Summary Water relations and growth of maritime pine (Pinus pinaster Ait.) were investigated in 2-year-old seedlings of French (‘Landes’), Iberian (‘Iberian’) and Moroccan (‘Tamjoute’) origin raised for 67 days in a flowing solution culture system containing 0, 50, 150 or 250 mM NaCl. Height growth, and stem, needle and root dry matter were reduced by salinity with minor differences among geographic origins. Predawn needle water potential was decreased by salinity and corresponded approximately to the osmotic potential of the nutrient solution. Stomatal conductance was reduced accord-ing to the amount of salinity applied. Whole-plant hydraulic conductance was also reduced, even when expressed on a root dry weight basis. The osmotic potential of xylem sap was five-to sixfold lower than that of the nutrient solution. Seedlings of the most southerly origin (Tamjoute) exhibited a greater ability to decrease osmotic potential under saline conditions than seedlings of more northerly origin (Landes and Iberian) as a result of higher mineral cation transport to the shoot.
Keywords: geographic origin, hydraulic conductance, needle water potential, osmotic potential, salt stress, stomatal con-ductance.
Introduction
Intrusions of a salinized water table during spring are sus-pected to be the cause of pine forest dieback in the Vendée and Pays de Loire coastal range (Guyon 1991). The major tree species in this region is maritime pine (Pinus pinaster Ait.) of which two geographic origins have been used for afforestation of the area, i.e., the local ‘Landes’ origin and a so-called ‘Iberian’ origin, the latter being a composite from various locations in Spain and Portugal. Nguyen and Lamant (1989) have shown that seedlings of Moroccan origin (‘Tamjoute’) exhibit a greater capacity for osmoregulation in response to polyethyleneglycol-induced water stress than seedlings of Landes or Iberian origin. Based on these studies and our unpublished observations, we hypothesize that trees of Moroc-can origin are better able to tolerate salinity than trees of Landes and Iberian origins. Because salt-tolerant genotypes of several Australian trees have been identified and successfully used to afforest salinized areas of Western Australia (Van der Moezel et al. 1991, Sun and Dickinson 1993), we reasoned that
pine forest dieback in the Vendée and Pays de Loire coastal range might be reduced if the area was afforested with a salt-tolerant genotype of P. pinaster.
Although there are few studies on the physiological re-sponses of P. pinaster to soil salinity, the effects of salinity have been investigated in several other Pinus species including P. radiata D. Don (Sands and Clarke 1977), P. contorta Dougl. ex Loud. (Bedunah and Trlica 1979) and P. taeda L. (Land 1974, Pezeshki 1992). All of these species are moderately salt-tolerant. Sands and Clarke (1977) concluded that P. ra-diata is better adapted to avoid rather than to tolerate an accumulation of Na+ and Cl− in the needles because a sudden increase in salinity of the root environment caused an abrupt and large increase in the concentrations of Na+ and Cl− ions in the needles, resulting in plant death, whereas a more progres-sive increase in salinity of the root environment resulted in a gradual decline in transpiration rate and water potential, and a concomitant decrease in leaf osmotic pressure, half of the latter being accounted for by an accumulation of mineral anions and cations.
We have investigated genetic variation in the response of 2-year-old P. pinaster seedlings to 67 days of exposure to salinized soil solution. The plant material used represents the range of ecological variation found for this species: (i) the Landes origin is from the French Atlantic coast and represents the most northerly distribution of P. pinaster and the most humid conditions; (ii) the Iberian origin developed from popu-lations separated from the Landes by the Pyrrennean Moun-tains; and (iii) the Tamjoute origin is from Morocco and represents the most southerly and dry distribution for this species (Baradat and Marpeau-Bezard 1988). Dry matter and height increment, stomatal and hydraulic conductances, and water relations were examined.
Materials and methods
Plant material and growth conditions
One-year-old seedlings of maritime pine (P. pinaster) were raised from seed in sand culture. Seeds of Landes and Iberian origin were obtained from the seed collection of the compara-tive inter-provenance trials, located in Vendée, France (Magnin 1990). Tamjoute seed was collected from its natural center of
Growth and water relations of three geographically separate origins of
maritime pine (Pinus pinaster) under saline conditions
D. LOUSTAU, S. CREPEAU, M. G. GUYE, M. SARTORE and E. SAUR
INRA, Laboratoire d’Ecophysiologie et Nutrition, BP 45, 33611 Gazinet cedex, France
Received September 19, 1994
origin in the Atlas Mountains, Morocco. Following germina-tion, 80 seedlings of each origin were grown in pure quartz sand for 1 year in 4-l plastic pots in an unheated greenhouse equipped with a cooling system. Ambient temperatures did not exceed 30 °C. An automated intermittent nutrient-flow system was used to irrigate each pot, and 20 ml of a nutrient solution (pH 5.6) was applied every 20 min during the light period, giving a total daily application of 720 ml. The composition of the nutrient solution (mM) was: NH4NO3 (2), KH2PO4 (0.5), CaCl2 (0.25), MgSO4 (0.25), Fe (0.1), B (8 × 10−3), Mn (1.5 × 10−3), Zn (0.15 × 10−3), Cu (0.15 × 10−3), Cu (0.0015 × 10−3), and Mo (0.0015 × 10−3). The pH was 4.5.
Salt treatments
Four salt treatments (0, 50, 150 and 250 mM NaCl) were added in a stepwise fashion to the nutrient solution so that the salt concentration increased at a rate of 40 mM every 48 h. The highest concentration was reached 12 days after the start of the experiment. Salt treatments were continued for 67 days from the beginning of application. The osmotic potential, pH and conductivity of the solution used in each treatment are given in Table 1. The salt treatments were distributed randomly among 16 blocks, giving a total population of 192 plants.
Growth analysis
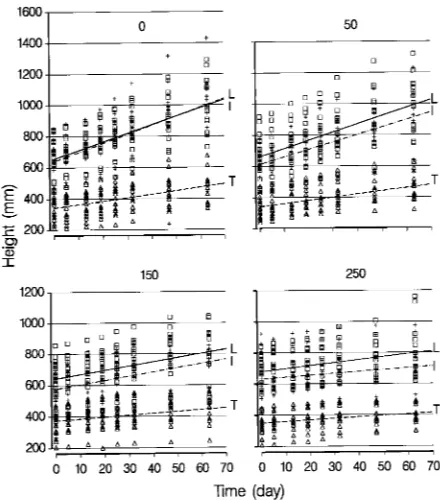
Growth was measured nondestructively as an increase in plant height over a 67-day period, expressed as mm day−1. Because height growth during the experiment was nearly linear (Fig-ure 1), a linear regression of height on time was fitted by the least squares method for each plant, and the linear growth rate in height was estimated for each plant. All seedlings were destructively harvested after 67 days, and total dry matter of root, stem and leaf tissues was determined.
Water relations
Four blocks were chosen randomly from among the 16 blocks for the determination of water status and transpiration. Sto-matal conductance and needle water potential were determined on each plant of the four blocks every 10 days. Predawn water potential (Ψb) was measured with a pressure chamber during the hour preceding dawn on two needles per plant. Between 0900 and 1130 h (IST), stomatal conductance (gs) was meas-ured with a steady-state porometer (LI-1600, Li-Cor Inc., Lincoln, NE) equipped with a cylindrical chamber. Ambient conditions during measurements were: temperature = 18--16 °C, vapor pressure deficit = 500--1200 Pa, and
photosyn-thetic photon flux density > 600 µmol m−2 s−1. Measurements were made on one pair of fully expanded needles of the current-year growth near the apex. Two to three measurements of stomatal conductance were made per plant, and each meas-urement was made on a single pair of needles.
At the end of the experiment, predawn osmotic potential (Ψπ) of needles was determined. Needles were collected 1 h before dawn and frozen in liquid nitrogen. After thawing, cellular sap was extracted by crushing the needles with a syringe. The osmolarity of the cellular sap was determined by freezing-point depression osmometry (automated micro-os-mometer, Herman-Roebling, Berlin). Turgor (Ψt) was com-puted as the difference between water potential (Ψb) and osmotic potential (Ψπ).
On Day 67, whole-plant hydraulic conductance (L, µmol s−1 gDW−1 MPa−1) was determined for three plants each of Landes and Tamjoute origin from the slope of the regression line of water flux through each plant expressed on a needle dry weight basis (J, µmol s−1 gDW−1 MPa−1) against the water potential difference between the flowing solution and the needle (Ψf−
Ψs, MPa−1) according to the general transport equation:
L = J (Ψf−Ψs)
. (1)
Disregarding water storage and release in plant parts, J was assumed to be equal to transpiration rate, which was deter-mined gravimetrically at four 30-min intervals during the morning. Transpiration was assumed to be constant during each interval. Leaf water potential was determined at the
Table 1. Main physical and chemical characteristics of NaCl treatment solutions.
Characteristic Control 50 mM 150 mM 250 mM
pH 5.3 5.8 5.1 5.2
π (MPa) −0.05 −0.25 −0.63 −1.1
[Na] (mM) 0 50 150 250
Conductivity (mS) 1.0 6.2 14.5 24.7
midpoint of each interval with a pressure chamber and the values obtained (Ψf′) were corrected with the values of the osmotic potential of the xylem sap (πx) according to:
Ψf=Ψf′+πx. (2)
The osmotic potential of stem xylem sap was determined by the method used for needles. Plants were detopped 5 cm above the ground and the bark and phloem were removed from the stem tissue attached to the root. Sap was expressed from the root system with a pressure chamber at a pressure of Ψf′ + 1 MPa. The root system was then rinsed with tap water, dried at 70 °C and weighed.
Mineral nutrient analysis
The mineral concentrations of needles (i.e., K, Na, Ca, N and P) were determined on ground dry leaf material following mineralization. Total nitrogen content was determined by mi-cro-Kjeldahl digestion, and subsequent colorimetric ammonia determinations were performed automatically with an Autoanalyzer II (Technicon Instruments, Tarrytown, NY). Phosphorus concentration was determined colorimetrically, based on ascorbic acid reduction of the ammonium phospho-molybdate complex. Mineral cation concentrations were de-termined by atomic absorption spectrophotometry. All data were expressed as mmol kgDW−1.
Experimental design and data analysis
The treatments were distributed randomly among 16 blocks to give a total population of 192 plants; however, because of the death of some plants during the experiment, a final population of 189 plants was used. Statistical analyses were made with the GLM procedure of the SAS software package (SAS Institute Inc., Cary, NC). Salinity was considered a continuous variable, and its interaction with origin was analyzed by covariance analysis. The block and interaction effects were also included. All significant effects were tested with α = 0.05. The effects on the variables measured repeatedly during the experiment, gs and Ψb, were analyzed by considering the between-subject effects, after correction for within-subject effects, based on an adjusted P-value calculated according to Huyn and Feldt (1970).
Results
Effects of salinity on growth
There were no significant block or interaction effects including the block factor for either growth rate or initial height (data not shown). Before the salt treatment, the Tamjoute seedlings were smaller than those of the more northerly origins (Table 2). The effects of origin, salinity and the interaction of these factors were all significant for linear growth rate (LGR). The growth rate of Tamjoute seedlings was lower and was less affected by salinity than the growth rates of seedlings of the more northerly origins (Figure 1). Salinity reduced the LGR of Tamjoute seedlings by 0.005 mm day−1 mmol−1 (0.25% of the control)
compared with reductions of 0.017 (0.30%) and −0.018 (0.31%) mm day−1 mmol−1 for the Landes and Iberian seed-lings, respectively.
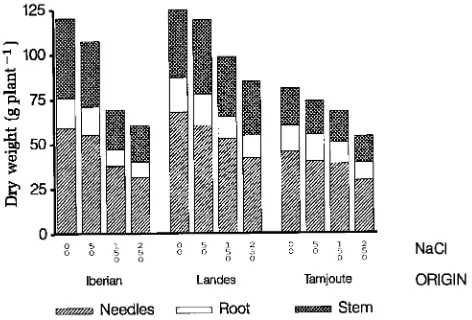
Salinity decreased plant dry weight (Figure 2), but it did not significantly affect the root/shoot ratio. The root/shoot ratio was highest for Tamjoute seedlings (0.23 versus 0.17 for seed-lings of the other two origins; F-test and Student-Newman-Keuls test, mean comparisons). The saline treatments caused a greater decrease in stem biomass, and consequently total biomass, in Iberian seedlings than in seedlings of the other genotypes (Table 3). Irrespective of genotype, salinity reduced needle biomass by 0.058 g mmol−1 NaCl, whereas it only reduced the biomass of root and stem tissues by 0.022 g mmol−1 NaCl (Table 3).
Effects of salinity on plant water relations
Salinity significantly reduced stomatal conductance and pre-dawn needle water potential (Figure 3), and the effects were independent of origin (Table 4). The effect of salinity on predawn water potential varied with time (Figure 3), particu-larly during the first phase of the experiment when the NaCl concentration was being increased stepwise. During the period when the NaCl concentrations were held constant, the be-tween-treatment differences in Ψb were stable and corre-sponded approximately to the differences in osmotic potentials of the irrigation solutions (Table 1). There were only minor day-to-day variations in predawn water potential throughout the 67-day experiment.
Table 2. Comparison of the mean initial plant height of each genotype. Values followed by the same letter are not significantly different; ANOVA and Student-Newman-Keuls test, α = 0.05.
Genotype Initial height (mm)
Tamjoute 351 c
Iberian 615 b
Landes 659 a
Because there was a large residual variation in the flux versus potential regressions used to estimate hydraulic con-ductance of the whole plant (Figure 4), the estimates of
hy-draulic conductance are not accurate and can only be used for comparative purposes. Plant hydraulic conductance decreased with increasing salinity independent of genotype (ANOVA, data not shown). There were few visible differences in the root systems of seedlings in the control and 50 mM NaCl treat-ments, although there were more lignified roots and less fine root tips in salt-treated plants. At salt concentrations above 50 mM, we observed a blackening of the whole root system and the disappearance of fine white root apices.
Although the osmotic potential of xylem sap was variable within the same origin × salinity combination, there was a significant decrease in osmotic potential of xylem sap of Tamjoute seedlings with increasing salinity (Table 5). Osmotic potential values as negative as −0.54 MPa were measured in xylem sap of Tamjoute seedlings in the 150 mM NaCl treat-ment, which was much less than the osmotic pressure of the irrigation solution (Table 1). The saline treatments caused only moderate changes in the osmotic potential of the xylem sap of Landes seedlings, from −0.11 MPa at 0 mM NaCl to −0.27 MPa at 250 mM NaCl.
There were significant differences in predawn osmotic po-tential of cellular sap between origins in response to salinity (Table 6). Because Tamjoute seedlings were more responsive to increases in salinity than Iberian and Landes seedlings (Figure 5), a higher turgor was maintained in Tamjoute seed-lings than in seedseed-lings of the other genotypes at high salinity. This response paralleled the accumulation of Na in needles (Figure 5). In response to increasing salinity, the Na concen-tration in needles increased four- to sixfold in seedlings of more northerly origin compared to 19-fold in Tamjoute seed-lings. Irrespective of genotype, the salt treatments also signifi-cantly increased N and K concentrations of the needles. The
Table 3. Genotype and salinity effects on dry matter production. The table gives the results of the analysis of variance (F-values) and the parameter values estimated for the salinity effect. The block effects were not significant and are not included in the variance model. The model is
W = Intercept +δi+ (β +γi)[NaCl ] +ε, where W is the total dry matter at the end of the experiment (g), which may refer to either stem, root, needle
or total dry matter per plant, [NaCl] is the salinity (mmol), δi is the genotype effect (g), where i denotes the genotype, β is the salinity effect (g
mmol−1), γi is the salinity × genotype interaction term (g mmol−1), ε is the residual (g), and intercept is the minimum mean value estimated from
the treatment × origin combinations.
Source df Root dry matter (g) Stem dry matter (g) Needle dry matter (g) Total dry matter (g)
Genotype 2 7.68**1 32.72** 11.68** 17.29**
Salinity 1 70.11** 46** 51.05** 55.26**
Interaction 2 1.51 ns 5.9* 1.95 ns 3.26*
Error 184
Total 189 17.7** 29** 17.7** 21.8**
Parameters estimates
Intercept 15.3 20.55 45.01 80.87
δ Tamjoute 0 0 0 0
Iberian 3.27 21.47 14.29 37.08
Landes 1.32 25.25 22.07 50.60
β −−0.0216 −−0.022 −−0.058 −−0.10
γ Tamjoute 0 0 0 0
Iberian −0.014 −−0.078 −0.061 −−0.15
Landes −0.006 −0.046 −0.041 −0.09
1 The parameters estimates that differ significantly from 0 (t-test) are bold; * = α < 0.5; ** = α < 0.01; ns = not significant.
Figure 3. Time course of the effects of salinity on stomatal conduc-tance (gs) and predawn water potential (Ψb) of Pinus pinaster. Data
other nutrients examined were not affected by the salt treat-ments (Saur et al. 1995).
Discussion
By the end of the 67-day experiment, a 300 mmol kg−1 increase in NaCl concentration of the plant tissue had resulted in less than a 1% increase in plant dry weight. We conclude, therefore,
Table 4. Analysis of variance of the effects of time, genotype and salinity on stomatal conductance.
(a) Test of hypotheses for between-subject effect
Source of variation df Sum of squares F-value P-value
Salinity 1 0.3421 131.9 0.0001
Genotype 2 0.0137 2.66 0.08
Interaction 2 0.008 1.62 0.21
Error 49 0.127
(b) Univariate tests of hypotheses for within-subject effects
Source of variation df Sum of squares F-value Adjusted P-value
Time 3 0.040 5.17 0.01
Time × salinity 3 0.017 2.23 0.10
Time × genotype 6 0.030 1.93 0.10
Time × salinity × genotype 6 0.007 0.43 0.81
Error 147 0.377
1 Significant effects (α = 0.05) are bold.
Figure 4. Flux--water potential diagrams for Landes and Tamjoute genotypes of Pinus pinaster. The lines are the regressions of the flux--potential relationship. Each line was calculated from the pooled data obtained from three seedlings giving a total of 12 plants per genotype.
Table 5. Mean values of the osmotic potential (MPa) of the xylem sap extracted at the end of the hydraulic conductance determinations. Each value is the mean of the values obtained from three seedlings. Covari-ance analysis showed the salinity and genotype × salinity effects were significant, and the rate of decrease in xylem sap osmotic potential with increasing xylem sap salinity was more pronounced in Tamjoute seedlings than in Landes seedlings.
Salinity (mM) Tamjoute Landes
0 (Control) −0.16 −0.11
50 −0.20 −0.36
150 −0.54 −0.14
250 −0.45 −0.27
Table 6. Estimates of model parameters and comparison of means for salinity and genotype effects on the predawn osmotic water potential of cellular sap. The model is Ψπ= Intercept + δi+(β+γi)[NaCl ] +ε,
where Ψπ is the osmotic potential (MPa), intercept is the minimum mean osmotic potential among genotypes at NaCl = 0 (mm day−1), [NaCl] is the salinity (mmol), δi is the genotype effect (MPa), where i denotes the genotype, β is the salinity effect (MPa mmol−1), γi is the
salinity × genotype interaction term (MPa mmol−1), and ε is the residual (MPa).
Parameter Genotype Estimate Standard error on the estimate
Intercept −−1.501 0.07
δ Tamjoute 0
Iberian −0.05 0.09 Landes 0.038 0.10
β −−0.0036 0.0004
γ Tamjoute 0
Iberian 0.0018 0.0006
Landes 0.0018 0.0006
1 Significant effects (according to covariance analysis, α = 0.05) are
that differences in dry matter accumulation of pine seedlings at the end of the experiment were due to differences in carbon balance of the plant and not to the accumulation of Na+ and Cl− ions. Salinity affected height growth, dry matter increment and water relations of the maritime pine seedlings. There were small genotypic differences in growth response to salinity. The dry matter increment of Iberian seedlings was slightly more affected by salinity than that of seedlings of the other geno-types (Table 3). Tamjoute seedlings exhibited a greater ability to maintain stem elongation under salt stress than seedlings of more northerly origin (Figure 1). This ability was associated with large increases in osmotic potentials of cellular and xylem saps (Table 6, Figure 5) that, in turn, may be partly explained by the ability of Tamjoute seedlings to maintain high turgor under salt stress (Figure 5).
Salinity induces drought-like effects in maritime pine in-cluding decreases in needle water potential and osmotic poten-tial (Nguyen and Lamant 1989), cessation of growth, and a reduction in gas exchange (Loustau et al. 1989). Stomatal conductance of maritime pine was sensitive to salinity (Fig-ure 3). Similar responses are shown by many other glycophytic species including Vitis vinifera L. (Downton et al. 1990), Lycopersicon species (Sanchez-Blanco et al. 1991), Triticum aestivum L. (Zerbi et al. 1990), Phaseolus vulgaris L.
(Brug-noli and Lauteri 1991), Fraxinus excelsior L. (Leonardi and Fluckiger 1986) and P. taeda (Pezeshki 1992). Little is known about the physiological processes involved in stomatal regula-tion of plants under salt stress. However, stomata exhibit a gradual response to root water deficit, which is mediated by abscisic acid (ABA) in the xylem sap (Zhang and Davies 1990, Khalil and Grace 1993). Moreover, Downton and Loveys (1981) and Walker and Dumbroff (1981) have shown that osmotic or saline stress induces the accumulation of ABA in leaves.
A reduction in photosynthesis by stomatal closure may partly explain the saline-induced reduction in dry matter incre-ment. Picon et al. (unpublished data) demonstrated that, in maritime pine, stomatal limitation of photosynthesis accounts for a major part of the reduction in dry matter increment observed in response to water deficit. However, additional salt-induced modifications of either nondiffusional processes of photosynthesis (Seeman and Sharkey 1986, Pezeshki 1992) or plant carbon balance (Shone and Gale 1983, Richardson and McCree 1985) might also contribute to decreased dry matter production.
The hydraulic conductance of the whole plant was affected at salt concentrations exceeding 50 mM (Figure 4). Because the salt treatments affected root dry weight similarly in each genotype (Table 3), the salinity-induced reduction in hydraulic conductance cannot be explained entirely by a reduction in size of the whole root system, although there were visible symptoms of root injury at the two highest salt concentrations. The effects of salinity on root systems have been investigated in Vigna radiata (L.) R. Wilcz. (Salim 1991), different Citrus rootstocks (Zekri and Parsons 1989, Garcia-Legaz et al. 1993) and excised maize roots (Azaizeh and Steudle 1991), and have been attributed to salt-induced suberization of cortical cells and membrane damage caused by excessive Na+ and Cl− con-centrations in the rhizosphere. However, Shalhevet et al. (1976) did not observe a salt-induced reduction in hydraulic conductance of roots in tomato or sunflower at salt concentra-tions exceeding those used here.
Because we did not use standard methods for determining pressure--volume curves, the differences in osmotic potential between origin and salinity (Figure 5) may not represent the true osmotic adjustment. The response of osmotic potential to salinity, including the differences among genotypes, can be mostly explained by the accumulation of mineral ions (pre-sumably including Cl−) in the needles (Figure 5). This expla-nation is corroborated by the differences in xylem sap osmotic potentials that were observed at the end of the experiment (Table 6). Similarly, Sands and Clarke (1977) concluded that cation accumulation accounted for half of the observed os-motic adjustment in P. radiata. The difference in total accumu-lation of foliar Na between Tamjoute and the other genotypes was much less when expressed on a foliar concentration basis, but it was still positive. Because whole-plant water uptake was presumably less in Tamjoute seedlings, as a result of their lower foliar biomass (Table 3), seedlings of this genotype were less able to avoid transport of salt to their aerial parts than seedlings of the other genotypes. Both accumulation of salt in
Figure 5. Relationship between flowing solution salinity ([NaCl]), turgor (Ψt) and needle predawn osmotic potential (Ψπ, upper panel)
and foliar concentration of sodium ([Na]f, lower panel). Landes = solid
the aerial part of trees (Foster and Sands 1977) and avoidance of salt transfer from roots (Greenway and Munns 1980) are adaptive mechanisms to saline environments (Cheeseman 1988). Although foliar accumulation of Na+ and Cl− ions has negative effects on growth in many nonhalophytic species (Greenway and Munns 1980, McKimmie and Dobrenz 1991, Garcia-Legaz et al. 1993) including P. radiata (Sands and Clarke 1977, Cromer et al. 1982), P. taeda and P. elliottii Engelm. (Land 1974), it may be of adaptive significance in halophytes. Thus, the enhanced transport of Na to needles in Tamjoute seedlings at low levels of salinity should not be interpreted as a disadvantage. The ability to sequester salt in plant parts less prone to salt damage, e.g., tracheids of the stem or epidermal cells for pine trees (Foster and Sands 1977), has adaptive significance in saline environments (Cheeseman 1988). In the present experiment, although the Tamjoute seed-lings were less able to avoid transport of salt to their aerial parts than seedlings of the other genotypes, their stem elongation response was reduced less by salinity than that of seedlings of the other genotypes. However, no differences among geno-types were found for root growth or seedling water transport capacity.
We conclude that the responses of the Tamjoute genotype to saline conditions differ from those of the other two genotypes; however, further studies are needed to elucidate the adaptive significance of this behavior. The other genotypes showed no adaptations to salt stress.
Acknowledgments
This project was supported by contract INRA-ONF ‘‘Dépérissement du Pin maritime sur le littoral Nord-Atlantique,’’ coordinated by J.P. Guyon. The authors thank A. Pauliac, C. Lambrot, N. Rotival and J.L. Grange for their collaboration during this experiment, J.P. Guyon as manager of the project, and Mme A. Nguyen-Queyrens for helpful comments on the manuscript.
References
Azaizeh, H. and E. Steudle. 1991. Effects of salinity on water transport of excised maize (Zea mays L.) roots. Plant Physiol. 97:1136--1145. Baradat, P. and A. Marpeau-Bezard. 1988. Le Pin maritime Pinus
pinaster Ait. Biologie et Génétique des terpènes pour la
connais-sance et l’amélioration de l’espèce. Thèse de Doctorat d’Etat. Uni-versité de Bordeaux I, Talence, France, 439 p.
Bedunah, D. and M.J. Trlica. 1979. Sodium chloride effects on carbon dioxide exchange rates and other plant and soil variables of ponder-osa pines. Can. J. For. Res. 9:349--353.
Brugnoli, E. and M. Lauteri. 1991. Effects of salinity on stomatal conductance, photosynthetic capacity, and carbon isotope discrimi-nation of salt-tolerant (Gossypium hirsutum L.) and salt-sensitive (Phaseolus vulgaris L.) C3 non-halophytes. Plant Physiol.
95:628--635.
Cheeseman, J.M. 1988. Mechanisms of salinity tolerance in plants. Plant Physiol. 87:247--550.
Cromer, R.N., K.G. Eldridge, D. Tompkins and N.J. Barr. 1982. Intraspecific variation in the response of Pinus radiata to saline and water waste. Aust. For. Res. 12:203-- 215.
Downton, W.J.S. and B.R. Loveys. 1981. Abscisic acid content and water relations of salt-stressed grapevine leaves. Aust. J. Plant Physiol. 8:443--453.
Downton, W.J.S., B.R. Loveys and W.J.R. Grant. 1990. Salinity effects on the stomatal behaviour of grapevine. New Phytol. 116:499--503. Foster, R.C. and R. Sands. 1977. Response of radiata pine to salt stress.
II. Localization of chloride. Aust. J. Plant Physiol. 4:863--875. Garcia-Legaz, M.F., J.M. Ortiz, A. Garcia-Lidon and A. Cerda. 1993.
Effect of salinity on growth, ion content and CO2 assimilation rate
in lemon varieties on different rootstocks. Physiol. Plant. 89:427--432.
Greenway, H. and R. Munns. 1980. Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol. 31:149--190.
Guyon, J.P. 1991. Dépérissement du Pin maritime en Vendée. Les causes écologiques. Ann. Sci. For. 48:333--346.
Huyn, H. and L.S. Feldt. 1970. Conditions under which mean square ratios in repeated measurements designs have exact F-distributions. J. Am. Stat. Assoc. 65:1582--1589.
Khalil, A.A.M. and J. Grace. 1993. Does xylem sap ABA control the stomatal behaviour of water stressed sycamore (Acer
pseudopla-tanus L.) seedlings. J. Exp. Bot. 264:1127--1134.
Land, S.G. 1974. Depth effects and genetic influences on injury caused by artificial sea water floods to loblolly pine and slash pine seedlings. Can. J. For. Res. 4:179--185.
Leonardi, S. and W. Fluckiger. 1986. The influence of NaCl on leaf water relations and the proportions of K, Na, Ca, Mg and Cl in epidermal cells of Fraxinus excelsior L. Tree Physiol. 2:115--121. Loustau, D., A. Granier and F. El Hadj Moussa. 1989. Evolution
saisonnière du flux de sève dans un peuplement de Pin maritime. Ann. Sci. For. 46:599--618.
Magnin, H. 1990. Dépérissement du Pin maritime sur la côte vendéenne. Etat sanitaire du peuplement et écologi du dépérisse-ment. Mémoire de fin d’étude ENITEF, ONF Le Mans, 80 p. McKimmie, T. and A.K. Dobrenz. 1991. Ionic concentrations and
water relations of alfalfa seedlings differing in salt tolerance. Agron. J. 83:363--367.
Nguyen, A. and A. Lamant. 1989. Variation in growth and osmotic regulation of roots of water-stressed maritime pine (Pinus pinaster Ait.) provenances. Tree Physiol. 5:123--133.
Pezeshki, S.R. 1992. Response of Pinus taeda L. to soil flooding and salinity. Ann. Sci. For. 49:148--158.
Richardson, S.G. and K.J. McCree. 1985. Carbon balance and water relations of sorghum exposed to salt and water stress. Plant Physiol. 79:1015--1020.
Salim, M. 1991. Change in water conducting properties of plant roots by nutrition and salt stress. J. Agron. Crop Sci. 166:285--287. Sanchez-Blanco, M.J., M.C. Bolarin, J.J. Alarcon and A. Torecillas.
1991. Salinity effects on water relations in Lycopersicon
esculen-tum and its wild tolerant relative species L. pennellii. Physiol. Plant.
83:269--274.
Sands, R. and A.R.P. Clarke. 1977. Reponse of radiata pine to salt stress. I. Water relations, osmotic adjustment and salt uptake. Aust. J. Plant Physiol. 4:637--646.
Saur, E., C. Lambrot, D. Loustau, N. Rotival and P. Trichet. 1995. The growth and uptake of mineral elements in response to sodium chloride of three provenances of maritime pine. J. Plant Nutr. 18:243--256.
Seeman, J.R. and T.D. Sharkey. 1986. Salinity and nitrogen effects on photosynthesis, ribulose-1,5-biphosphate carboxylase and metabo-lite pool sizes in Phaseolus vulgaris. Plant Physiol. 82:555--560. Shalhevet, J., E.V. Maas, G.J. Hoffman and G. Ogata. 1976. Salinity
Sun, D. and G. Dickinson. 1993. Responses to salt stress of 16
Eucalyptus species, Grevillea robusta, Lophostemon confertus and Pinus caribea var. hondurensis. For. Ecol. Manage. 60:1--14.
Van der Moezel, P.G., G.V.N. Pearce-Pinto and D.T. Bell. 1991. Screening for salt and waterlogging tolerance in Eucalyptus and
Melaleuca species. For. Ecol. Manage. 40:27--37.
Walker, M.A. and E.B. Dumbroff. 1981. Effect of salt stress on abscisic acid and cytokinin levels in tomato. Z. Pflanzenphysiol. 101:461--470.
Zekri, M. and L.R. Parsons.1989. Growth and root hydraulic conduc-tivity of several citrus rootstocks under salt and polyethylene glycol stresses. Physiol. Plant. 77:99--106.
Zerbi, G., D.R. Lecain and J.A. Morgan. 1990. Concurrent action of salinity and water stress on leaf gas exchange and water relations in tomato. J. Hortic. Sci. 65:675--681.
![Table 3. Genotype and salinity effects on dry matter production. The table gives the results of the analysis of variance (Fmmolthe treatment or total dry matter per plant, [NaCl] is the salinity (mmol), values estimated for the salinity effect](https://thumb-ap.123doks.com/thumbv2/123dok/1014887.923641/4.612.56.279.361.631/genotype-salinity-production-fmmolthe-treatment-salinity-estimated-salinity.webp)
![Table 6. Estimates of model parameters and comparison of means forsalinity and genotype effects on the predawn osmotic water potentialsalinity [NaCl]where denotes the genotype, mean osmotic potential among genotypes at NaCl = 0 (mm dayof cellular sap](https://thumb-ap.123doks.com/thumbv2/123dok/1014887.923641/5.612.68.238.282.561/estimates-parameters-comparison-forsalinity-potentialsalinity-genotype-potential-genotypes.webp)
![Figure 5. Relationship between flowing solution salinity ([NaCl]),turgor (and foliar concentration of sodium ([Na]line, Iberian = dashed-dotted line, and Tamjoute = dashed line](https://thumb-ap.123doks.com/thumbv2/123dok/1014887.923641/6.612.57.265.76.376/figure-relationship-flowing-solution-salinity-concentration-iberian-tamjoute.webp)