Contributions of the Bean/Cowpea CRSP to cultivar and
germplasm development in common bean
J.S. Beaver
a,*, J.C. Rosas
b, J. Myers
c, J. Acosta
d, J.D. Kelly
e, S. Nchimbi-Msolla
f,
R. Misangu
f, J. Bokosi
g, S. Temple
h, E. Arnaud-Santana
i, D.P. Coyne
jaDepartment of Agronomy and Soils, University of Puerto Rico, P.O. Box 9030, Mayaguez, PR 00681, USA bDepartment of Agricultural Science and Production, Escuela Agrı´cola Panamericana, P.O. Box 93, Tegucigalpa, Honduras
cDepartment of Horticulture, Oregon State University, 4017 Agriculture and Life Sciences, Corvallis, OR 97331, USA dINIFAP, Apartado Postal, C.P. 56230, Chapingo, Edo Mexico, Mexico
eDepartment of Crop and Soil Sciences, Michigan State University, East Lansing, MI 48824, USA fDepartment of Crop Science and Production, Sokoine University of Agriculture, Morogoro, Tanzania
gBunda College of Agriculture, Lilongwe, Malawi
hDepartment of Agronomy and Range Science, University of California at Davis, Davis, CA 95616, USA iCESIAF, San Juan de la Maguana, Dominican Republic
jDepartment of Horticulture, University of Nebraska, 386 Plant Sciences, East Campus, Lincoln, NE 68583, USA
Abstract
Disease and abiotic stress are important factors limiting bean production wherever beans are grown. The development of bean cultivars having resistance to these stresses is a cost-effective and sustainable means to address these constraints. During the past 20 years, the Bean/Cowpea Collaborative Research Support Project (B/C CRSP) has supported common bean cultivar development and germplasm improvement programs in the USA and developing countries. Plant breeders have developed and released in Central America and the Caribbean bean cultivars and germplasm with one or more of the following traits; resistance to bean golden yellow mosaic virus (BGYMV), bean common mosaic necrotic virus (BCMNV), rust, web blight and common bacterial blight (CBB) and greater tolerance to high temperatures. In the highlands of Mexico and Ecuador bean cultivars with resistance to anthracnose, rust, root rots and bean common mosaic virus (BCMV), greater biological nitrogen fixation and improved adaptation to intermittent drought have been released. The bean breeding programs in East Africa have developed and released bean cultivars and germplasm with resistance to BCMNV, rust and bruchid seed weevils. Participation in the B/C CRSP has permitted USA bean breeding programs to develop and release bean cultivars and germplasm with resistance to BGYMV, BCMNV, anthracnose, rust, CBB, architectural avoidance to white mold and greater yield potential. Numerous plant breeders, plant pathologists and agronomists from developing countries have received advanced degree training in the USA, which has enhanced the capacity to develop improved bean cultivars for Latin America and Africa. The lack of sustainable seed production and delivery systems continues to limit the impact of the release of improved bean cultivars in many parts of Latin America and East Africa.
#2003 Elsevier Science B.V. All rights reserved.
Keywords: Phaseolus vulgaris; BGYMV; BCMV; Rust; Anthracnose; Yield; Abiotic stress; Drought; Heat tolerance; Bean cultivars
*Corresponding author.
1. Introduction
Diseases such as anthracnose, bean rust, CBB and bean common mosaic and abiotic stresses such as drought and low soil fertility are important constraints to bean production wherever the crop is grown (Schwartz et al., 1996; Van Schoonhoven and Voysest, 1989; Wortmann et al., 1998). Angular leaf spot is a serious bean disease in the tropics, whereas white mold is more important in temperate bean growing regions. Halo blight and root rots are production constraints in the tropical highlands and temperate bean production zones (Murillo et al., 1997). In the lowland tropics, BGYMV, web blight and high tem-peratures can threaten bean production.
Since 1981, the USAID funded Bean/Cowpea Col-laborative Research Support Project (B/C CRSP) has supported common bean (Phaseolus vulgarisL.) cul-tivar development and germplasm improvement pro-grams in Latin America, the Caribbean, East Africa and the USA. A network for bean research is needed because no single institution can address all of the factors constraining bean production and utilization in each region. Because a number of these stresses affect production in both the USA and developing countries, bean breeders within the B/C CRSP share common interests and research results are often mutually ben-eficial (Table 1).
Long-term support by USAID provides continuity of effort, a key to the success of cultivar development programs. Bean breeders and plant pathologists, for example, have been able to identify sources of durable resistance to BGYMV, rust and anthracnose. The B/C CRSP has provided opportunities for bean breeders from developing countries to obtain advanced degree training and has permitted scientists from Latin Amer-ica and AfrAmer-ica to have access to laboratories where marker-assisted selection, and other advanced techni-ques can be conducted.
Most of the genetic diversity of beans is found in Latin America (Gepts and Debouk, 1991) where bean diseases and pests are often more virulent. This has enabled B/C CRSP supported bean breeders to iden-tify tropical germplasm with valuable genes for dis-ease resistance. Key to the B/C CRSP programs has been the use of elite lines from USA bean breeding programs and landrace cultivars from Latin America, the Caribbean and Eastern Africa. This has broadened
the genetic base of bean cultivars released in the USA and developing countries. B/C CRSP cultivar devel-opment programs have benefited greatly from the availability of breeding lines developed by the Centro Internacional de Agricultura Tropical (CIAT) in Cali, Colombia. CIAT scientists have mostly collaborated with bean breeding programs associated with Minis-tries of Agriculture, whereas B/C CRSP projects have tended to associate with institutions of higher educa-tion including the Escuela Agrı´cola Panamericana (EAP) in Honduras, the University of Costa Rica, Sokoine University of Agriculture in Tanzania, and Bunda College of Agriculture in Malawi. This differ-ence in focus by CIAT and the B/C CRSP has helped to broaden the base of bean research expertise in devel-oping countries (Table 2).
The development of bean cultivars with resistance to the most important abiotic and biotic stresses represents a cost-effective and sustainable means to address these constraints. The following sections con-tain examples where bean breeding programs sup-ported by the B/C CRSP have had significant impact in developing countries and the USA.
2. Biotic constraints
2.1. Bean golden yellow mosaic virus
Bean golden yellow mosaic is a whitefly transmitted geminivirus that threatens dry bean production in Central America and the Caribbean and snap bean production in southern Florida (Blair et al., 1995; Morales, 1994). Genetic progress in breeding for BGYMV resistance has been incremental. CIAT bean breeders, working in Guatemala, made significant advances in the identification of sources of resistance to BGYMV and developed black and small red culti-vars with resistance to the virus (Beebe, 1994). At the University of Puerto Rico, a greenhouse screening technique was developed that allowed inheritance studies to be conducted (Adames-Mora et al., 1996).
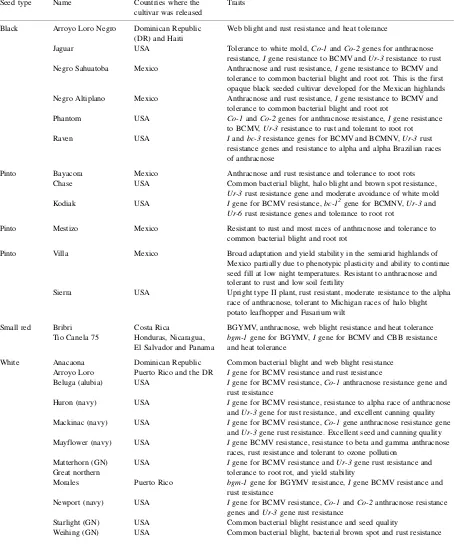
Table 1
Bean cultivars developed with participation by B/C CRSP scientists Seed type Name Countries where the
cultivar was released
Traits
Black Arroyo Loro Negro Dominican Republic (DR) and Haiti
Web blight and rust resistance and heat tolerance
Jaguar USA Tolerance to white mold,Co-1andCo-2genes for anthracnose resistance,Igene resistance to BCMV andUr-3resistance to rust Negro Sahuatoba Mexico Anthracnose and rust resistance,Igene resistance to BCMV and
tolerance to common bacterial blight and root rot. This is the first opaque black seeded cultivar developed for the Mexican highlands Negro Altiplano Mexico Anthracnose and rust resistance,Igene resistance to BCMV and
tolerance to common bacterial blight and root rot
Phantom USA Co-1andCo-2genes for anthracnose resistance,Igene resistance to BCMV,Ur-3resistance to rust and tolerant to root rot Raven USA Iandbc-3resistance genes for BCMV and BCMNV,Ur-3rust
resistance genes and resistance to alpha and alpha Brazilian races of anthracnose
Pinto Bayacora Mexico Anthracnose and rust resistance and tolerance to root rots Chase USA Common bacterial blight, halo blight and brown spot resistance,
Ur-3rust resistance gene and moderate avoidance of white mold Kodiak USA Igene for BCMV resistance,bc-12gene for BCMNV,Ur-3and
Ur-6rust resistance genes and tolerance to root rot
Pinto Mestizo Mexico Resistant to rust and most races of anthracnose and tolerance to common bacterial blight and root rot
Pinto Villa Mexico Broad adaptation and yield stability in the semiarid highlands of Mexico partially due to phenotypic plasticity and ability to continue seed fill at low night temperatures. Resistant to anthracnose and tolerant to rust and low soil fertility
Sierra USA Upright type II plant, rust resistant, moderate resistance to the alpha race of anthracnose, tolerant to Michigan races of halo blight potato leafhopper and Fusarium wilt
Small red Bribri Costa Rica BGYMV, anthracnose, web blight resistance and heat tolerance Tio Canela 75 Honduras, Nicaragua,
El Salvador and Panama
bgm-1gene for BGYMV,Igene for BCMV and CBB resistance and heat tolerance
White Anacaona Dominican Republic Common bacterial blight and web blight resistance Arroyo Loro Puerto Rico and the DR Igene for BCMV resistance and rust resistance
Beluga (alubia) USA Igene for BCMV resistance,Co-1anthracnose resistance gene and rust resistance
Huron (navy) USA Igene for BCMV resistance, resistance to alpha race of anthracnose andUr-3gene for rust resistance, and excellent canning quality Mackinac (navy) USA Igene for BCMV resistance,Co-1gene anthracnose resistance gene
andUr-3gene rust resistance. Excellent seed and canning quality Mayflower (navy) USA Igene BCMV resistance, resistance to beta and gamma anthracnose
races, rust resistance and tolerant to ozone pollution Matterhorn (GN)
Great northern
USA Igene for BCMV resistance andUr-3gene rust resistance and tolerance to root rot, and yield stability
Morales Puerto Rico bgm-1gene for BGYMV resistance,Igene BCMV resistance and rust resistance
Newport (navy) USA Igene for BCMV resistance,Co-1andCo-2anthracnose resistance genes andUr-3gene rust resistance
Starlight (GN) USA Common bacterial blight resistance and seed quality
to pod deformation in the presence of BGYMV. Identification of a codominant RAPD marker linked to thebgm-1gene has proved very useful for indirect selection for BGYMV resistance (Urrea et al., 1996). In addition to the major gene resistance, quantitative trait loci (QTL) associated with the BGYMV resis-tance in DOR 364 have also been identified (Miklas et al., 1996). Markers linked to resistance sources have been utilized to breed for resistance to BGYMV and
pyramid resistance sources (seeKelly et al. (2003)in this issue).
Rosas et al. (2000a)also reported success in devel-oping BGYMV resistant small red bean cultivars for Central America. Tio Canela 75, released in Hon-duras, Nicaragua and El Salvador, combines BGYMV resistance with a commercially acceptable small red seed type, heat tolerance and moderate levels of CBB resistance (Rosas et al., 1997). In 2000, more than
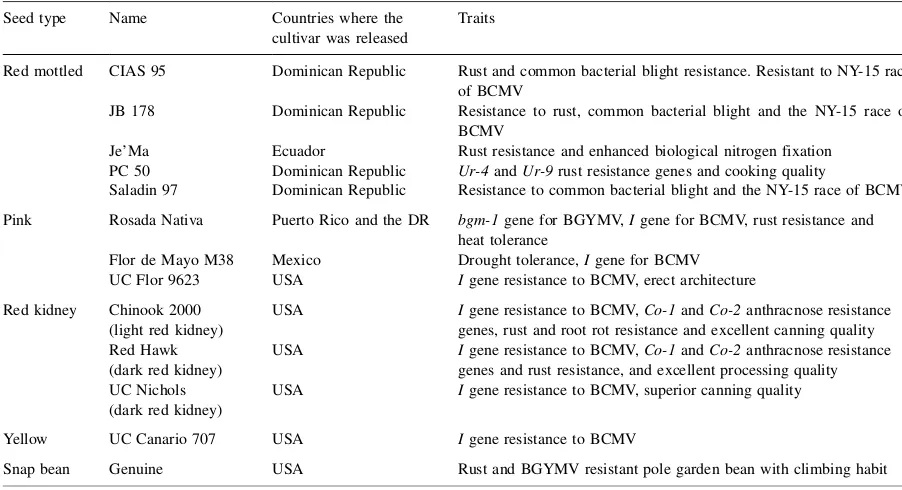
Table 1 (Continued)
Seed type Name Countries where the cultivar was released
Traits
Red mottled CIAS 95 Dominican Republic Rust and common bacterial blight resistance. Resistant to NY-15 race of BCMV
JB 178 Dominican Republic Resistance to rust, common bacterial blight and the NY-15 race of BCMV
Je’Ma Ecuador Rust resistance and enhanced biological nitrogen fixation PC 50 Dominican Republic Ur-4andUr-9rust resistance genes and cooking quality
Saladin 97 Dominican Republic Resistance to common bacterial blight and the NY-15 race of BCMV Pink Rosada Nativa Puerto Rico and the DR bgm-1gene for BGYMV,Igene for BCMV, rust resistance and
heat tolerance
Flor de Mayo M38 Mexico Drought tolerance,Igene for BCMV UC Flor 9623 USA Igene resistance to BCMV, erect architecture Red kidney Chinook 2000
(light red kidney)
USA Igene resistance to BCMV,Co-1andCo-2anthracnose resistance genes, rust and root rot resistance and excellent canning quality Red Hawk
(dark red kidney)
USA Igene resistance to BCMV,Co-1andCo-2anthracnose resistance genes and rust resistance, and excellent processing quality UC Nichols
(dark red kidney)
USA Igene resistance to BCMV, superior canning quality
Yellow UC Canario 707 USA Igene resistance to BCMV
Snap bean Genuine USA Rust and BGYMV resistant pole garden bean with climbing habit
Table 2
Bean germplasm lines developed with participation by B/C CRSP scientists Seed type Name Country where germplasm
was developed
Traits
Black MUS-N-8 Dominican Republic Web blight resistance
White BelMiDak RR 1-12 (navy) USA Pyramided rust resistance genes BelMiNeb RR 1-7 (GN) USA Pyramided rust resistance genes
Red kidney PR9443-4 Puerto Rico bgm-2gene for BGYMV and CBB resistance Pinto BelDakMi RR 1-18 USA Pyramided rust resistance genes
Red mottled PR9745-232 Puerto Rico bgm-1gene for BGYMV resistance PR9909-5 Puerto Rico bgm-1gene for BGYMV resistance
20% of the bean producers in Honduras planted Tio Canela 75 (Rosas, pers. commun.). Farmers in Haiti have expressed an increased interest in planting small red beans based on the yield and adaptation of Tio Canela 75 in on-farm trials (Prophete, pers. commun.). ‘Bribri’, a small red bean cultivar that combines BGYMV, web blight resistance and tolerance to heat, was released in Costa Rica in 2000 (Rosas et al., 2003). The BGYMV, BCMV and rust resistant white bean cultivar Morales has become the most popular bean cultivar for green-shelled bean production in Puerto Rico (Beaver and Miklas, 1999). Rosada Nativa, a pink bean cultivar that combines BGYMV, BCMV and rust resistance and heat tolerance was released in Puerto Rico (Beaver et al., 1999b). The bgm-1 gene for BGYMV resistance has also been incorporated into tropically adapted, light red kidney, red mottled and pinto bean lines (Beaver et al., 1999a). Collaboration among researchers at the University of Puerto Rico, University of Florida and USDA-ARS led to develop-ment of BelDade RGMR 4, 5 and 6, which are McCa-slan type pole (type IV) beans that combine superior culinary traits with BGYMV and rust resistance ( Sta-vely et al., 2001). BelDade RGMR 5 is being marketed by a private company in southern Florida as the cultivar Genuine (Shamrock Seed Co., Salinas, CA).
Research supported by the B/C CRSP to develop transgenic beans with BGYMV resistance was unsuc-cessful. Although a few transgenic beans were devel-oped at great effort and expense, susceptibility of the transgenic lines to BGYMV was attributed to a failure of the plants to express the viral coat protein (Azzam et al., 1996). This experience belies the widely held assumption (Victor and Runge, 2002) that biotechnol-ogy provides a more rapid and efficient approach to achieve plant breeding objectives.
2.2. Bean common mosaic virus and bean common mosaic necrotic virus
Bean common mosaic virus (BCMV) and bean common mosaic necrotic virus (BCMNV) are aphid-transmitted viruses that can cause significant yield loss (Ga´lvez and Morales, 1989). The viruses can be seed-borne, allowing long distance spread and compromis-ing bean seed increase and dissemination programs.
B/C CRSP supported research in East Africa led to the discovery of BCMNV as a separate virus species
from BCMV (Mink et al., 1994). BCMNV causes whole plant systemic top necrosis (black root reaction) in bean lines possessing the I gene, independent of temperature, whereas BCMV isolates either do not cause systemic necrosis, or only do so when tempera-tures exceed 308C (Drijfhout, 1978). Greater genetic diversity in BCMNV is evident in East Africa than in other bean production regions. BCMNV isolates have occasionally been transported to other continents through the green bean seed trade based in Arusha, Tanzania.
Most bean cultivars released in Central America and the Caribbean carry the dominant I gene for resistance to BCMV and this source of resistance has proven to be effective in controlling the virus in the region. Unfortunately, the recent arrival of BCMNV in the Dominican Republic and Haiti will require that future Caribbean bean breeding lines possess additional genes to protect against the necrotic reaction to BCMNV. Due to similar problems in the USA, the black bean cultivar ‘Raven’ was developed to resist both viruses by combiningIandbc-3 resis-tance genes (Kelly et al., 1994). Marker-assisted selection was used to incorporate thebgm-1gene into black and white-seeded breeding lines with the reces-sivebc-3gene confirmed through inoculation with the NL3 strain of BCMNV.
In Central America, bean lines with theIgene are susceptible to bean severe mosaic, a beetle-trans-mitted comovirus (Morales and Singh, 1997). Bean researchers from the University of Puerto Rico and Michigan State University collaborated to eliminate the I gene from local germplasm and, concurrently incorporate thebgm-1 andbc-3genes into small red beans. Sequence characterized amplified regions (SCAR) markers for both thebgm-1andIgenes were multiplexed to simultaneously select for the presence of bgm-1 and the absence of the dominant I allele (Kelly et al., 2003).Breeding lines without theIgene were inoculated with the NL3 strain of BCMNV to detect the presence of bc-3gene. This collaboration resulted in the release of the small red bean germplasm line PR9357-107 with resistance to BGYMV and BCMNV (Beaver et al., 1998).
specific recessive resistance genes alone or in combi-nation with theI gene. The latter strategy is termed ‘‘protectedIgene resistance’’ (Kelly et al., 1995b). By combining one of the recessive resistance genes (pre-ferablybc-22or bc-3) with the I gene, the systemic necrosis reaction is prevented (Kelly, 1997). The I gene prevents more virulent virus strains from over-coming the recessive gene resistance thereby creating a more durable form of resistance than conferred by either gene alone. Rojo, released from Sokoine Uni-versity of Agriculture in Tanzania in 1997 is an example of a cultivar developed using this approach. Studies byMartin and Adams (1987)demonstrated the importance of landraces with distinct seed types and plant characteristics for Malawian bean production and consumption. Researchers at the University of Califor-nia at Davis and Malawi’s Bunda College of Agriculture used successive backcrosses to incorporatebc-3 resis-tance to BCMV and BCMNV into six contrasting land-race types. National disease surveys had also confirmed significant losses due to the foliar pathogen angular leaf spot (ALS), caused by the fungus Phaeoisariopsis griseola. Initial crosses to transferbc-3were therefore made as three-way crosses, using CIAT lines A 286 and BAT 477 as sources of ALS resistance. These parents were selected based on results from multi-location tests of many potential BCMNVand ALS resistance sources. Deployment of durable ALS resistance into certain Malawian landraces was complicated by the exis-tence of specific gene pools withinP. vulgaris(Gepts, 1998).Guzman et al. (1995)demonstrated a coevolu-tionary relationship between host resistance genes and pathogenicity genes identified in Phaeoisariop-sis. A low frequency of offspring that combined resistance to Andean and Mesoamerican races of ALS slowed the development of Malawian landrace types with ALS and BCMNV resistance. Progeny from the third backcross to landrace parental types have been selected for BCMVN and ALS resistances. Several lines, currently in multiplication and multi-site testing, have grain and plant types nearly identical to their landrace progenitors.
The Malawi/UC Davis breeding and BCMV testing activities have also produced three new varieties for the USA that carry both the I-gene resistance to BCMV, and significant amounts of subtropical genetic background. The Dark Red Kidney variety UC Nichols, released in 2000 (Temple et al., 1999), has
a very strong root system, and has demonstrated high yields and excellent canning quality. Canario 707, a sulfur yellow bean with high yield, large seed size, and BCMV resistance, represents a new seed type for producers in the USA. Similarly, UC Flor 9623, a representative of the ‘‘Flor de Mayo’’ class popular in some areas of Mexico, combines BCMV resistance with very erect architecture and high yield (Temple et al., 2002).
A ‘‘protectedIgene’’ approach has been increasingly used in the USA as a resistance strategy with a number of germplasm and cultivar releases such as Kodiak pinto that combine theIgene with either thebc-12,bc-22, or bc-3 genes (Kelly et al., 1999a; Miklas et al., 1997;
Myers et al., 2001;Pastor-Corrales et al., 2001). Several rust and BCMV resistant great northern germplasm (GN) and pinto lines have been released. GN BelNeb 1 and 2 combines resistance to CBB, halo blight, bacterial brown spot, rust andI gene BCMV (Stavely et al., 1992). TheUr-5,Ur-6andUr-7genes for rust resistance from donor parents B-190, Pinto Olathe and GN 1140, respectively, were backcrossed into Harris, a GN cultivar developed at the University of Nebraska. Subsequently, GN BelMiNeb 4 and 5 (Ur-4,Ur-6, Ur-11) and 7 (Ur-3, Ur-4,Ur-11) and pinto BelDakMi 18 with resistance (Ur-3,Ur-4,Ur-6, Ur-11). Germplasm lines from this backcross program were released which have resistance to all of the rust and BCMV races present in the US (Pastor-Corrales et al., 2001).
2.3. Common bacterial blight
Chase (Type II) is the first pinto bean to combine resistance to CBB, bacterial brown spot, halo blight and some avoidance of white mold due to a porous canopy (Coyne et al., 1994). The cultivar GN Starlight with excellent seed size and bright white seed coat along with moderate resistance to CBB was released in 1991 (Coyne et al., 1991). The large, bright, white-seeded Weihing (Type IIb) is the first GN cultivar to combine resistance to CBB, halo blight, bacterial brown spot, BCMV (I gene), rust (Ur-3, Ur-6) and architectural avoidance to white mold (Coyne et al., 2000). Efforts by researchers from the University of Nebraska to pyramid resistance to CBB using molecular markers are discussed byKelly et al. (2003)andMiklas et al. (2000a). High levels of CBB resistance of VAX lines developed at CIAT are the result of an effort to pyramid different sources of resistance (Singh et al., 2001).
2.4. Bean rust
Bean rust caused by Uromyces appendiculatus (Pers.) Unger var. appendiculatus is an important disease in most bean production regions of the world (Stavely and Pastor-Corrales, 1989). Because Hon-duras has among the most virulent pathotypes of rust in Central America and the Caribbean, it has proven to be an ideal site to screen beans for resistance (Araya, 1996). An effective field screening technique for bean rust has been developed at the Escuela Agrı´cola Panamericana where bean lines growing in benches are inoculated with a mixture of virulent pathotypes of bean rust followed by a frequent moistening of the leaf canopy using micro-irrigation. Twelve navy (BelMiDak), 18 pinto (BelDakMi) and 7 great north-ern (BelMiNeb) bean germplasm lines that pyramid specific genes for resistance to bean rust were devel-oped (Pastor-Corrales et al., 2001). The most recent germplasm releases such as BelDakMi RMR 18 com-bineIandbc-3gene resistance to BCMVand BCMNV with the Ur-3, Ur-4,Ur-6 andUr-11 genes for rust resistance. Pinto beans have become popular in the Caribbean following the distribution of this seed type by food assistance programs. Tropically adapted pinto bean lines that combine the Ur-6 and Ur-11 rust resistance genes and thebgm-1gene for BGYMV resistance have been developed in Puerto Rico. Because pinto beans have a higher yield potential than Caribbean red mottled beans, these lines may benefit
subsistence farmers in the Dominican Republic and Haiti. PC-50, a selection from the Dominican Repub-lic landrace Pompadour Checa (Saladin et al., 2000), possesses specific resistance to rust determined by the Ur-9 gene, adult plant resistance conditioned by the Ur-12 gene, which is hypostatic to Ur-9, and also a dominant genePu-afor dense abaxial leaf pubescence (Bokosi, 1996). These three genes are inherited inde-pendently (Bokosi, 1996) and were mapped byJung et al. (1998). Adult plant resistance was also detected in other landraces in the Dominican Republic (Mmbaga et al., 1992a) and in Andean germplasm from Malawi (Bokosi, 1996). The genetic merit of adult plant resistance and pubescence to reduce yield losses in rust epidemics when specific resistance breaks down needs to be determined. Dense abaxial leaf pubescence in Caribbean germplasm is thought to contribute to reduced rust infection through trapping of spores on long dense hairs (Mmbaga et al., 1992b). The rust resistant red mottled bean cultivars JB-178 and CIAS-95 were released in the Dominican Repub-lic (Arnaud-Santana et al., 2000a,c).
Pinto Chase was developed in response to a request from the Nebraska Dry Bean Growers Association because of epidemics of rust in southwestern Nebraska and the lack of pinto cultivars with resistance to rust pathotypes endemic in the region (Coyne et al., 1994). The annual value of the release of Chase, based on higher (7–10%) yields and reduced use of fungicides was estimated to be US$ 5 million (Perrin et al., 2000). Bean rust is a constraint in Africa as well as in the New World. Germplasm and cultivars with resistance to rust races in East Africa have been identified and released. Examples of resistant cultivars include SUA 90 (originally a germplasm accession obtained from CIAT) and Rojo in Tanzania, and Kalima (also ori-ginally obtained from CIAT) released by Bunda Col-lege in Malawi. Efforts to systematically identify the rust races of importance in East Africa are in early stages, although it appears that the AndeanUr-4gene would not be effective.Ur-3does provide resistance to most African races, and when combined with Ur-5, provides resistance against all known African races.
2.5. Anthracnose
lindemuthianum (Sacc. and Magn.) Scrib. Given the substantial variability in the pathogen (Balardin et al., 1997), bean breeders have to combine multiple resis-tance genes to generate lines with durable resisresis-tance. Two black bean cultivars, Jaguar and Phantom, a navy cultivar, Newport, a light red kidney cultivar, Chinook 2000 and a dark red kidney cultivar, Red Hawk, carry both theCo-1andCo-2resistance genes to anthracnose (Kelly et al., 1995, 1998a, 1998b, 2000, 2001). The INIFAP bean breeding program in highland Mexico has also been active in the development of bean cultivars with anthracnose resistance. The black bean cultivars Negro Sahuatoba and Negro Altiplano and the pinto bean cultivars Bayacora, Mestizo and Pinto Villa are resistant to most anthracnose pathotypes in Mexico (Acosta-Gallegos et al., 1995a,b, 2001a,b,c,d). Balar-din and Kelly (1998)found the red mottled cultivar PC 50 selected in the Dominican Republic to be among the most anthracnose resistant bean lines of Andean origin.
2.6. Web blight
Web blight, caused by Thanatephorus cucumeris (Frank) Donk (anamorph:Rhizoctonia solaniKu¨hn) is one of the most important bean diseases in the warm and humid tropics, and when climatic conditions favor disease development, can significantly reduce both seed yield and quality (Godoy-Lutz et al., 1998). Only moderate levels of resistance to web blight are avail-able in common bean. The black bean cultivar Arroyo Loro Negro and the small red cultivar Bribri are reported to have some resistance to web blight (Arnaud-Santana et al., 2000b; Rosas et al., 2003). Because erect plant architecture contributes to disease avoidance, it is difficult to separate physiological resistance and disease avoidance in field trials. Further, field evaluations for web blight are limited to seasons when climatic conditions favor disease development. In Puerto Rico, Takegami and Beaver (2001) inoculated field trials with a suspension of mycelia prepared from a virulent isolate of the web blight pathogen, then applied short periods of over-head irrigation early in the morning for 2 weeks after inoculation. These practices helped to ensure a uni-form web blight infection in the field. Bean lines have also been screened for physiological resistance in a greenhouse by applying droplets of a suspension of mycelia on leaflets followed by frequent misting
(Polanco et al., 1996) and the inoculation of detached leaves (Bautista-Pe´rez and Echa´vez-Badel, 2000).
Quantitative inheritance and low to moderate her-itabilities have been observed for web blight resistance (Arnaud-Santana et al., 1998; Montoya et al., 1997; Takegami and Beaver, 2000). Screening for web blight resistance would be more effective evaluating advanced lines in replicated trials. Jung et al. (1996) detected QTLs for resistance to web blight, rust, and common blight in a population of RILs derived from the cross BAC 6/HT 7719.
Isolates ofR. solani collected from different bean production regions of Central America and the Car-ibbean vary in morphological characteristics, anasto-mosis group and virulence (Godoy-Lutz et al., 1996, 2000). Only two lines from the CIAT core germplasm collection, Talamanca and BAT 93, had useful levels of resistance to web blight in both Panama and Puerto Rico (Beaver, pers. commun.). Bean breeders at the University of Puerto Rico and the EAP have initiated a recurrent selection program to accumulate alleles for resistance to web blight. The base population con-sisted of sources with moderate levels of web blight resistance from diverse origins. The lines are being evaluated for web blight reaction in both Honduras and Puerto Rico.
Interspecific crosses may also be used to transfer web blight resistance from scarlet runner bean (P. coccineus) and tepary bean (P. acutifolius). Two accessions ofP. coccineusfrom the CIAT core germplasm collection, G35066 and G35006, have been identified in Puerto Rico as potential sources of resistance to web blight (Takegami and Beaver, 2000). Climatic conditions in the natural habitat ofP. coccineuscan favor the devel-opment of web blight. Wild bean plants with web blight symptoms have been observed at an altitude of 1400 m near Guinope, Honduras. The USDA core germplasm collection was also screened in the field in Puerto Rico for web blight resistance. Only three lines (<1%) of the Phaseolus core collection were selected for further evaluation. One of the lines selected was a tepary bean.
2.7. Bruchid (bean weevil) resistance
with insecticides, tumbling infested bags (Quentin et al., 1991) and storing in containers to physically exclude the insects. Physical barriers are less likely to be effective againstA. obtectus because this species will infest seeds in the field before harvest. Genetic resistance toZabrotes, first discovered in wild bean accessions from Mexico, has been transferred into cultivated germplasm by CIAT scientists (Cardona et al., 1992; Kornegay et al., 1993). Resistance is conferred by lectin proteins known as phytohemag-glutinin, conditioned by alleles of the ‘arcelin’ seed protein present only in wildP. vulgaris(Osborne et al., 1988) and by the a-amylase inhibitor gene family proteins. Arcelin 1 (arc1) is particularly effective against Z. subfasciatus, but not against A. obtectus. CIAT transferredarc1into the RAZ germplasm lines. This resistance gene has been backcrossed at Sokoine University of Agriculture into elite local cultivars and four lines with resistance toZ. subfasciatushave been developed (Nchimbi-Nsolla, pers. commun.). One or two of these lines should be released after completion of on-farm testing in 2003. Current studies emphasize arc4, which confers limited resistance to both bruchid species, and the transfer of this gene into advanced breeding lines, as well as interspecific transfer of bruchid resistance from tepary bean into common bean. When used in conjunction with other storage techniques, genetic resistance should be effective in preventing seed loss due to bruchids.
3. Abiotic stress
3.1. Drought
Drought is a common cause of yield loss in beans, with two types of drought distinguished. Farmers in Central America plant beans toward the end of the rainy season in relay intercrops with maize. These bean plantings often suffer yield losses due to terminal drought. In the highlands of Mexico beans are sub-jected to extended periods of intermittent drought. The only traits that have proven to be valuable in both terminal and intermittent drought are earliness and partitioning toward reproductive structures, resulting in greater harvest index (Acosta-Gallegos and Adams, 1991; Foster et al., 1994). A disadvantage of selection based on harvest index is that the trait can only be
measured at harvest maturity. Crop cover during the middle of pod fill, has been found to have intermediate to high correlations with bean seed yield under both stress and non-stress conditions (Acosta et al., 1999). The most reliable crop cover readings are obtained when bean lines are grouped by similar phenology and growth habit. Genetic improvement for these dissim-ilar types of drought will, in large part, require the selection of different traits.
The collaborative structure of the B/C CRSP has permitted the regional testing of germplasm for the different types of drought stress present in individual countries.Beaver and Rosas (1998)found that selec-tion for earlier flowering, a greater rate of partiselec-tioning and a shorter reproductive period permitted the selec-tion of small red bean breeding lines having 1 week earlier maturity without sacrificing yield potential. These combinations of phenological and physiologi-cal traits contribute to the genotypic avoidance of terminal drought. Researchers in Mexico found that selection for high seed yield potential under irrigation may permit indirect selection for greater tolerance to terminal drought (Acosta, pers. commun.).
Resistance to drought is confounded by root health and vigor and with resistance to soil-borne root rot pathogens such asFusariumspp. andR. solani( Navar-rete-Maya and Acosta-Gallegos, 1999). The lack of adequate levels of root rot resistance contributes to the increased susceptibility of bean cultivars to intermit-tent drought in highland production regions. Likewise, tolerance to terminal drought is associated with resis-tance to ashy stem blight caused by Macrophomina phaseolina(Tassi) Goid. Bean cultivars for the low-lands of Mexico require resistance to ashy stem blight in combination with tolerance to terminal drought and low P soils (Acosta, pers. commun.). It has yet to be determined how selection for shallow vigorous adven-titious root growth to enhance tolerance to low P soils (Liao et al., 2001) might affect drought tolerance. Tio Canela 75, has been observed to have greater drought tolerance than other recently released small red culti-vars (Rosas, pers. commun.). Ashy stem blight resis-tance of a bean line is often limited to a specific geographic region due to variability in virulence pat-terns of the pathogen (Mayek-Perez et al., 2001). Nevertheless, a few cultivars such as the pink bean Rosada Nativa and BAT 477 were found to be resistant in both Puerto Rico and Mexico.Echavez-Badel et al. (2000)developed a greenhouse technique for screen-ing bean lines for resistance to ashy stem blight. This technique can also be used to screen lines for reaction toMacrophominaisolates from different geographical regions.Miklas et al. (1998a) identified tepary bean lines with resistance to ashy stem blight and QTL that could be used for indirect selection for resistance (Miklas et al., 1998b).
3.2. Heat
In lowland environments, terminal drought stress can be aggravated by high temperatures. In Central Amer-ica and the Caribbean, breeders have focused on heat as a constraint to expanding bean production in the low-land tropics. They have made significant progress in the development of bean cultivars with improved levels of heat tolerance (Rosas et al., 2000b). Heat tolerance, combined with BGYM, CBB and web blight resis-tance, would permit increased bean production during non-traditional growing seasons when rainfall distri-bution patterns are more favorable. The release of Tio Canela 75 has permitted the expansion of bean
production at lower altitudes in Central America (Rosas et al., 1997, 2000a). The heat tolerant pink bean cultivar Rosada Nativa was released in Puerto Rico and the Dominican Republic (Beaver et al., 1999b). The small red cultivar Bribri released in Costa Rica combines heat tolerance with web blight resistance. Indeterminate Jamaica Red, a pink striped bean of Andean origin, was identified in Puerto Rico as a promising source of heat tolerance (Baiges et al., 1996). Narrow sense heritabilities of heat tolerance in populations derived from Indeterminate Jamaica Red were intermediate in magnitude (h2ranged from 0.4 to 0.6 in Puerto Rico during 1999 and 2000) meaning that breeders will probably need to screen advanced generation lines in replicated trials to identify heat tolerant lines ( Roman-Avile´s and Beaver, 2001). Indeterminate Jamaica Red is currently being used to improve the heat tolerance of kidney beans in the USA (Miklas et al., 2000b).
4. Yield potential and stability
base population included large-seeded determinate parents of Andean origin and small-seeded Mesoamer-ican bean lines with greater yield potential and erect plant architecture. The progeny were evaluated for yield in the F5generation using replicated hill-plots. After two cycles of recurrent selection, several inde-terminate lines with commercially acceptable seed for the Caribbean were selected that yielded 30% more than determinate check cultivars. However, the lines selected for yield were 10 days later in maturity (Beaver and Kelly, 1994).
Breeding for physiological efficiency was proposed as another approach to improve bean yield (Wallace et al., 1993). Limited adaptation is the major constraint to breeding for yield, since a cultivar must fit the environment in which it will be grown. Days to maturity is the most important physiological trait affecting that outcome. All bean plants have a specific growth rate. Through genetic manipulation, this growth rate can be increased. Increasing growth rate does not necessarily translate into higher seed yield, however, unless greater amounts of the photosynthate from biomass are partitioned to the seed. Indirect selection for the three major physiological traits affect-ing yield, namely plant biomass, harvest index (HI), and days to maturity, should result in improved yield (Wallace et al., 1993). Simultaneous selection is required because genetically established interrelation-ships occur among these three traits. An increase in days to maturity, which is undesirable in some envir-onments, results in an increase in biomass. An increase in HI results in a decrease in days to maturity and a decrease in biomass. All three physiological traits and the correlations among them can be quantified by a yield system analysis (YSA) of yield trial data (Wallace et al., 1993; Wallace and Yan, 1998). Selec-tion for biomass within the constraints of maturity is equivalent to actual selection for a higher rate of biomass accumulation. Selection for yield per day should help compensate for the tendency for higher biomass to result in longer duration of growth. Selec-tion solely for greater biomass will give later maturity, lower HI and higher yield, whereas selection solely for HI results in early maturity with an accompanying reduction in yield. Wallace et al. (1993) noted that selection for yield can result in increased harvest index with little or no increase in biomass. The use of YSA led to the release of RedKanner light red kidney bean
in the USA which has more yield potential than check cultivars because of a greater number of seed per pod (Wallace and Shardlow, 2001).
Given the diversity of approaches to improving yield of common bean,Kelly et al. (1998c)proposed a more integrated approach that employed a three-tiered pyr-amid where breeding activities differed at each level of the pyramid. The purpose of the breeding pyramid is to better utilize the genetic diversity present within P. vulgaris, yet to integrate and optimize ways to improve yield for bean breeders who must work within the constraints of growth habit, maturity and seed class restrictions. The pyramid offers a structured metho-dology to exploit genetic variability both within the cultivated and among the wild members ofP. vulgaris. Since the domestication of common bean may have reduced genetic variability, wild bean accessions may be a potential source of novel genes for adaptation and yield (Koinange et al., 1996). A BC4F4:7line derived from a cross between an elite black bean cultivar Negro Tacana (Lopez-Salinas et al., 1997) and a wild bean accession G24423 from Colombia produced the highest recorded bean yield in a Michigan State Uni-versity yield trial (5790 kg ha 1). The original cross and backcrosses were made at CIAT and the inbred progeny were evaluated through the B/C CRSP net-work in Mexico and the USA. The highest yielding BC4F4:7line outperformed the recurrent parent, Tacana by 27% in this study (Kelly, pers. commun.).
distribution patterns. Breeding beans for specific envir-onments would permit the exploitation of genotype environment interaction and may reduce the number of traits that need to be selected for each environment (Beaver, 1999). A multiplicative interaction (AMMI) model was used by Macchiavelli and Beaver (1999)
to identify red mottled lines with greater yield potential and stability for lowland tropical regions.
5. Challenges
5.1. Sustainable seed production and distribution systems
The absence of formal seed production and distri-bution systems in many countries in Latin America and Africa has limited the impact of bean cultivar development programs supported by the B/C CRSP. Traditionally, bean seed production in Central Amer-ica has been managed by the government. During the last 10 years, several initiatives for artisan seed pro-duction have been promoted by state agencies and non-governmental organizations (NGOs) to improve the quality of the bean seed utilized by small farmers (Rosas and Castro, 1999). These efforts, however, are still few in number and rather isolated. The lack of an effective seed multiplication system also makes farm-ers more vulnerable to seed shortages when natural disasters occur. In 1998, hurricanes Mitch and George depleted bean seed supplies in Central America and the Caribbean. A drought during 2000 also reduced seed supplies in Central America. After these disas-ters, seed of B/C CRSP bean cultivars were multiplied and used to relieve the shortage of beans in Haiti and Honduras.
The B/C CRSP has used different strategies to promote the adoption of bean cultivars. Bean grower associations formed to multiply and distribute seed, assured the widespread adoption of the cultivar, Pinto Villa in highland Mexico. The Escuela Agrı´cola Pana-mericana (EAP) in Zamorano, Honduras has been very successful distributing seed of elite bean breeding lines and recently released bean cultivars through collaboration with NGOs. The Zamorano bean pro-gram provides basic seed stocks and technical bulle-tins; the NGOs provide assistance in the testing of promising breeding lines on small farms. Participatory
plant breeding (PPB) approaches that involve an active participation of farmers in the development of improved varieties, have been used since 1999 by the Zamorano breeders in two bean producing regions of Honduras (Rosas, 2001). Although these efforts are still experi-mental, simplified PPB methodologies can help to increase the adoption of improved varieties among small farmers, especially in communities having lim-ited access to existing extension systems or improved cultivars. Awell-organized PPB program requires train-ing and close collaboration among breeders, extension agencies, NGO representatives and farmers.
Land and personnel on research stations in devel-oping countries is often underutilized. The EAP in Honduras, Bunda College in Malawi and the Univer-sity of Puerto Rico produce basic seed stocks of bean cultivars developed by the B/C CRSP. Profit from the sale of seed can be used to support bean breeding activities. Basic seed stocks can also be produced on research stations during the dry season which can help to reduce the incidence of certain seed-borne diseases such as CBB (Beaver and Molina, 1997).
5.2. Training in plant breeding
in plant breeding courses taught in the USA and the developing world.
In Central America, the Caribbean and Eastern Africa, training should be planned and conducted on a regional basis. The B/C CRSP, CIAT and regional networks should be encouraged to continue to coor-dinate informal training activities such as workshops. In addition, bean breeders from both the USA and developing countries should have opportunities to meet on a frequent basis to plan and discuss colla-borative research and to participate in scientific meet-ings such as the Bean Improvement Cooperative (BIC) in the USA and the Programa Centroamericano Coop-erativo de Mejoramiento de Cultivos y Animales (PCCMCA) in Central America.
References
Acosta, J.A., Acosta, E., Padilla, S., Goytia, M.A., Rosales, R., Lopez, E., 1999. Mejoramiento de la resistencia a la sequia del frijol comu´n en Me´xico. Agronomia Mesoamericana 10 (1), 83–90.
Acosta-Gallegos, J.A., Adams, M.W., 1991. Plant traits and yield stability of dry bean (Phaseolus vulgarisL.) cultivars under drought stress. J. Agric. Sci. (Cambridge) 117, 213–219. Acosta-Gallegos, J.A., Kohashi-Shibata, J., 1989. Effect of water
stress on growth and yield of indeterminate dry-bean (Phaseolus vulgaris) cultivars. Field Crops Res. 20, 81–93. Acosta-Gallegos, J.A., Ochoa-Marquez, R., Arrieta-Montiel, M.P.,
Ibarra-Perez, F., Pajarito Ravelero, A., Sanchez Valdez, I., 1995a. Registration of Pinto Villa common bean. Crop Sci. 35, 1211. Acosta-Gallegos, J.A., Castellanos, J.Z., Nunez-Gonzalez, S.,
Ochoa-Marquez, R., Rosales-Serna, R., Singh, S.P., 1995b. Registration of Flor de Mayo M38 common bean. Crop Sci. 35, 941–942.
Acosta-Gallegos, J.A., Ibarra-Pe´rez, F.J., Rosales-Serna, R., Castillo-Rosales, A., Kelly, J.D., 2001a. Registration of Negro Sahuatoba opaque black bean. Crop Sci. 41, 1646–1647. Acosta-Gallegos, J.A., Ibarra-Perez, F.J., Rosales-Serna, R.,
Fernan-dez-Hernandez, P., Castillo-Rosales, A., Kelly, J.D., 2001b. Registration of Mestizo pinto bean. Crop Sci. 41, 1645–1646. Acosta-Gallegos, J.A., Ibarra-Perez, F.J., Rosales-Serna, R.,
Castillo-Rosales, A., Ca´zares-Enrı´quez, B., Fernandez-Hernan-dez, P., Kelly, J.D., 2001c. Registration of Negro Altiplano common bean. Crop Sci. 41, 1650.
Acosta-Gallegos, J.A., Ibarra-Perez, F.J., Rosales-Serna, R., Ca´zares-Enrı´quez, B., Fernandez-Hernandez, P., Castillo-Ro-sales, A., Kelly, J.D., 2001d. Registration of Bayacora pinto bean. Crop Sci. 41, 1645–1646.
Adames-Mora, C., Beaver, J.S., Diaz, O., 1996. Una metologia de evaluacion del virus del mosaico dorado de habichuela en el invernadero. J. Agric. Univ. Puerto Rico 80, 65–72.
Adams, M.W., 1982. Plant architecture and yield breeding in
Phaseolus vulgarisL. Iowa State J. Res. 56, 225–254. Araya, C.M., 1996. Pathogenic and molecular variability and telia
production ofU. appendiculatusisolates from the Andean and Middle American centers of domestication of bean. Ph.D. dissertation. University of Nebraska, Lincoln, NE, 159 pp. Arnaud-Santana, E., Coyne, D.P., Steadman, J.R., 1998. Inheritance
and heritabilities of the reaction to web blight disease. Ann Rep. Bean Improv. Coop. 41, 29–30.
Arnaud-Santana, E., Nin, J.C., Saladin, F., Godoy-Lutz, G., Beaver, J.S., Coyne, D.P., Steadman, J.R., 2000a. Registration of JB-178 red mottled bean. Crop Sci. 40, 857–858.
Arnaud-Santana, E., Nin, J.C., Saladin, F., Godoy-Lutz, G., Coyne, D.P., Beaver, J.S., Steadman, J.R., 2000b. Registration of Arroyo Loro Negro black bean. Crop Sci. 40, 856–857. Arnaud-Santana, E., Nin, J.C., Saladin, F., Godoy-Lutz, G., Coyne,
D.P., Beaver, J.S., Steadman, J.R., 2000c. Registration of CIAS-95 red mottled bean. Crop Sci. 40, 857.
Azzam, O., Dı´az, O., Beaver, J.S., Gilbertson, G.L., Russell, D.R., Maxwell, D.P., 1996. Transgenic beans with bean golden mosaic gemnivirus coat protein gene are susceptible to virus infection. Ann. Rep. Bean Improv. Coop. 39, 276–277. Baiges, S., Beaver, J.S., Miklas, P.N., Rosas, J.C., 1996. Evaluation
and selection of Andean beans for heat tolerance. Ann. Rep. Bean Improv. Coop. 39, 88–89.
Balardin, R.S., Kelly, J.D., 1998. Interaction among races of
Colletotrichum lindemuthianum and diversity in Phaseolus vulgaris. J. Am. Soc. Hort. Sci. 123, 1038–1047.
Balardin, R.S., Jarosz, A.M., Kelly, J.D., 1997. Virulence and molecular diversity in Colletotrichum lindemuthianum from South, Central and North America. Phytopathology 87, 1184– 1191.
Bautista-Pe´rez, M., Echa´vez-Badel, R., 2000. Methodology for screening common bean for resistance to web blight. J. Agric. Univ. Puerto Rico 84, 91–94.
Beaver, J.S., 1999. Improvement of large-seeded race Nueva Granada cultivars. In: Singh, S.P. (Ed.), Common Bean Improvement in the Twenty-First Century. Kluwer Academic Publishers, Boston, MA, pp. 275–288.
Beaver, J.S., Kelly, J.D., 1994. Comparison of two selection methods for the improvement of dry bean populations derived from crosses between gene pools. Crop Sci. 34, 34–37. Beaver, J.S., Miklas, P.N., 1999. Registration of Morales small
white bean. Crop Sci. 39, 1257.
Beaver, J.S., Molina, A., 1997. Mejoramiento de frijol para el Caribe. In: Singh, S.P., Voysest, O. (Eds.), Taller de Mejoramiento de Frijol para el Siglo. XXI. Bases para una Estrategia para America Latina. CIAT, Cali, Colombia, pp. 353–376.
Beaver, J.S., Rosas, J.C., 1998. Heritability of length of reproductive period and rate of seed mass accumulation in common beans. J. Am. Soc. Hort. Sci. 123 (3), 407–411. Beaver, J.S., Steadman, J.R., Coyne, D.P., 1992. Field reaction of
landrace components of red mottled beans to common bacterial blight. HortScience 27, 50–51.
Beaver, J.S., Miklas, P.N., Kelly, J.D., Steadman, J.R., Rosas, J.C., 1998. Registration of PR9357-107 small red germplasm resistant to BCMV, BCMNV and rust. Crop Sci. 38, 1406–1407. Beaver, J.S., Zapata, M., Miklas, P.N., 1999a. Registration of bean
PR9443-4, germplasm resistant to bean golden mosaic, common bacterial blight and rust. Crop Sci. 39, 1257. Beaver, J.S., Miklas, P.N., Echavez-Badel, R., 1999b. Registration
of Rosada Nativa pink bean. Crop Sci. 39, 1257.
Beebe, S., 1994. Breeding for resistance to bean golden mosaic virus: history and perspectives. In: Morales, F.J. (Ed.), Bean Golden Mosaic 1994: Research Advances. CIAT, Cali, Colombia, pp. 148–150.
Blair, M.W., Bassett, M.J., Abouzid, A.M., Hiebert, E., Polston, J.E., McMillan, R.T., Graves, W., Lamberts, M., 1995. Occurrence of bean golden mosaic virus in Florida. Plant Dis. 79, 529–533. Bokosi, J.M., 1996. Sources of rust resistant germplasm and
inheritance of rust reaction and hybrid plant abnormalities in common bean. Ph.D. dissertation. University of Nebraska, Lincoln, NE, p. 170.
Brothers, M.E., Kelly, J.D., 1993. Interrelationship of plant architecture and yield components in the pinto bean ideotype. Crop Sci. 33, 1234–1238.
Cardona, C., Dick, K., Posso, C.E., Ampofo, K., Nadhy, S.M., 1992. Resistance of a common bean (Phaseolus vulgaris L.) cultivar to post-harvest infestation by Zabrotes subfasciatus
(Boheman) (Coleoptera: Bruchidae). 2. Storage test. Trop. Pest Manage. 38, 173–175.
Coyne, D.P., Steadman, J.R., D, T., Nuland, D.S., 1991. Starlight great northern bean. HortScience 26, 441–442.
Coyne, D.P., Nuland, D.S., Lindgren, D.T., Steadman, J.R., 1994. Chase pinto bean. HortScience 29, 44–45.
Coyne, D.P., Nuland, D.S., Lindgren, D.T., Steadman, J.R., Smith, D.W., Gonzales, J., Schild, J., Reiser, J., Sutton, L., Carlson, C., 2000. Weihing great northern disease resistant dry bean. HortScience 35, 310–312.
Drijfhout, E., 1978. Genetic interaction between Phaseolus vulgarisand bean common mosaic virus with implications for strain identification and breeding for resistance. Doctoral thesis, Agric. Res. Rep. 872. Wageningen.
Echavez-Badel, R., Alameda, M., Beaver, J.S., 2000. Methodology for screening bean germplasm for charcoal rot resistance. Ann. Rep. Bean Improv. Coop. 43, 176–177.
Foster, E.F., Pajarito, A., Acosta-Gallegos, J.A., 1994. Moisture stress impact on N partitioning N remobilization and N-use efficiency in beans (Phaseolus vulgarisL.). J. Agric. Sci. 124, 27–37.
Ga´lvez, G.E., Morales F.J., 1989. Aphid-transmitted viruses. In: Schwartz, H.F., Pastor-Corrales, M.A. (Eds.), Bean Production Problems in the Tropics. CIAT, Cali, Colombia, pp. 333–362. Gepts, P., 1998. Origin and evolution of common bean: past events
and recent trends. HortScience 33, 1124–1130.
Gepts, P., Debouk, D., 1991. Origin, domestication and evolution of the common bean (Phaseolus vulgaris L.). In: Common Beans: Research for Crop Improvement. CIAT, Cali, Colombia, pp. 7–54.
Godoy-Lutz, G., Arias, J., Saladin, F., Steadman, J.R., Carling, D.E., 1996. Characterization of isolates ofRhizoctonia solani
that can cause web blight on common bean in Central America and the Caribbean with implications for disease management. Ann. Rep. Bean Improv. Coop. 39, 154–155.
Godoy-Lutz, G., Arias, J., Arnaud-Santana, E., Steadman, J.R., 1998. Web blight affects seed yield and quality of red mottled bean lines and cultivars in the Dominican Republic. Ann. Rep. Bean Improv. Coop. 41, 72–73.
Godoy-Lutz, G., Steadman, J.R., Powers, K., Higgins, B., 2000. DNA variation and virulence among isolates causing web blight on common beans. Ann. Rep. Bean Improv. Coop. 43, 72–73. Guzman, P., Gilbertson, R.L., Nodari, R., Johnson, W.C., Temple, S., Mandala, D., Mkandawire, A.B.C., Gepts, P., 1995. Characterization ofPhaeoisariopsis griseola isolates by ran-dom amplified polymorphic DNA (RAPD) markers suggests pathogen coevolution with its Phaseolus vulgaris host. Phytopathology 85, 600–607.
Jung, G., Coyne, D.P., Skroch, P.W., Nienhuis, J., Arnaud-Santana, E., Bokosi, J., Ariyarathne, H.M., Steadman, J.R., Beaver, J.S., Kaeppler, S.M., 1996. Molecular markers associated with plant architecture and resistance to common blight. J. Am. Soc. Hort. Sci. 121, 794–803.
Jung, G., Coyne, D.P., Bokosi, J., Steadman, J.R., 1998. Mapping genes for specific and adult plant resistance to rust and abaxial leaf pubescence and their genetic relationship using randomly amplified polymorphic DNA (RAPD) markers in common bean. J. Am. Soc. Hort. Sci. 123, 859–863.
Kelly, J.D., 1997. A review of varietal response to bean common mosaic potyvirus inPhaseolus vulgaris. Plant Varieties Seeds 10, 1–6.
Kelly, J.D., 2000. Remaking bean plant architecture for efficient production. Adv. Agron. 71, 109–143.
Kelly, J.D., Adams, M.W., 1987. Phenotypic recurrent selection in ideotype selection in ideotype breeding of pinto beans. Euphytica 36, 69–80.
Kelly, J.D., Adams, M.W., Varner, G.V., 1987. Yield stability of determinate and indeterminate dry bean cultivars. Theor. Appl. Genet. 74, 516–521.
Kelly, J.D., Adams, M.W., Saettler, A.W., Hosfield, G.L., Varner, G.V., Beaver, J.S., Uebersax, M.A., Taylor, J., 1989. Registra-tion of Mayflower navy bean. Crop Sci. 29, 1571–1572. Kelly, J.D., Adams, M.W., Saettler, A.W., Hosfield, G.L., Varner,
G.V., Uebersax, M.A., Taylor, J., 1990. Registration of Sierra pinto bean. Crop Sci. 30, 745–746.
Kelly, J.D., Hosfield, G.L., Varner, G.V., Uebersax, M.A., Miklas, P.N., Taylor, J., 1992. Registration of Alpine great northern bean. Crop Sci. 32, 1509–1510.
Kelly, J.D., Hosfield, G.L., Varner, G.V., Uebersax, M.A., Haley, S.D., Taylor, J., 1994. Registration of Raven black bean. Crop Sci. 34, 1406–1407.
Kelly, J.D., Hosfield, G.L., Varner, G.V., Uebersax, M.A., Afanador, L.K., Taylor, J., 1995a. Registration of Newport navy bean. Crop Sci. 35, 1710–1711.
Kelly, J.D., Hosfield, G.L., Varner, G.V., Uebersax, M.A., Taylor, J., 1998b. Registration of Mackinac navy bean. Crop Sci. 38, 280. Kelly, J.D., Kolkman, J.M., Schneider, K., 1998c. Breeding for yield
in dry bean (Phaseolus vulgarisL.). Euphytica 102, 343–356. Kelly, J.D., Hosfield, G.L., Varner, G.V., Uebersax, M.A., Taylor, J.,
1999a. Registration of Kodiak pinto bean. Crop Sci. 39, 292–293. Kelly, J.D., Hosfield, G.L., Varner, G.V., Uebersax, M.A., Taylor, J., 1999b. Registration of Chinook 2000 light red kidney bean. Crop Sci. 39, 293.
Kelly, J.D., Hosfield, G.L., Varner, G.V., Uebersax, M.A., Taylor, J., 1999c. Registration of Beluga alubia bean. Crop Sci. 39, 294.
Kelly, J.D., Hosfield, G.L., Varner, G.V., Uebersax, M.A., Taylor, J., 1999d. Registration of Matterhorn great northern bean. Crop Sci. 39, 589–599.
Kelly, J.D., Schneider, K.A., Kolkman, J.M., 1999e. Breeding to improve yield. In: Singh, S.P. (Ed.), Developments in Plant Breeding: Common Bean Improvement in the Twenty-First Century, vol. 7. Kluwer Academic Publishers, Dordrecht, The Netherlands.
Kelly, J.D., Hosfield, G.L., Varner, G.V., Uebersax, M.A., Taylor, J., 2000. Registration of Phantom black bean. Crop Sci. 40, 572.
Kelly, J.D., Hosfield, G.L., Varner, G.V., Uebersax, M.A., Taylor, J., 2001. Registration of Jaguar black bean. Crop Sci. 41, 1649–1650.
Kelly, J.D., Gepts, P., Miklas, P.N., Coyne, D.P., 2003. Tagging and mapping of genes and molecular marker-assisted selection for traits of economic importance in bean and cowpea. Field Crops Res., this issue.
Koinange, E.M.K., Singh, S.P., Gepts, P., 1996. Genetic control of the domestication syndrome in common bean. Crop Sci. 36, 1037–1045.
Kornegay, J., Cardona, C., Posso, C.E., 1993. Inheritance of resistance to Mexican bean weevil in common bean, determined by bioassay and biochemical tests. Crop Sci. 33, 589–594. Liao, H., Rubio, G., Yan, X., Cao, A., Brown, K.M., Lynch, J.P.,
2001. Effect of phosphorus availability on basal root shallow-ness in common bean. Plant Soil 232, 69–79.
Lopez-Salinas, E., Acosta-Gallegos, J.A., Becerra-Leor, E.N., Frayre-Vazquez, G., Orozco, S.H., Beebe, S.E., 1997. Registra-tion of Negro Tacana common bean. Crop Sci. 37, 1022. Macchiavelli, R., Beaver, J.S., 1999. Analysis of
genotype-by-environment interaction with AMNI models using SAS PROC Mixed. In: Proceedings of the XI Conference on Applied Statistics in Agriculture, Manhattan, KS.
Martin, G.B., Adams, M.W., 1987. Landraces of Phaseolus vulgaris (Fabaceae) in northern Malawi. II. Generation and maintenance of variability. Econ. Bot. 41 (2), 204–215. Mayek-Perez, N., Lopez-Castaneda, C., Gonzalez-Chavira, M.,
Garcia-Espinosa, R., Acosta-Gallegos, J., Martinez de la Vega, O., Simpson, J., 2001. Variability of Mexican isolates of
Macrophomina phaseolinabased on pathogenesis and AFLP genotype. Physiol. Mol. Plant Pathol. 59, 257–264.
Miklas, P.N., Johnson, E., Stone, V., Beaver, J.S., Montoya, C., Zapata, M., 1996. Selective mapping of QTL conditioning disease resistance in common bean. Crop Sci. 36, 1344–1351.
Miklas, P.N., Beaver, J.S., Steadman, J.R., Silbernagel, M.J., Freytag, G.F., 1997. Registration of three bean common mosaic virus-resistant navy bean germplasm. Crop Sci. 37, 1025. Miklas, P.N., Schwartz, H.F., Nina, R., Beaver, J.S., 1998a.
Reaction of select tepary bean to ashy stem blight and Fusarium wilt. HortScience 33, 136–139.
Miklas, P.N., Stone, V., Urrea, C.A., Johnson, E., Beaver, J.S., 1998b. Inheritance and QTL analysis of field resistance to ashy stem blight in common bean. Crop Sci. 38, 916–921. Miklas, P.N., Zapata, M., Beaver, J.S., Grafton, K.F., 1999.
Registration of four dry bean germplasms resistant to common bacterial blight: ICB-3, ICB-6, ICB-8, and ICB-10. Crop Sci. 39, 594.
Miklas, P.N., Smith, J.R., Riley, R., Grafton, K.F., Singh, S.P., Jung, G., Coyne, D.P., 2000a. Marker-assisted breeding for pyra-mided resistance to common bacterial blight in common bean. Ann. Rep. Bean Improv. Coop. 43, 39–40.
Miklas, P.N., Hannan, R., Smith, J.R., Beaver, J.S., Riley, R., Antonius, S., 2000b. Transferring heat tolerance and indeter-minancy from Indeterminate Jamaica Red (PI 163122) to kidney bean. Ann. Rep. Bean Improv. Coop. 43, 66–67. Mink, G.I., Vetten, J., Ward, C.W., Berger, P., Morales, F., Myers,
J.R., Silbernagel, M.J., Barnett, O.W., 1994. Taxonomy and classification of legume infecting potyviruses. A proposal from the Potyviridae Study Group of the Plant Virus Subcommittee of ICTV. Arch. Virol. 139, 231–235.
Mmbaga, M.T., Arnaud-Santana, E., Steadman, J.R., Coyne, D.P., 1992a. New sources of nonspecific resistance to rust and common bacterial blight in the dry bean landrace Pompadour. Euphytica 61, 137–144.
Mmbaga, M.T., Arnaud-Santana, E., Steadman, J.R., Coyne, D.P., 1992b. Adult plant resistance associated with leaf pubescence in common bean. Plant Dis. 76, 1230–1236.
Molina Castaneda, A., Beaver, J.S., 1998. Inheritance of normal pod development in bean golden mosaic resistant common beans. Ann. Rep. Bean Improv. Coop. 41, 5–6.
Montoya, C.A., Beaver, J.S., Rodriguez, R., Miklas, P.N., Godoy-Lutz, G., 1997. Heritability of resistance to web blight in five common bean populations. Crop Sci. 37, 780–783.
Morales, F.J., 1994. Current situation of bean golden mosaic in Latin America. In: Morales, F.J. (Ed.), Bean Golden Mosaic 1994: Research Advances. CIAT, Cali, Colombia, pp. 100–101.
Morales, F.J., Singh, S.P., 1997. Inheritance of mosaic and necrosis reactions induced by bean severe mosaic comoviruses in
Phaseolus vulgarisL. Euphytica 93, 223–226.
Murillo, A., Peralta, E., Pinzo´n, J., Lepiz, R., 1997. Logros, problemas y perspectivas para el mejoramiento del frijol arbustivo en el Ecuador. In: Singh, S.P., Voysest, O. (Eds.), Taller de mejoramiento de frijol para el Siglo. XXI. Bases para una estrategia para America Latina. CIAT, Cali, Colombia, pp. 405–414.
Myers, J.R., Stewart-Williams, K.D., Hayes, R.E., Kolar, J.J., Singh, S.P., 2001. Registration of UI 259 red dry bean. Crop Sci. 41, 1642–1643.
solanien el Altiplano de Me´xico. Agronomia Mesoamericana 10 (1), 37–46.
Osborne, T.C., Burow, M., Bliss, F.A., 1988. Insecticidal activity and lectin homology of arcelin seed protein. Science 240, 207–210.
Pastor-Corrales, M.A., Stavely, J.R., Kelly, J.D., Grafton, K.F., Steadman, J.R., Coyne, D.P., Lindgren, D.T., Scully, B.T., 2001. Rust and mosaic resistant bean germplasm releases, 1997– 1999. Ann. Rep. Bean Improv. Coop. 44, 101–102.
Perrin, R., Poor, J., Coyne, D.P., 2000. Economic impact of the chase variety of pinto bean. University of Nebraska Coopera-tive Extension RB 338, pp. 1–8.
Polanco, T., Rodriguez, R.P., Beaver, J.S., 1996. Microgotas: Metodo de inoculacion conRhizoctonia solaniKuhn para la evaluar la reaccion de lineas de habichuela (Phaseolus vulgaris
L.). J. Agric. Univ. Puerto Rico 80, 111–122.
Quentin, M.E., Spencer, J.L., Miller, J.R., 1991. Bean tumbling as a control measure for the common bean weevil,Acanthoscelides obtectus(Say). Entomol. Exp. Appl. 60, 105–109.
Roman-Avile´s, B., Beaver, J.S., 2001. Heritability of heat tolerance of an Andean bean population. Ann. Rep. Bean Improv. Coop. 44, 49–50.
Rosales-Serna, R., Kohashi-Shibata, J., Acosta-Gallegos, J.A., Trejo-Lo´pez, C., Ortiz-Cereceres, J., Kelly, J.D., 2002. Yield and phenological adjustment in four drought-stressed common bean cultivars. Ann. Rep. Bean Improv. Coop. 45, 198–199. Rosas, J.C., 2001. Aplicacio´n de metodologı´as participativas para
el mejoramiento gene´tico del frijol en Honduras. Agronomia Mesoamericana 12, 219–228.
Rosas, J.C., Castro, A., 1999. Experiencias en produccio´n artesanal de semilla de frijol en Centro Ame´rica. Memoria del Taller de Produccio´n y Distribucio´n de Semillade Frijol en Centro Ame´rica. Zamorano, Honduras, August 3–6, 1998, 101 pp.
Rosas, J.C., Varela, O.I., Beaver, J.S., 1997. Registration of Tio Canela 75 small red bean. Crop Sci. 37, 1391.
Rosas, J.C., Castro, J.A., Flores, E.D., 2000a. Mejoramiento genetico del frijol rojo y negro mesoamericano para Centro America y El Caribe. Agronomia Mesoamericana 11 (2), 37–46.
Rosas, J.C., Castro, A., Beaver, J.S., Perez, C.A., Morales, A., Lepiz, R., 2000b. Mejoramiento genetico para tolerancia a altas temperaturas y resistencia a mosaico dorado en frijol comun. Agronomia Mesoamericana 11 (1), 1–10.
Rosas, J.C., Castro, J.A., Herna´ndez, J.C., Araya, R., 2003. Registration of Bribri small red bean (race Mesoamerica). Crop Sci. 43, 430–431.
Saettler, A.W., 1989. Common bacterial blight. In: Schwartz, H.F., Pastor-Corrales, M.A. (Eds.), Bean Production Problems in the Tropics. CIAT, Cali, Colombia, pp. 261–284.
Saladin, F., Arnaud-Santana, E., Nin, J.C., Godoy-Lutz, G., Beaver, J.S., Coyne, D.P., Steadman, J.R., 2000. Registration of PC-50 red mottled bean. Crop Sci. 40, 858.
Schneider, K.A., Rosales-Serna, R., Ibarra-Perez, F., Cazares-Enriquez, B., Acosta-Gallegos, J.A., Ramirez-Vallejo, P., Wassimi, N., Kelly, J.D., 1997. Improving common bean performance under drought stress. Crop Sci. 37, 43–50.
Schwartz, H.F., Brick, M.A., Nuland, D.S., Franc, G.D., 1996. Dry bean production and pest management. Regional Bulletin 562A. Cooperative Extension Resource Center. Colorado State University, Fort Collins, CO, 106 pp.
Singh, S.P., Mun˜oz, C.G., Tera´n, H., 2001. Registration of common bacterial resistant dry bean germplasm VAX 1, VAX 3, VAX 4. Crop Sci. 41, 275–276.
Stavely, J.R., Pastor-Corrales, 1989. Rust. In: Schwartz, H.F., Pastor-Corrales, M.A. (Eds.), Bean Production Problems in the Tropics. CIAT, Cali, Colombia, pp. 159–194.
Stavely, J.R., Steinke, J., McMillan, R.T., Grafton, K.F., Steadman, J.R., Kelly, J.D., Coyne, D.P., Lindgren, D.T., Silbernagel, M.J., 1992. Rust resistant bean germplasm release. Ann Rep. Bean Improv. Coop. 35, 228–229.
Stavely, J.R., McMillan, R.T., Beaver, J.S., Miklas, P.N., 2001. Release of three McCaslan type, indeterminate, rust and golden mosaic resistant snap bean germplasm lines BelDade RGMR 4, 5 and 6. Ann. Rep. Bean Improv. Coop. 44, 197–199. Takegami, J.C., Beaver, J.S., 2000. Heritability of web blight
resistance in common bean. Ann. Rep. Bean Improv. Coop. 43, 43–44.
Temple, S.R., Helms, D.M., Sanchez, L.A., Bellaloui, N.I., 1999. New dry bean varieties for California producers. In: Proceed-ings of the American Society of Agronomy Annual MeetProceed-ings, Salt Lake City, p. 78 (agronomy abstracts).
Temple, S., Helms, D., Smith, S., Pinzon, M., Bensen, T., Roberts, P., Matthews, B., Gilbertson, B., de Carvalho, E., Guzman, P., 2002. UC Davis 2001 Grain Legume Improvement Program. In: University of California Dry Bean Research, 2001 Progress Report, pp. 1–23.
Urrea, C.A., Miklas, P.N., Beaver, J.S., Riley, R.H., 1996. A codominant RAPD marker useful for indirect selection of BGMV resistance in common bean. J. Am. Soc. Hort. Sci. 121, 1035–1039.
Van Schoonhoven, A., Voysest, 1989. Common beans in Latin America and their constraints. In: Schwartz, H.F., Pastor-Corrales, M.A. (Eds.), Bean Production Problems in the Tropics. CIAT, Cali, Colombia, pp. 33–57.
Velez, J., Bassett, M.J., Beaver, J.S., Molina, A., 1998. Inheritance of resistance to bean golden mosaic virus in common bean. J. Am. Soc. Hort. Sci. 123 (4), 628–631.
Victor, D.G., Runge, C.F., 2002. Farming the genetic frontier. Foreign Affairs 81 (3), 107–121.
Wallace, D.H., Shardlow, D.K., 2001. Description of RedKanner light red kidney (NY 10195). Annu. Rep. Bean Improv. Coop. 44, 200.
Wallace, D.H., Yan, W., 1998. Plant Breeding and Whole System Crop Physiology, Improving Adaptation, Maturity and Yield. CAB International, Wallingford, Oxon, UK.