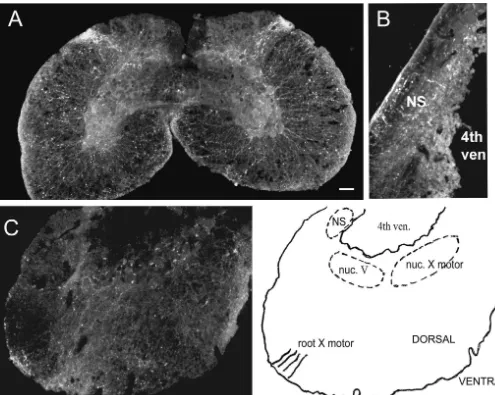
Fig. 7. PACAP immunoreactivity in sections of spinal cord and brainstem. Horizontal sections (16mm) from the cervical spinal cord (A) and caudal brainstem (C) were labeled with antibody to PACAP-38 (1:800). Longitudinal sections of brainstem were also examined (B). PACAP-ir nerve fibers can be found entering the dorsal horn of the spinal cord (A) and innervating the region of the nucleus solitarius (B, arrow). No evidence for PACAP-ir cell somas or fibers were observed in the region of the dorsal motor nucleus in a colchicine-treated preparations (C, arrow). PACAP-ir fibers were observed in the dorsal regions of the brainstem sections in the region where the vagus nerve enters / exits. Calibration bar5100mm in A and B, 50mm in C. Abbreviations: NS5nucleus solitarius; 4th ven5fourth ventricle; nuc. V5nucleus of cranial nerve V; nuc. X motor5motor nucleus of cranial nerve X; root X motor5roots of motor fibers from cranial nerve X. Nuclei and fiber tract localization from Herrick [5].
that PACAP is positioned to function as an important in part by NIH AREA grant R15 HL60619. The tyrosine
sensory peptide in the regulation of cardiac function in the hydroxylase antibody developed by LeDourain and Ziller
mudpuppy. was obtained from the Developmental Studies Hybridoma
Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Acknowledgements
We would like to thank Reshma Kumar, Kristen Sager References
and Meghan Rossi for their technical assistance in this
project. We would also like to thank Rodney Parsons for [1] K.M. Braas, V. May, S.A. Harakall, J.C. Hardwick, R.L. Parsons,
modulation of neuronal excitability in guinea pig cardiac ganglion, [13] T.W. McKeon, R.E. Carraway, L.M. Konopka, R.L. Parsons, Dis-J. Neurosci. 18 (1998) 9766–9779. tribution of galanin-like peptide in various tissues of Necturus [2] H.C. Champion, J.A. Santiago, E.A. Garrison, D.Y. Cheng, maculosus, Cell Tissue Res. 262 (1990) 461–466.
D.H. Coy, W.A. Murphy, R.J. Ascuitto, N.T. Ross-Ascuitto, D.B. [14] T.W. McKeon, R.L. Parsons, Galanin immunoreactivity in the McNamara, P.J. Kadowitz, Analysis of cardiovascular responses to mudpuppy cardiac ganglion, J. Autonom. Nerv. Syst. 31 (1990) PACAP-27, PACAP-38, and vasoactive intestinal peptide, Ann. NY 135–140.
Acad. Sci. 805 (1996) 429–442. [15] U.J. McMahan, D. Purves, Visual identification of two kinds of [3] N.J. Dun, T. Miyazaki, H. Tang, E.C. Dun, Pituitary adenylate nerves cells and their synaptic contacts in a living autonomic cyclase activating polypeptide immunoreactivity in the rat spinal ganglion of the mudpuppy (Necturus maculosus), J. Physiol. 254 cord and medulla: implication of sensory and autonomic functions, (1976) 405–425.
Neuroscience 73 (1996) 677–686. [16] A. Miyata, A. Arimura, R.R. Dahl, N. Minamino, A. Uehara, L. ¨
[4] T. Elsas, R. Uddman, H. Mulder, F. Sundler, Pituitary adenylate Jiang, M.D. Culler, D.H. Coy, Isolation of a novel 38 residue-cyclase activating polypeptide and nitric oxide synthase are ex- hypothalamic polypeptide which stimulates adenylate cyclase in pressed in the rat ciliary ganglion, Br. J. Ophthalmology 81 (1997) pituitary cells, Biochem. Biophys. Res. Commun. 164 (1989) 567–
223–227. 575.
[5] C.J. Herrick, The medulla oblongata of Necturus, J. Comp. Neurol. [17] H. Mulder, R. Uddman, K. Moller, T. Elsas, E. Ekblad, J. Alumets, 50 (1930) 1–96. R. Sundler, Pituitary adenylate cyclase activating polypeptide is [6] M. Hirose, Y. Furukawa, Y. Nagashima, K. Yamazaki, Y. Hoyano, S. expressed in autonomic neurons, Reg. Peptides 59 (1995) 121–128. Chiba, Effects of PACAP-38 on the SA nodal pacemaker activity in [18] D.S. Neel, R.L. Parsons, Catecholamine, serotonin, and substance autonomically decentralized hearts of anesthetized dogs, J. Cardio- P-like peptide containing intrinsic neurons in the mudpuppy para-vasc. Pharmacol. 29 (1997) 216–221. sympathetic cardiac ganglion, J. Neurosci. 6 (1986) 1970–1975. [7] C.H.V. Hoyle, Neuropeptide families: evolutionary perspectives, [19] R.L. Parsons, D.S. Neel, Distribution of calcitonin gene-related
Reg. Peptides 73 (1998) 1–33. peptide immunoreactive nerve fibers in the mudpuppy cardiac [8] C. Kimura, K. Ohkubo, K. Ogi, M. Hosoya, Y. Itoh, H. Onda, A. septum, J. Autonom. Nerv. Syst. 21 (1987) 135–143.
Miyata, L. Jiang, R.R. Dahl, H.H. Stibbs, A. Arimura, M. Fujino, A [20] R.L. Parsons, D.S. Neel, L.M. Konopka, T.W. McKeon, The novel peptide which stimulates adenylate cyclase: molecular cloning presence and possible role of a galanin-like peptide in the mudpuppy and characterization of the ovine and human cDNAs, Biochem. heart, Neuroscience 29 (1989) 749–759.
Biophys. Res. Commun. 166 (1990) 81–89. [21] R.L. Parsons, D.S. Neel, T.W. McKeon, R.E. Carraway, Organiza-[9] L. Klimaschewski, C. Hauser, C. Heym, PACAP immunoreactivity tion of a vertebrate cardiac ganglion: A correlated biochemical and
in the rat superior cervical ganglion in comparison to VIP, Neuro- histochemical study, J. Neurosci. 7 (1987) 837–846.
Report 7 (1996) 2797–2801. [22] S. Roper, The acetylcholine sensitivity of the surface membrane of [10] G. Legradi, S. Shioda, A. Arimura, Pituitary adenylate cyclase- multiply-innervated parasympathetic ganglion cells in the mudpuppy activating polypeptide-like immunoreactivity in autonomic regula- before and after partial dennervation, J. Physiol. 254 (1976) 455– tory areas of the rat medulla oblongata, Neurosci. Lett. 176 (1994) 473.
193–196. [23] J. Seebeck, W.E. Schmidt, H. Kilbinger, J. Neumann, N.
[11] J. Lydie, Y. Laurent, C. Nicolas, G. Bruno, F. Alain, C.J. Michael, V. Zimmermann, S. Herzig, PACAP induces bradycardia in guinea-pig Hunbert, Characterization and localization of pituitary adenylate heart by stimulation of atrial cholinergic neurones, Naunyn-cyclase-activating polypeptide (PACAP) binding sites in the brain of Schmiedeberg’s Arch. Pharmacol. 354 (1996) 424–430.
the frog Rana ridibunda, J. Comp. Neurol. 412 (1999) 218–228. [24] L. Yon, M. Feuilloley, N. Chartel, A. Rimura, J.M. Conlon, A. [12] K. Matsuda, Y. Takei, J.-I. Katoh, S. Shioda, A. Arimura, M. Fournier, H. Vaudry, Immunohistochemical distribution and bio-Uchiyama, Isolation and structural characterization of pituitary logical activity of pituitary adenylate cyclase-activating polypeptide adenylate cyclase activating polypeptide (PACAP)-like peptide from (PACAP) in the central nervous system of the frog Rana ridibunda, the brain of a teleost, stargazer, Uranoscopus japonicus, Peptides 18 J. Comp. Neurol. 324 (1992) 485–499.