Environmental and Experimental Botany 43 (2000) 131 – 139
Interaction of CO
2enrichment and drought on growth,
water use, and yield of broad bean (Vicia faba
)
Dong-Xiu Wu
1, Gen-Xuan Wang *
State Key Laboratory of Arid Agroecology,Lanzhou Uni6ersity,Lanzhou730000,People’s Republic of China
Received 10 April 1999; received in revised form 28 September 1999; accepted 9 October 1999
Abstract
Broad bean (Vicia fabacv. Lincan II) were grown in pots at two CO2concentrations (350 and 700 parts per million
by volume (ppmv)) and three soil water levels (80, 60 and 40% field water capacity) in field open-top chambers (OTCf). Water deficit reduced plant shoot dry weight, bean yield, and water use efficiency (WUE) by over 40, 30, and
15%, respectively, with higher relative reduction under elevated CO2. High CO2significantly increased leaf
photosyn-thesis, plant growth, bean yield and WUE. The increase is significant only at sufficient water supply. Both CO2
enrichment and water deficit influenced bean yield mainly through bean number. Harvest index was increased by both high CO2and drought. There were significant interactions between CO2enrichment and soil water deficit on plant
growth and yield. On the basis of above results, it is concluded that the effects of CO2enrichment on plants depend
on soil water status, and the negative effects induced by drought will be relatively more serious in the future at increased CO2concentrations. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Broad bean; Carbon dioxide; Drought; Growth; Harvest index;Vicia faba; Water use; Yield
www.elsevier.com/locate/envexpbot
1. Introduction
Atmospheric CO2 concentration ([CO2]) is
cur-rently increasing at a rate of 1.8 parts per million by volume (ppmv) per year (Houghton et al., 1990). It has been estimated that within the next century the [CO2] will be double the pre-industrial
level of 280 ppmv. That increase along with pro-jected rises in other ‘greenhouse’ trace gases is
likely to cause a change in climate (IPCC, 1996). Global circulation models predict a rise in global surface temperature by 1.5 – 5.9°C, changes in pre-cipitation patterns and cloud cover in the next 50 – 70 years (Washington and Meehl, 1984; Wilson and Mitchell, 1987). Further, the fre-quency of extreme climatic events such as heat and drought stresses are predicted to increase (Mearns et al., 1984). CO2 enrichment and the
climate change could affect agricultural produc-tivity. Precipitation is limited in large parts of the Loess Plateau in China and water is and will be a limiting factor for agricultural productivity in this area and many other regions. Thus, it is
impor-* Corresponding author.
E-mail address:[email protected] (D.-X. Wu) 1Current address: Biology Department, Henan University, Kaifeng 475001, People’s Republic of China.
tant to consider both elevated CO2concentrations
and differences in soil water in order to assess the possible effects of climate change on crops.
Numerous experiments have demonstrated that in many C3 species high atmospheric [CO2] leads
to increases in the photosynthetic rate, whole plant growth and water use efficiency (WUE) and decreases in stomatal conductance and transpira-tion and photosynthesis is the most sensitive pro-cess to CO2 enrichment (Kimball, 1983; Drake
and Leadley, 1991; Bowes, 1993; Poorter, 1993; Idso and Idso, 1994; Jiang, 1995; Wang et al., 1998). While results of studies on the plant canopy water use requirements are conflicting (Al-len, 1990) water deficit, on the other hand, is well established to constrain leaf photosynthesis, plant growth and water use requirements with the most sensitive process being cell growth (Hsiao, 1973; Turner, 1987). However, on the interactive effects of CO2 and other environmental factors on
plants, publications are relatively fewer, and among these there are two contradictory views. Some authors proposed that high [CO2] effects on
plants were not affected by environmental stress factors (Idso and Idso, 1994) whereas other au-thors have reported or theoretically concluded that high [CO2] effects vary among plant species
under different environmental conditions (Kim-ball, 1983; Poorter, 1993, 1998; Thompson and Woodward, 1994; Hunt et al., 1995; Ziska et al., 1996; Bunce, 1998). Some authors have even sug-gested that the positive effects of CO2 can not be
maintained when other environmental factors are limiting (Kramer, 1981; Poorter, 1998). So plant growth and yield response to CO2 can depend on
the availability of soil water (Stronach et al., 1994). However, judging by the available data on the interactions between CO2 and other
environ-mental factors, water stress, which is probably the most important of the environmental interactions with elevated CO2, is one of the least well studied
(Bowes, 1993; Picon et al., 1997).
In this study, broad beans were grown under different combinations of CO2 concentration and
soil water levels and focused on the effect of long-term exposure of plants to elevated CO2and
drought on photosynthesis, growth and water use. It was hypothesized that: (1) there would be
inter-action between CO2 and drought on growth and
yield, and the effects of CO2enrichment on plants
depend on soil water status; (2) CO2 enrichment
would promote plant canopy water use require-ments due to the decrease in transpiration being over-offset by an increase in leaf area; (3) WUEi
and WUE would be increased by CO2enrichment.
2. Materials and methods
2.1. Plant materials and growth conditions
The experiment was conducted in field open-top chambers (OTCf), at Lanzhou (103.9° E, 36.0° N),
Gansu, in semi-arid region of Loess Plateau of China. Seeds of the local common-used broad bean cultivar (Vicia fabaL., Lincan II) were sown in March 1997 in 17.2 l black plastic pots (27 cm in diameter, and 30 cm in height) 14 beans per pot, filled with field loessial soil. Fertilizer (com-pound fertilizer, carbamide and ammonium phos-phate) was applied prior to planting to reach local favourable nutrient level. Then, the soil was sam-pled and analyzed at the Soil and Plant Chemical Testing Laboratory in the State Key Laboratory of Arid Agroecology. Based upon the results of that analysis, the soil properties are: pH 7.5, organic matter 3.2%, available N 170.7 mg kg−1
(i.e. hydrolytic N, 1 N NaOH hydrolysis), avail-able P 214.8 mg kg−1 (0.5 M NaHCO
3
extrac-tion), available K 228.5 mg kg−1 (1 N
CH3COONH4 extraction), field water capacity
33%. Before sowing the soil was irrigated to 80% field water capacity (FWC) (favourable soil water level for broad bean). Six treatments consisting of factorial combinations of two [CO2] levels and
three soil water levels commenced 20 days after sowing (DAS). Treatments are designated HA, MA, LA, HD, MD, and LD, where H, M, and L stand for high, medium, and low soil water levels, A and D stand for ambient and double ambient [CO2], respectively. Due to the use of
field-col-lected soil sufficient Rhizobium infection was found.
Plants were grown in six OTCfs (F1.5 m×2
m), three with ambient [CO2] (a seasonal average
ambi-D.-X.Wu,G.-X.Wang/En6ironmental and Experimental Botany43 (2000) 131 – 139 133
ent [CO2]. CO2 was supplied from three 25 t
storage tanks with vaporization facilities. Elevated atmospheric CO2was maintained for 10 h day
−1
at photoperiod (08:00 – 18:00 h, local time). Indi-vidual blowers made the air inside each chamber changing twice per minute. Nine pots were placed into each chamber. CO2concentrations were
con-tinuously monitored by CO2infrared gas analyzer
(CID, USA) and controlled by a computer. A WHM3 thermo-hygrograph (Tianjin, China) was fixed in each chamber to record temperature and relative humidity continuously. In the mean time the photosynthetic active radiation, leaf and air temperature and relative humidity in chambers were periodically examined using a CI-301 portable photosynthesis system (CID, USA). Dur-ing the broad bean growth season (April – July) in the chambers, average photosynthetic active radi-ation (PAR) was 672 mmol m−2 s−1, average
day/night temperature was 24.5/12.9°C, average relative humidity was 39.5% (Table 1).
Three soil water levels, 40, 60 and 80% FWC, were applied to each chamber (three pots per water treatment) from seedling stage onwards. The soil water contents were controlled by com-mon-used weight method. Before sowing soil wa-ter content and soil field wawa-ter capacity were measured. The total control weight for each pot was derived from the pot weight, soil dry weight in it and the expected soil water content level. The pots were weighted every other day and supple-mented a determinate quantity of water calculated from the controlled weight minus the actual weight. In the last growth phase of broad bean when the estimated total plant wet weight in each pot was more than 0.5 percent of control weight the pot control weight was periodically corrected by adding total plant wet weight in each pot to its initial control weight.
2.2. Measurements
Leaf net photosynthesis, transpiration and stomatal resistance were periodically measured. At each time, upper most fully expanded leaves of nine plants in each treatment were selected for the photosynthesis measurement using a CI-301 portable photosynthesis system (CID, USA).
Pho-tosynthesis (mmol CO2 m
−2 s−1) was calculated
on a leaf area basis determined by the window size of certain leaf chamber and manually put into photosynthesis system while measuring.
This bean cultivar is self-pollinating. Plant growth was assessed by periodical destructive growth analysis of three plants randomly selected from each pot. All component dry weights were obtained following oven-drying to constant weight at 85°C. Leaf area was determined using CI-203 area meter (CID, USA). Plants were har-vested on 10 July. Total shoot dry weight, bean dry weight per plant, bean number per plant and average bean dry weight in each pot were deter-mined at harvest.
Instantaneous water use efficiency (WUEi), that
is transpiration efficiency, is defined as the ratio of photosynthetic rate/transpiration rate. Whole growth season water use efficiency (WUE) was calculated from shoot dry weight per plant at harvest divided by cumulative consumption of water per plant, thus leaf transpiration and soil evaporation.
Standard deviation (S.D.) of each treatment was calculated. The significance analyses of indi-vidual and interactive effect of CO2 and drought
were performed using two-way analysis of vari-ance (ANOVA) with replicates and t-test at PB
0.05 using software developed by Statistical W5.0 (Statistics Inc. USA).
3. Results
3.1. Leaf photosynthesis
Leaf net photosynthesis (Pn) in different
combi-nations of [CO2] and soil water level treated
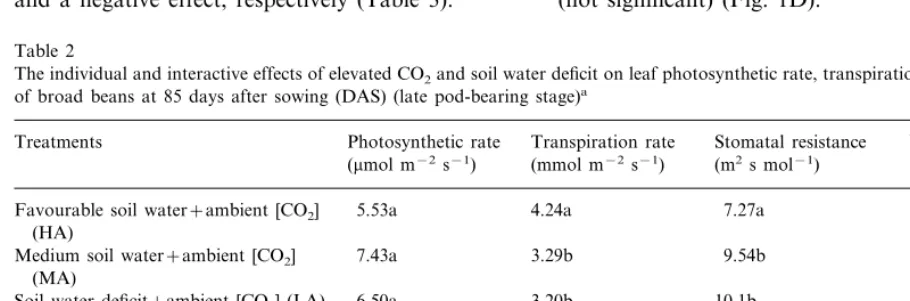
plants at pod-bearing stage is shown in Table 2. High [CO2] increased the leaf net photosynthesis
by over 90% for plants grown under either fa-vourable soil water level (HD) or less than that (MD, LD) (PB0.01). However, soil drought had
a significant negative effect on photosynthetic rate only under double [CO2] (PB0.01). Based on the
results of two-way ANOVA with replicates, there was a significant interaction between high [CO2]
D
.-X
.
Wu
,
G
.-X
.
Wang
/
En
6
ironmental
and
Experimental
Botany
43
(2000)
131
–
139
Table 1
The dynamics of photosynthetic active radiation (PAR), temperature, and relative humidity in the chambers during growth season of broad beans
Average maximum Average maximum
Daytime mean
Days after sow- Daytime mean Night mean tem- Average minimum Daytime mean
rel-ative humidity temperature (°C)
perature (08:00–
temperature temperature (°C)
PAR (08:00–
ing PAR (mmol m−2
s−1) (08:00–20:00) (°C) 20:00) (°C)
18:00) (mmol m−2 (08:00–20:00) (%)
s−1)
661 1110 19.1 24.1 5.4 15.5
0–30 7.1
9.0 24.5
27.2
23.2 11.8
31–60 684 1250
31.7
689 1260 27.6 15.9 14.5 33.0
61–90
18.8 32.5 16.8 36.1
643
D.-X.Wu,G.-X.Wang/En6ironmental and Experimental Botany43 (2000) 131 – 139 135
3.2. Plant growth
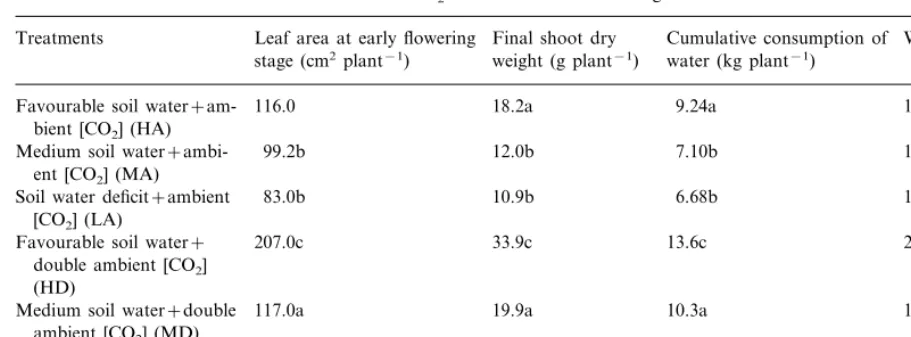
High [CO2] significantly increased the shoot dry
weight at harvest at high and medium soil water levels (HD vs. HA, MD vs. MA) (Table 3). However, under low soil water level, the effect of high [CO2] on growth was not significant (LD vs.
LA). The shoot dry weight at harvest was signifi-cantly reduced by 40% by water deficit at ambient [CO2] (LA vs. HA) and by 69% at high [CO2] (LD
vs. HD). Based on the results of two-way ANOVA with replicates, high [CO2] and drought
showed significant interaction effect on growth (PB0.01).
3.3. Water use
Since high [CO2] significantly increased
photo-synthesis and decreased transpiration, high [CO2]
significantly increased transpiration efficiency (WUEi) (PB0.001). On the other hand, drought
had no significant effects on WUEi due to its
simultaneous decreasing effect on photosynthesis and transpiration. The negative effect of high [CO2] and drought on transpiration was closely
associated with increase in stomatal resistance (Table 2). As for whole season water consumption and WUE, high [CO2] and drought had a positive
and a negative effect, respectively (Table 3).
3.4. Yield and yield components
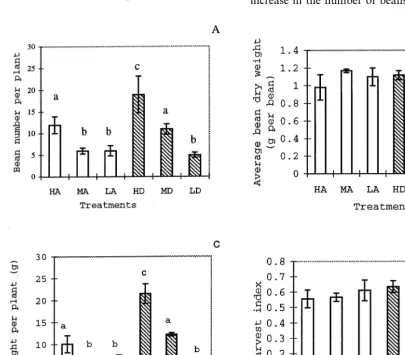
CO2 enrichment markedly increased the
num-ber of beans per plant at high and medium water levels (HD vs. HA, MD vs. MA) (Fig. 1A), while under low soil water levels, the effect of high [CO2] was not significant. Water deficit severely
decreased the number of seeds per plant by 50% at ambient [CO2] (LA vs. HA), by 78% at doubled
CO2 (LD vs. LD). There were significant interac-tions between high [CO2] and drought on bean
number. In the current experiment, the average bean number per pod was 1.5, and relatively stable.
Both CO2 enrichment and drought had no
sig-nificant effect on individual bean weight (Fig. 1B). CO2 enrichment significantly increased the
total-bean weight per plant by 113% at high water level (HD vs. HA), 80% at medium water level (MD vs. MA) (Fig. 1C), while under low soil water level, the effect of high [CO2] was not significant. Water
deficit significantly reduced bean production by 34% at ambient [CO2] (LA vs. HA) and 77% at
high CO2 (LD vs. HD). Drought and high [CO2]
showed significant interactive effects on bean weight per plant.
The proportion of plant dry biomass allocated to beans (harvest index, HI) increased by over 10% by high [CO2] (HD) and water deficits (LA)
(not significant) (Fig. 1D).
Table 2
The individual and interactive effects of elevated CO2and soil water deficit on leaf photosynthetic rate, transpiration rate and WUEi of broad beans at 85 days after sowing (DAS) (late pod-bearing stage)a
Photosynthetic rate Stomatal resistance
Treatments Transpiration rate WUEi
(m2s mol−1)
(mmol m−2s−1) (mmol m−2s−1) (mmol mmol−1)
Favourable soil water+ambient [CO2] 5.53a 4.24a 7.27a 1.51a (HA)
Medium soil water+ambient [CO2] 7.43a 3.29b 9.54b 1.94a (MA)
6.50a
Soil water deficit+ambient [CO2] (LA) 3.20b 10.1b 2.35a 21.0b 25.3b
Favourable soil water+double ambient 1.43c 22.1c [CO2] (HD)
35.7d 19.0b
Medium soil water+double ambient 21.8b 0.99d [CO2] (MD)
36.1d 20.0b
Soil water deficit+double ambient 12.6c 0.75d [CO2] (LD)
Table 3
The individual and interactive effects of elevated CO2and soil water deficit on growth and water use of broad beansa
Cumulative consumption of
Treatments Leaf area at early flowering Final shoot dry WUE (g kg−1) weight (g plant−1)
stage (cm2plant−1) water (kg plant−1)
116.0 18.2a
Favourable soil water+am- 9.24a 1.96a
bient [CO2] (HA)
Medium soil water+ambi- 99.2b 12.0b 7.10b 1.69b
ent [CO2] (MA)
83.0b 10.9b 6.68b
Soil water deficit+ambient 1.63b
[CO2] (LA)
2.50c
207.0c 33.9c
Favourable soil water+ 13.6c
double ambient [CO2] (HD)
117.0a
Medium soil water+double 19.9a 10.3a 1.93a
ambient [CO2] (MD)
89.1b 10.6b 5.88b 1.79ab
Soil water deficit+double ambient [CO2] (LD)
aWithin columns, values followed by different letters are significantly (PB0.05) different (n=9).
4. Discussion
4.1. The effect of CO2 enrichment and drought on
growth
CO2enrichment markedly increased leaf
photo-synthesis under all three soil water levels and significantly increased plant growth and yield un-der relatively favourable soil water levels (Tables 2 and 3, Fig. 1). This observation agrees with many other reports that CO2 enrichment
influ-ences plant growth through photosynthesis (Pearcy and Bjo3rkman, 1983; Bowes, 1993; Wheeler et al., 1996; Deng and Woodward, 1998). On the other hand, water deficit had a significant negative influence on leaf net photosynthesis only at double [CO2], but significantly reduced plant
growth under both ambient and double [CO2]
(Tables 2 and 3). This means that water deficit directly regulate plant growth and yield, not nec-essarily through photosynthesis (Hsiao, 1973; Day, 1981; Pei and An, 1985; Turner, 1987). Based on many previous reports on plant re-sponses to water deficit, Hsiao (1973) has pro-posed that the most sensitive response of plants to water deficit is cell growth. Therefore, water and CO2 may affect plant growth in different ways
though there are interactions between them. There is a positive feedback cycle between
photosyn-thetic products, growth and leaf area. The effec-tiveness of this cycle is among others undoubtedly influenced by water status. Under relatively fa-vourable water conditions, this positive feedback cycle is unblocked; CO2 enrichment may increase
plant growth by stimulating photosynthetic rate and thereby accelerating the cycle (Pearcy and Bjo3rkman, 1983; Bowes, 1993; Rogers et al., 1996a). Under drought conditions, both cell wall extension and water uptake — the two process involved in cell expansion — are inhibited (Boyer, 1968); additionally, plant as a whole unit has to allocate much more energy for tolerating the water deficit in the environment. This will inevitably make the above positive feedback cycle impeded or even invalidated. So, the positive ef-fect of high [CO2] on plant growth was relatively
greater under favourable water condition than under less favourable water condition, and water deficit-induced reduction in crop growth and yield were relatively greater at double [CO2] than at
ambient [CO2] (Table 3, Fig. 1).
4.2. The effect of CO2 enrichment and drought on
water use
Both high [CO2] and water deficit reduce
D.-X.Wu,G.-X.Wang/En6ironmental and Experimental Botany43 (2000) 131 – 139 137
agrees with most of earlier reports (Morison, 1985, 1998). However, the reduction in transpira-tion under double [CO2] was over-offset by a
larger leaf area, so that CO2enrichment increased
rather than decreased whole-season water con-sumption (Table 3). The high [CO2]-induced
in-crease in growth was greater than that in water consumption, so that high [CO2] increased WUE.
This is very beneficial to crops grown in
water-limited environments. While drought decreased WUE, the decrease under double [CO2] was more
serious.
4.3. The effect of high CO2 and drought on yield
The positive yield response of broad bean to CO2 enrichment was closely associated with an
increase in the number of beans per plant. This is
Fig. 1. Effects of elevated CO2concentration and soil water deficit on the yield of broad bean. (A) Number of beans per plant; (B) average bean dry weight; (C) bean dry weight per plant; (D) harvest index (HI). Values are means of nine observations. In A and C, columns marked by different letters are significantly (PB0.05) different (n=9). In B, both [CO2] and soil water has no significant effects. The interactive effects of high [CO2] and water deficit are significant in A, C and D. Vertical bars indicate standard deviation (S.D.) of the means. HA, high soil water level (80% field water capacity (FWC)) and ambient [CO2] (350 parts per million by volume
supported by earlier findings that increases in yield with CO2 enrichment largely resulted from
an increase in grain number (Downton et al., 1987; Sung and Chen, 1991; Thompson and Woodward, 1994; Rogers et al., 1996b). In the current experiment, the bean number per pod is relatively stable, so bean numbers per plant practically reflect the flowers per plant. The ex-tra carbon assimilates produced at high [CO2]
may ensure the full development of flowers and seeds (Deng and Woodward, 1998). Drought – yield responses also largely resulted from the changes in bean number. Water deficit may cause the abscission of flowers, reducing the ef-fective pollination period and leading to failure of fertilization (Clifford et al., 1993).
Both CO2 enrichment and water deficit
in-creased HI (Fig. 1D). This means that beans allocated a greater proportion of carbon assimi-lates for growth at high [CO2] (Lawlor and
Mitchell, 1991; Wheeler et al., 1996) or at low water supply. The increased HI associated with high [CO2] may be due to the higher
sensitive-ness of bean development to extra leaf photo-synthesis induced by high [CO2] than vegetative
growth. It is evolutionarily advantageous and natural that annual plants make full use of ex-tra carbon assimilates to promote fitness. On the other hand, drought-induced high HI may be a result of the relatively weaker constraints of drought on bean growth than on vegetative growth. Under stress conditions, plants firstly ensure reproduction, this is also of evolutionary advantage.
In conclusion, high [CO2] is of benefit to crop
growth, WUE and yield. Water stress, on the other hand, decreases the plant growth and yield. But both high [CO2] and water stress
in-crease HI. From the analysis of present experi-mental results, it was suggest that [CO2] and
water affect plants by different ways, but inter-act with each other. Crops may benefit much more from CO2 enrichment if sufficient water is
supplied. Water deficits will cause relatively more serious reduction in plant growth and yield under future high [CO2] conditions.
Acknowledgements
This work was granted by the National Natu-ral Science Foundation of China (39670139), the Major State Basic Research Development Pro-gram of China, and Henan Natural Science Foundation.
References
Allen, L.H. Jr, 1990. Plant responses to rising carbon dioxide and potential interactions with air pollutants. J. Environ. Qual. 19, 15 – 34.
Bowes, G., 1993. Facing the inevitable: plants and increasing atmospheric CO2. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 309 – 332.
Boyer, J.S., 1968. Relationship of water potential to growth of leaves. Plant Physiol. 43, 1056 – 1062.
Bunce, J.A., 1998. The temperature dependence of the stimula-tion of photosynthesis by elevated carbon dioxide in wheat and barley. J. Exp. Bot. 49, 1555 – 1561.
Clifford, S.C., Stomach, I.M., Mohamed, A.D., Azam-Ali, S.N., Crout, N.M.J., 1993. The effects of elevated atmo-spheric carbon dioxide and water stress on light intercep-tion, dry matter production and yield in stands of groundnut (Arachis hypogaeaL.). J. Exp. Bot. 44, 1763 – 1770.
Day, W., 1981. Water stress and crop growth. In: Johnson, C.B. (Ed.), Physiological Processes Limiting Plant Produc-tivity. Butterworths, London, pp. 199 – 215.
Deng, X., Woodward, F.I., 1998. The growth and yield re-sponses of Fragaria ananassa to elevated CO2 and N supply. Ann. Bot. 81, 67 – 71.
Downton, W.J.S., Grant, W.J.R., Loveys, B.R., 1987. Carbon dioxide enrichment increases yield of Valencia Orange. Aust. J. Plant Physiol. 14, 493 – 501.
Drake, B.G., Leadley, P.W., 1991. Canopy photosynthesis of crops and native plant communities exposed to long-term elevated CO2. Plant Cell Environ. 14, 853 – 860.
Houghton, J.T., Jenkins, G.J., Ephraums, J.J. (Eds.), 1990. Climate change. The IPPC scientific assessment. Intergov-ernmental Panel on Climate Change, World Meteorologi-cal Organization? United Nations Environmental Programme. Cambridge University Press, Cambridge, p. 365.
Hsiao, T.C., 1973. Plant responses to water stress. Annu. Rev. Plant Physiol. 24?, 519 – 570.
Hunt, R., Hand, D.W., Hannah, M.A., Neal, A.M., 1995. Temporal and nutritional influences on the response to elevated CO2 in selected British grasses. Ann. Bot. 75, 207 – 216.
D.-X.Wu,G.-X.Wang/En6ironmental and Experimental Botany43 (2000) 131 – 139 139
IPCC, 1996. Climate change 1995. In: Houghton, J.T., Meira Filho, L.G., Callander, B.A., Harris, N., Kattenberg, A., Maskell, K. (Eds.), The Science of Climate Change, Cam-bridge University Press, CamCam-bridge, p. 572.
Jiang, G.M., 1995. The impact of global increasing CO2 on plants (in Chinese). Chin. Bull. Bot. 12, 1 – 7.
Kimball, B.A., 1983. Carbon dioxide and agricultural yield: an assemblage and analysis of 430 prior observations. Agron. J. 75, 779 – 788.
Kramer, P.J., 1981. Carbon dioxide concentration, photosyn-thesis, and dry matter production. BioScience 31, 29 – 33. Lawlor, D.W., Mitchell, R.A.C., 1991. The effects of
increas-ing CO2 on crops photosynthesis and productivity: a re-view of field studies. Plant Cell Environ. 14, 807 – 818. Mearns, L.O., Katz, R.W., Schneider, S.H., 1984. Changes in
the probabilities of extreme high temperature events with changes in global mean temperature. J. Clim. Appl. Meteo-rol. 23, 1601 – 1613.
Morison, J.I.L., 1985. Sensitivity of stomata and water use efficiency to high CO2. Plant Cell Environ. 8, 467 – 474. Morison, J.I.L., 1998. Stomatal response to increased CO2
concentration. J. Exp. Bot. 49, 443 – 452.
Pearcy, R.W., Bjo3rkman, O., 1983. Physiological effects. In: Lemon, E.R. (Ed.), CO2 and Plants, The Response of Plants to Rising Levels of Atmospheric Carbon Dioxide. Westview Press, Colorado, pp. 65 – 105.
Pei, B.X., An, S.Q., 1985. The results of experimental study on the relationship of winter wheat yield and field soil water (in Chinese). Chin. Bull. Sci. 20, 1599 – 1600.
Picon, C., Ferhi, A., Guehl, J.-M., 1997. Concentrtion and
d13C of leaf carbophydrates in relation to gas exchange in Quercus robur under elevated CO2 and drought. J. Exp. Bot. 48, 1547 – 1556.
Poorter, H., 1993. Interspecific variation in the growth re-sponse of plants to an elevated ambient CO2 concentra-tion. Vegetatio 104/105, 77 – 97.
Poorter, H., 1998. Do slow-growing species and nutrient-stressed plants respond relatively strongly to elevated CO2. Global Change Biol. 4, 693 – 697.
Rogers, G.S., Milham, P.J., Gillings, M., Conroy, J.P., 1996a. Sink strength may be the key to growth and nitrogen
responses in N-deficient wheat at elevated CO2. Aust. J. Plant Physiol. 23, 253 – 264.
Rogers, G.S., Milham, P.J., Thibaud, M.C., Conroy, J.P., 1996b. Interactions between rising CO2concentration and nitrogen supply in cotton. I. Growth and leaf nitrogen concentration. Aust. J. Plant Physiol. 23, 119 – 125. Stronach, I.M., Clifford, S.C., Mohamed, A.D.,
Singleton-Jones, P.R., Azam-Ali, S.N., Crout, N.M.J., 1994. The effects of elevated carbon dioxide, temperature and soil moisture on the water use of stands of groundnut (Arachis hypogaeaL.). J. Exp. Bot. 45, 1633 – 1638.
Sung, F.J.M., Chen, J.J., 1991. Gas exchange rate and yield response of strawberry to carbon dioxide enrichment. Sci-entia Horticult. 48, 241 – 251.
Thompson, G.B., Woodward, F.J., 1994. Some influences of CO2 enrichment, nitrogen nutrition and competition on grain yield and quality in spring wheat and barley. J. Exp. Bot. 45, 937 – 942.
Turner, N.C., 1987. Crop water deficits: a decade of progress. Adv. Agron. 39, 1 – 51.
Wang, X.L., Xu, S.H., Liang, H., 1998. The experimental study of the effects of CO2 concentration enrichment on growth, development and yield of C3 and C4 crops(in Chinese). Sci. Agric. Sin. 31, 55 – 61.
Washington, W.M., Meehl, G.A., 1984. Seasonal scale experi-ment on the climatic sensitivity due to a doubling of CO2 with an atmospheric general circulation model coupled to a simple mixed-layer ocean model. J. Geophys. Res. 89 (D6), 9475 – 9503.
Wheeler, T.R., Hong, T.D., Ellis, R.H., Batts, G.R., Morison, J.I.L., Hadley, P., 1996. The duration and rate of grain growth, and harvest index, of wheat (Triticum aesti6amL.)
in response to temperature and CO2. J. Exp. Bot. 47, 623 – 630.
Wilson, C.A., Mitchell, J.F., 1987. A doubled CO2 climate sensitivity experiment with global climate model including a simple ocean. J. Geophys. Res. 92, 13315 – 13343. Ziska, L.H., Manalo, P.A., Ordonez, R.A., 1996. Intraspecific
variation in the response of rice (Oryza sati6a L.) to
increased CO2and temperature: growth and yield response of 17 cultivars. J. Exp. Bot. 47, 1353 – 1359.