Plant cell walls as targets for biotechnology
Clint Chapple
∗
and Nick Carpita
†
Plants are the sources of major food, feed, and fiber products that are used globally. This past year has seen advances in our understanding of the enzymes that modify wall architecture, the cloning of the first cellulose synthase gene, and revisions to the lignin biosynthetic pathway. These discoveries have facilitated the development of new strategies to alter cell wall properties in transgenic plants.
Addresses
∗Department of Biochemistry and†Department of Botany and Plant
Pathology, Purdue University, West Lafayette, Indiana 47907, USA
∗e-mail: [email protected]
†e-mail: [email protected]
Current Opinion in Plant Biology1998,1:179–185 http://biomednet.com/elecref/1369526600100179
Current Biology Ltd ISSN 1369-5266 Abbreviations
4CL hydroxycinnamoyl CoA ligase
CAD hydroxycinnamoyl alcohol dehydrogenase CCR hydroxycinnamoyl CoA reductase C4H cinnamate-4-hydroxylase EST expressed sequence tag FSH ferulate-5-hydroxylase
OMT caffeic acid/5-hydroxyferulic acidO-methyltransferase PAL phenyl ammonia-lyase
PGase polygalacturonase PME pectin methylesterase
XET xyloglucan endotransglycosylase
Introduction
Herbicide- and insect-resistant plants are now in mass production, and the first genetically engineered plant oils and other products are reaching the market-place. As the range of products produced by transgenic plants continues to broaden, plant cell walls have now become the targets for engineering. Recently, many enzymes that function in the assembly, modification, and turnover of wall polysaccharides have been purified and their cDNAs have been cloned but we have only recently begun to identify the genes responsible for polysaccharide synthesis. A major milestone just passed was the identification of a gene encoding a catalytic subunit of cellulose synthase [1••] and proof of its function by complementation of a temperature-sensitive synthase mutant [2••]. These discoveries have indicated that many previously unknown and unrecognized genes are present in the Arabidopsis expressed sequence tag (EST) database that may encode synthases of many non-cellulosic polysaccharides [3]. In comparison, it had been thought that the pathway of lignin biosynthesis was better understood than those that generate cell wall polysaccharides. The analysis of plants that are downregulated in the expression of lignification-associated enzymes, or mutated in their
corresponding genes, indicate that this may not be the case, and that there is much yet to be learned about all aspects of cell wall synthesis and structure. In this brief review, we highlight a few of the advances in the identification of the relevant genes and gene products that either are being or could be manipulated to alter cell wall structures in our crop plants and trees.
Cell walls as food products
Nutritionally, plant cell wall polysaccharides are important dietary fibers. They are used widely in the food industry as gelling and thickening agents. Along with storage proteins, unique features of the cell walls in different seed flours alter baking properties and crumb textures. Cell walls are the principal textural components of fruits and vegetables, and this texture changes markedly during ripening and/or cooking. We are just now learning which components are the important determinants of texture, how these components are assembled, and how the many wall enzymes change wall characteristics.
has ceased and the walls begin to soften.
In addition to large-scale changes in major cell wall polysaccharides, alterations in some of the more minor cell wall polysaccharides also have an impact on cell wall properties. For example, in contrast to what happens in the tomato pericarp, xylans and pectic polysaccharides are extensively degraded and solubilized in the locule during liquefaction [13]. Rhamnogalacturonan I (RG I) is another major pectic polysaccharide of the fruit wall and one that is highly branched with (1→4)-β-D-galactans that can affect wall texture. These RG-I-decorating galactans were immunolocalized to all cells of the pericarp but not the locule or epidermis [14]. Similarly, the de-esterification of methylated pectins catalyzed by PME does not occur uniformly but occurs in distinct block-like domains, indicating that PME activity is spatially restricted [15]. Infrared microspectroscopy is able to non-invasively map esterified and non-esterified pectin domains and orientation of polysaccharides in 10×10µm sections of underivatized tissues [16•]. This technique will be brought to bear on the problems of localized alterations in wall metabolism that impact overall fruit quality.
Not all the architectural changes can be traced strictly to the structure of individual polysaccharides. Waldron et al. [17] have shown that diferulic acid crosslinks of arabinoxylans are critical to cell wall structural rigidity and methods to enhance cross-linking may enhance textural qualities during processing. Another important processing characteristic is wall softening, which is correlated with wall swelling. Redgwell et al. [18•] showed that isolated walls from ripe kiwi fruit swell almost ten-fold greater than do walls from unripe fruit, and this enormous volume change occurs without significant pectin depolymerization. How to prevent breakage of polysaccharide cross-linkages must be included in engineering strategies.
Cellulose biosynthesis and improvement of
fibers
Improvement of plant fibers for use in the textile industry will require an understanding of how glucan chains are packed into a para-crystalline cellulose microfibril, how the microfibrils are oriented around the fiber cells and how, in some instances, non-cellulosic polysaccharides space the microfibrils apart and contribute to unique textures of the fiber. Cellulose synthesis is associated with six-membered particle rosettes located at the plasmamembrane. The full pathway of cellulose biosynthesis, from translocated sugar to the synthesis of a para-crystalline array of several dozen (1→4)-β-D-glucan chains into a single microfibril, is still unknown [19]. For over 30 years, investigators have been stymied in their attempts to stabilize cellulose synthesisin vitro.
Two principal fiber crops are cotton and flax. Despite the lack of a stable cellulose synthase complex in vitro
thought to encode the catalytic subunit for cellulose synthase were first described in the past year [1••]. A bacterial cellulose synthase operon was first described several years ago [20] but use of the bacterial clone as probe for higher plant cellulose synthase proved fruitless. The identification of four domains essential for binding of the UDP-glucose substrate in many (1→4)-β-D-glucosyl transferases [20] proved to be the breakthrough needed to identify the plant homolog [1••] (Figure 1). Arioli et al.[2••] have identified a temperature-sensitive cellulose synthesis mutant of Arabidopsis that is defective in a gene which is a homolog of the cotton CelA genes. In this mutant, the rosette particle arrays were disoriented at non-permissive temperatures and reintroduction of the CelA gene into the mutant restored normal cellulose synthesis at elevated temperatures.
The celA protein is probably only one of several proteins comprising the complete cellulose synthase complex. The finding of a Zn2+-binding domain near the amino
terminus of celA is consistent with the hypothesis that the synthase interacts with at least one other protein [21]. One candidate is a recently-discovered, extracellular, membrane-associated endo-β-D-glucanase [22••]. A similar membrane-associated glucanase gene is also found within an operon essential for cellulose biosynthesis inAcetobacter [23]; however, the role for such an enzyme in cellulose synthesis is unknown. Hydrolases associated with the cel-lulose synthesis machinery may function in a proof-reading capacity to excise mistake linkages and ensure formation of only (1→4)-β-D-glucosyl units.
Not all the biotechnological advances that impact cell walls need to be targeted to the wall by the living cell. Beyond the efforts to understand how cellulose is made, researchers are now considering how the cotton fiber can be modified to impart special properties important to the textile industry [24]. For example, transgenic cotton fibers that make the thermoplastic polyhydroxyalkanoate, were shown to have improved insulating properties [25•]. In this instance, the plastic accumulated in the cytosol of the cotton hair and was heat-pressed into the cellulose after harvest of the mature fibers.
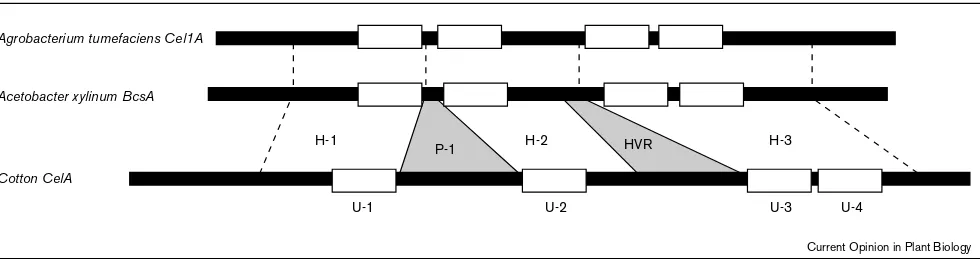
Figure 1
Agrobacterium tumefaciens Cel1A
Acetobacter xylinum BcsA
Cotton CelA
H-2 H-3
H-1
U-1 U-2 U-3 U-4
P-1 HVR
Current Opinion in Plant Biology
Comparison of the bacterial cellulose synthase genesBcsAfromAcetobacter xylinumandCelAfromAgrobacterium tumifacienswithCelA1
from cotton. Three regions (H-1, H-2, and H-3) in the deduced amino acid sequences of the plantCelAgene products possess high similarity with the proteins that are encoded by the bacterial genes [1••]. Within the conserved regions are four highly conserved subdomains (U-1, U-2, U-3, and U-4) previously suggested by Saxenaet al.[20] to be critical for catalysis and/or binding of the substrate UDP-Glucose. The plant
CelAgenes also contain two internal insertions of sequence, one conserved among the plantCelAgenes (P-1) and one hypervariable (HVR), that are not found in the bacterial genes.
are cemented tightly together in late development [26]. Many of the polysaccharides surrounding the cellulose microfibrils in flax are acetylated, a feature that must impact fiber qualities [27]. Among the pectin substances of the developing fiber is an unbranched β-D-galactan that turns over during the maturation of the fiber [28]. This unbranched galactan may prevent the cementing of the fiber bundles until after intrusive growth is complete. Suppression of galactan degradation may facilitate the combining of high-quality fibers used for linen. Flax is readily transformed by Agrobacterium tumefaciens, and, therefore, is particularly amenable to improvement by genetic engineering. Efforts must be directed towards the identification of fiber-specific genes.
Gene discovery through cell wall mutants
The discovery of a temperature-sensitive cellulose syn-thase mutant has been pivotal in the confirmation of the celAgene by complementation [2••]. Cutler and Somerville [3] reported that Arabidopsis contains several homologs of cotton CelA. They also found that a number of other sequences share significant identity to one or more of the suspected UDP-glucose-binding domains. Why plants have so many different CelA and related genes is now a focus of investigation in a number of laboratories. The glucan chains in cellulose microfibrils made by primary and secondary walls are distinguished by differences in their degree of polymerization, which may eventually be traced to different modes of catalysis and organization into para-crystalline arrays. A family of CelA genes may represent the diversity of cellulose synthases needed to build the different kinds of walls that plants make. For example, another Arabidopsis mutant was described whose xylem cells incompletely form secondary walls [29], apparently due to a defect in cell-specific cellulose
deposition. In addition, some of the other related genes may well encode synthases of β-D-xylans, mannans, the backbone of xyloglucan, mixed-linkage β-D-glucans, or callose (1→3β-D-glucan). Almost two dozen cell wall mutants representing 11 loci were discovered by screening a mutagenized Arabidopsis population [30•]. The first of these mutations to be analyzed is the mur1 mutant, which is characterized by the lack of fucose in any polymer of the aerial portion of the plant. The mutation has been traced to a defective gene encoding GDP-D -mannose-4,5-dehydratase, the first step towards formation of GDP-fucose [31]. Interestingly, blockage at this step shunts the carbon to GDP-L-galactose, which is added to a few of the 2-linked D-galactose units of xyloglucan instead of L-fucose [32•]. Whereas such a substitution imparts the mutant with a brittle influorescence stem, the oligosaccharide containing L-galactose in place of L-fucose is able to inhibit auxin-induced growth.
The biotechnological modification of
lignification
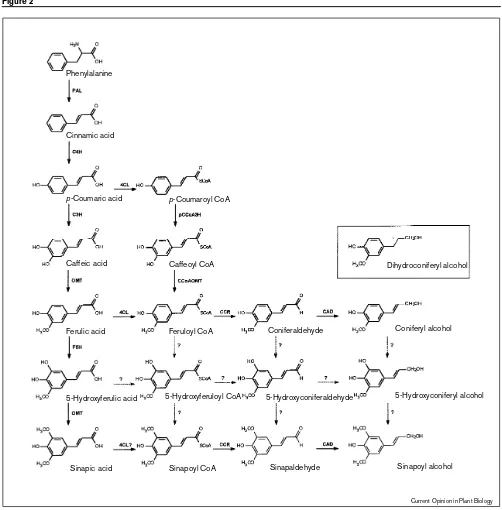
Phenylalanine
Cinnamic acid
p-Coumaric acid p-Coumaroyl CoA
Caffeic acid
Ferulic acid
5-Hydroxyferulic acid
Sinapic acid
Caffeoyl CoA
Feruloyl CoA
Sinapoyl CoA
Coniferaldehyde
5-Hydroxyconiferaldehyde
Sinapaldehyde
Coniferyl alcohol
5-Hydroxyconiferyl alcohol
Sinapoyl alcohol Dihydroconiferyl alcohol
5-Hydroxyferuloyl CoA
Current Opinion in Plant Biology
The biosynthetic pathway leading to the production of monolignols in dicotyledonous plants. The enzymatic steps required include phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H),p-coumarate-3-hydroxylase (C3H), caffeic acid/5-hydroxyferulic acidO-methyltransferase (OMT) ferulate-5-hydroxylase (F5H), hydroxycinnamoyl CoA ligase (4CL)p-coumaroyl CoA-3-hydroxylase (pCCoA3H), caffeoyl CoA
O-methyltransferase (CCoAOMT), hydroxycinnamoyl CoA reductase (CCR), and hydroxycinnamoyl alcohol dehydrogenase (CAD). Reactions shown with question marks and dotted arrows represent reactions that may occur in plants, or appear to occur in transgenic plants that have been altered in the expression of lignification-related genes. The activation of sinapic acid to sinapoyl CoA by 4CL is shown with a question mark to reflect the recent finding of Leeet al.[36••]. The structure of the unusual lignin monomer dihydroconiferyl alcohol found in the pinecad
mutant [45••,46••] is illustrated in the inset, although the route of its biosynthesis is still unclear.
Many attempts at the biotechnological modification of lignification have been aimed at decreasing the total quantity of lignin in plant tissues by targeting the enzymes required for the synthesis of all lignin precursors. Other research has addressed the modification of lignin
makeup of the lignin polymer is far more chemically plastic than was previously supposed.
Manipulation of lignin quantity
Tobacco plants with sense-suppressed levels of pheny-lalanine ammonia-lyase (PAL), the intial enzyme of the phenylpropanoid pathway, were the first plants to be generated with an engineered lignin [34]. These plants were characterized in greater detail in combination with plants downregulated in the second enzyme of the phenylpropanoid pathway, cinnamate-4-hydroxylase (C4H) [35•]. As expected, downregulation of PAL or C4H decreased stem lignin content; however, these two strategies had opposite effects with respect to lignin monomer composition. It is not clear why suppression of these two enzymatic activities led to different phenotypes; however, the authors speculate that they may reflect the perturbance of pathway channeling involved in the flux of phenylalanine toward monolignols.
Experiments aimed at downregulation of 4-coumarate CoA ligase (4CL) activity have also been attempted in several species [36••,37,38••,39]. Lee et al. [36••] found that antisense suppression of 4CL inArabidopsisdecreased lignin guaiacyl content, whereas syringyl content was essentially unchanged. These observations are consistent with the inability of Arabidopsis 4CL preparations to activate sinapate to sinapoyl CoA and indicate that there may be an alternative route for sinapate activation, at least in some plants. Alternatively, there may be other sinapate-utilizing CoA ligases that are sufficiently different in sequence and enzymatic character that they have not been identified to date. The activity of 4CL is also necessary for the so-called ‘alternative’ pathway of lignin biosynthesis. This pathway was originally described in the context of the synthesis of compounds in plant/pathogen interactions [39,40], but has recently been implicated in the biosynthesis of lignin monomers [41]. Although no mutants have been reported for genes of the alternative pathway, and there have been no published reports of the impact of antisensed or co-suppressed alternative pathway gene expression, the pattern of expression of alternative pathway genes is consistent with a role in lignification.
The recent cloning of the gene encoding hydroxycin-namoyl CoA reductase (CCR) [42] has permitted the examination of the utility of CCR-downregulation on lignin quality and quantity [43]. The primary phenotype of tobacco plants carrying antisense CCR constructs was a decrease in total lignin content, although the incorporation of guaiacyl units was somewhat more sensitive to CCR downregulation than was the deposition of syringyl units. The xylem of these plants had an orange-brown coloration, which may be at least partly explained by the elevated levels of ester-linked ferulic acid in the xylem cell walls. Collapse of tracheary elements was observed in the most severely downregulated lines, indicating that the walls of these cells were not sufficiently lignified to withstand the tension generated during transpiration.
Hydroxycinnamoyl alcohol dehydrogenase (CAD) has been downregulated in transgenic tobacco with little effect on the amount of lignin accumulated but with a striking change in lignin composition [44]. When extractable CAD activity was depressed to <10% of wild-type levels, a substantial amount of the lignin polymer was derived from hydroxycinnamaldehydes. An even more extreme example of CAD downregulation and lignin chemistry perturbation was reported recently when a CAD-deficient pine was identified and characterized [45•,46••]. Homozygous cadmutants are relatively normal in appearance but their wood is brown and contains large amounts of ethanol-soluble coniferaldehyde and vanillin. Surprisingly, dihydroconiferyl alcohol — which is normally only a very minor component — was the most abundant component of the lignin of thecadmutant [45•]. Although it is clear that a certain amount of lignin is essential for viability, the normal appearance of these CAD-suppressed plants again indicates that, at least in certain respects, lignin chemistry can be changed dramatically relative to what is normally found in Nature.
Manipulation of lignin quality
Modification of caffeic acid /5-hydroxyferulic acid O-methyltransferase (OMT) expression was aimed at mod-ifying both total lignin content and composition. In those cases where the greatest degree of endogenous OMT sup-pression was achieved [47,48], the lignin of the transgenic plants had a decreased syringyl content and contained novel 5-hydroxyguaiacyl units. In contrast, there was no effect on lignin guaiacyl content, presumably because the alternative pathway provides an OMT-independent route for guaiacyl lignin synthesis. The vascular tissue of the transgenic poplar trees produced in one of these studies [48] was also wine-red in color, similar to that of the brown-midrib-3 mutant of maize, which is defective in the gene encoding OMT [49]. The fact that this strategy did not modify lignin content demonstrates how a thorough knowledge of the interconnecting pathways of lignin biosynthesis will be required for the rational design of metabolic engineering strategies.
C4H promoter [52] was much more efficacious and the syringyl content of these transgenic plants was as high as 95%. Considering that most previous antisense and cosuppression efforts aimed at lignin modification have employed theCaMV 35Spromoter, it would be interesting to revisit these strategies to determine how the use of a lignification-associated promoter might improve the degree of suppression achieved.
Conclusions
The biotechnological improvement of the yield and quality of commercially useful cell wall derived products is now drawing on diverse areas of research including plant genome sequencing and mutant analysis as well as more traditional biochemical approaches. Improvement and maintenance of desirable characteristics, and the introduction of novel traits will require a thorough un-derstanding of the complement of enzymes that function in the biosynthesis, modification and degradation of plant wall components. We are experiencing a period of gene discovery of synthetic enzymes for both lignin and polysaccharides; discoveries that will pave the way for improvement through metabolic engineering.
Acknowledgements
We would like to acknowledge support from the Department of Energy, Division of Energy Biosciences (Contract FG02-94ER20138 to C Chapple, and FG02-88ER13903 to N Carpita). Journal paper No. 15,623 of the Purdue University Agricultural Experiment Station.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
••
1. Pear JR, Kawagoe Y, Schreckengost WE, Delmer DP, Stalker DM: Higher plants contain homologs of the bacterial celA genes encoding the catalytic subunit of cellulose synthase.Proc Natl Acad Sci USA1996,93:12637-12642.
This article reports the cloning of the first cellulose synthase gene (CelA) from a higher plant. The gene was recognized by deduced amino acid se-quence identity of four domains critical for UDP–Glc binding. The higher plantCelAgene contains two additional domains that are not found in bac-terial cellulose synthase genes.
••
2. Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, H ¨ofte H, Plazinski J, Birch R, Cork Aet al.: Molecular analysis of cellulose biosynthesis inArabidopsis.Science1997, 279:717-720.
A temperature-sensitive root-tip swelling mutant ofArabidopsiswas unable to make crystalline cellulose at elevated temperatures. Upon chromosome walking to the gene, this group showed that the gene affected was a homo-log of the cottoncelAgene. They were the first to demonstrate the function of theCelAgene by complementation.
3. Cutler S, Somerville CR:Cellulose synthase: cloningin silico.
Curr Biol1997,7:R108-R111.
4. Thakur BR, Singh RK, Handa AK: Chemistry and uses of pectin. A review.Crit Rev Food Sci Nutr1997,37:47-73.
5. Brummell DA, Labavitch JM:Effect of antisense suppression of endopolygalacturonase activity on polyuronide molecular weight in ripening tomato fruit and in fruit homogenates.Plant Physiol1997,115:717-725.
6. Dominguez-Puigjaner E, Llop I, Vendrell M, Prat S:A cDNA clone highly expressed in ripe banana fruit shows homology to pectate lyases.Plant Physiol1997,114:1071-1076.
β
locules of wild-type and mutant tomato fruit.Plant Physiol
1996,111:1313-1319.
8. O’Donoghue EM, Somerfield EM, deVre LA, Heyes JA: Developmental and ripening-related effects on the cell wall pepino (Solanum muricatum) fruit.J Sci Food Agricul1997, 73:455-463.
9. Carpita N, McCann M, Griffing LR:The plant extracellular matrix: news from the cells frontier.Plant Cell1996,8:1451-1463. 10. Cosgrove DJ:Relaxation in a high-stress environment: the
molecular bases of extensible cell walls and cell enlargment.
Plant Cell1997,9:1031-1041.
11. Nishitani K:The role of endoxyloglucan transferase in the organization of plant cell walls.Intern Rev Cytol1997, 173:157-206.
•
12. Rose JKC, Lee HH, Bennett AB:Expression of a divergent expansin gene is fruit-specific and ripening-regulated.Proc Natl Acad Sci USA1997,94:5955-5960.
The discovery of a tissue-specific expansin gene and the association of its expression with ripening suggests that wall softening may occur without extensive depolymerization of the wall matrix polymers.
13. Cheng CW, Huber DJ:Alterations in structural polysaccharides during liquefaction of tomato locule tissue.Plant Physiol1996, 111:447-457.
14. Jones L, Seymour GB, Knox JP: Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-D-galactan. Plant Physiol1997,113:1405-1412. 15. Steele NM, McCann MC, Roberts K:Pectin modification in cell
walls of ripening tomatoes occurs in distinct domains.Plant Physiol1997,114:373-381.
•
16. McCann MC, Chen L, Roberts K, Kemsley EK, S ´en ´e C, Carpita NC, Stacey NJ, Wilson RH:Infrared microspectroscopy: sampling heterogeneity in plant cell wall composition and architecture. Physiol Plant1997,100:729-738.
The authors of this paper review the utility of Fourier transform infrared mi-croscopy to determine the cellular mapping of pectins and certain polysac-charides to 10 by 10µm resolution non-invasively in underivatized samples. Polarizers can also be used to determine the orientation of specific polysac-charides.
17. Waldron KW, Smith AC, Parr AJ, Ng A, Parker ML: New approaches to understanding and controlling cell separation in relation to fruit and vegetable texture. Trends Food Sci Technol
1997,8:213-221.
•
18. Redgwell RJ, MacRae E, Hallet I, Fischer M, Perry J, Harker R:
In vivoandin vitroswelling of cell walls during fruit ripening.
Planta1997,203:162-173.
An evaluation of the role of the swelling of the cell wall during ripening of certain fruits and its possible relationship to wall softening and cell sepa-ration. An imaginative swelling assayin vitrodemonstrates the remarkable changes that can occur in the fruit wall without significant depolymerization of its components.
19. Brown RM, Saxena IM, Kudlicka K:Cellulose biosynthesis in higher plants.Trends Plant Sci1996,1:149-156.
20. Saxena I, Brown RM Jr, Fevre M, Geremia RA, Henrissat B: Multidomain architecture ofβ-glycosyl transferases: implications for mechanism of action.J Bacteriol1995, 177:1419-1424.
21. Kawagoe Y, Delmer DP:Cotton CelA has a LIM-like Zn binding domain in the N-terminal cytosolic region.Plant Physiol1997, 114(suppl):85.
••
22. Brummell DA, Catala C, Lashbrook CC, Bennett AB:A membrane-anchored E-type endo-1,4-β-glucanse is localized on Golgi and plasma membranes of higher plants.Proc Natl Acad Sci USA1997,94:4794-4799.
Unlike most extracellular polysaccharide hydrolases, a unique membrane-an-chored glucanase was found in developing tomato. The membrane-anchor would place the enzyme at the interface of the plasma membrane and cell wall where it may function in growth-related cell wall metabolism. 23. Matthysse AG, White S, Lightfoot R:Genes required for
cellulose synthesis in Agrobacterium-tumefaciens.J Bacteriol
1995,177:1069-1075.
•
25. John ME, Keller G:Metabolic pathway engineering in cotton: biosynthesis of polyhydroxybutryate in fiber cells.Proc Natl Acad Sci USA1996,93:12768-12773.
Bacterial genes encoding an acetoacetyl-CoA reductase and a polyhydro-xyalkanoate synthase were fused with a cotton fiber-specific promoter. To-gether with the cotton’s ownβ-ketothiolase, a new pathway to this thermo-plastic was generated in the developing fibers.
26. Girault R, Bert F, Rihouey C, Janeau A, Morvan C, Jarvis M: Galactans and cellulose in flax fibres: putative contributions to the tensile strength.Int J Biol Macromol1997,21:179-188. 27. Van Hazendonk JM, Reinerink EJM, deWaard P, van Dam JEG:
Structural analysis of acetyled hemicellulose polysaccharides from fibre flax (Linum usitatissimumL.).Carbohydr Res1996, 291:141-154.
28. Gorshkova TA, Chemikosova SB, Lozovaya VV, Carpita NC: Turnover of galactans and other polysaccharides during development of flax fibers.Plant Physiol1997,114:721-729. 29. Turner SR, Somerville CR:Collapsed xylem phenotype of
Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall.Plant Cell19979:689-701.
•
30. Reiter WD, Chapple C, Somerville CR:Mutants ofArabidopsis thalianawith altered cell wall polysaccharide composition.
Plant J1997,12:335-345.
This is the first comprehensive description of several dozen cell-wall mutants selected by screening of a mutagenized population ofArabidopsis. 31. Bonin CP, Potter I, Vanzin GF, Reiter WD:TheMUR1gene of
Arabidopsis thalianaencodes an isoform of GDP-D -mannose-4,6-dehydratase, catalyzing the first step in the de novo synthesis of GDP-L-fucose.Proc Natl Acad Sci USA1997, 94:2085-2090.
•
32. Zablackis E, York WS, Pauly M, Hantus S, Reiter WD, Chapple CCS, Albersheim P, Darvill A:Substitution ofL-fucose byL -galactose in cell walls of Arabidopsis mur1.Science1996, 272:1808-1810.
Demonstration that an oligosaccharide fragment of xyloglucan with a substi-tution ofL-galactose forL-fucose is able to inhibit auxin-induced growth in excised sections of plant tissue.
••
33. Campbell MM, Sederoff RR:Variation in lignin content and composition. Mechanisms of control and implications for the genetic improvement of plants.Plant Physiol1996,110:3-13. Although lignin biosynthesis has been surveyed many times, this article pro-vides an up-to-date review of our understanding of the pathway, with an emphasis on factors that are thought to control lignin quality and quantity. 34. Elkind Y, Edwards R, Mavandad M, Hedrick SA, Ribak O, Dixon
RA, Lamb CJ:Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene.Proc Natl Acad Sci USA1990,87:9057-9061.
•
35. Sewalt VJH, Ni W, Blount JW, Jung HG, Masoud SA, Howles PA, Lamb C, Dixon RA:Reduced lignin content and altered lignin composition in transgenic tobacco down-regulated in expression of L-phenylalanine ammonia-lyase or cinnamate-4-hydroxylase.Plant Physiol1997,115:41-50.
In this study, cinnamate-4-hydroxylase-suppressed plants showed a de-crease in syringyl residues whereas their guaiacyl residue content was rel-atively unaffected. In contrast, phenyl-ammonia-lyase-suppressed plants de-posited lignins that were enriched in syringyl residues.
••
36. Lee D, Meyer K, Chapple CC, Douglas CJ:Down-regulation of 4-coumarate:CoA ligase (4CL) inArabidopsis: effect on lignin composition and implications for the control of monolignol biosynthesis.Plant Cell1997,9:1985-1998.
In this study, manipulation of lignin content by downregulation of 4CL expres-sion was predicated on a model of the lignin biosynthetic pathway where activation of hydroxycinnamic acids to their corresponding CoA esters is required prior to their two-step reduction to the corresponding alcohols. The fact that syringyl lignin was essentially unchanged in these 4CL downregu-lated plants suggests that there may be an alternative route for sinapic acid activation, at least inArabidopsis.
37. Kajita S, Katayama Y, Omori S:Alterations in the biosynthesis of lignin in transgenic plants with chimeric genes for 4-coumarate:coenzyme A ligase.Plant Cell Physiol1996, 37:957-965.
••
38. Kajita S, Hishiyama S, Tomimura Y, Katayama Y, Omori S: Structural characterization of modified lignin in transgenic tobacco plants in which the activity of 4-coumarate:coenzyme A ligase is depressed.Plant Physiol1997,113:871-879. The two papers [37,38••] describe experiments in tobacco similar to those described inArabidopsisby Leeet al.[36••]. 4CL suppression in tobacco has generated plants with a pigmented xylem that has a high content of ester-bound hydroxycinnamic acids. These plants had a lower level of sy-ringyl residues in their lignin as determined by pyrolysis-mass spectrometry, opposite to the effect seen in 4CL-suppressedArabidopsis.
39. Kneusel RE, Matern U, Nicolay K:Formation of trans-caffeoyl CoA fromtrans-4-coumaroyl-CoA by Zn2+-dependent enzymes
in cultured plant cells and its activation by an elictor-induced pH shift.Arch Biochem Biophys1989,269:455-462.
40. Schmitt D, Pakusch A-E, Matern U:Molecular cloning, induction, and taxonomic distribution of caffeoyl-CoA 3-O -methyltransferase, an enzyme involved in disease resistance.
J Biol Chem1991,266:17416-17423.
41. Ye A-H, Kneusel RE, Matern U, Varner JE:An alternative methylation pathway in lignin biosynthesis inZinnia.Plant Cell
1994,6:1427-1439.
42. Lacombe E, Hawkins S, Van Doorsselaere J, Piquemal J, Goffner D, Poeydomenge O, Boudet AM, Grima-Pettenati J:Cinnamoyl CoA reductase, the first committed enzyme of the lignin branch biosynthetic pathway: cloning, expression and phylogenetic relationships.Plant J1997,11:429-441. 43. Piquemal J, Lapierre C, Myton K, O’Connell A, Schuch W,
Grima-Pettenati J, Boudet A:Down-regulation of cinnamoyl-CoA reductase induces significant changes of lignin profiles in transgenic tobacco plants.Plant J1997,13:71-83.
44. Halpin C, Knight ME, Foxon GA, Campbell MM, Boudet AM, Boon JJ, Chabbert B, Tollier M-T, Schuch W:Manipulation of lignin quality by downregulation of cinnamyl alcohol dehydrogenase.
Plant J1994,6:339-350.
•
45. Ralph J, MacKay JJ, Hatfield RD, O’Malley DM, Whetten RW, Sederoff RR:Abnormal lignin in a loblolly pine mutant.Science
1997,277:235-239. See annotation [46••].
••
46. MacKay JJ, O’Malley DM, Presnell T, Booker FL, Campbell MM, Whetten RW, Sederoff RR:Inheritance, gene expression, and lignin characterization in a mutant pine deficient in cinnamyl alcohol dehydrogenase.Proc Natl Acad Sci USA
1997,94:8255-8260.
This paper, together with [45•], reports the characterization of a CAD-de-ficient mutant, and the elegant NMR characterization of its lignin, which is derived partly from dihydroconiferyl alcohol.
47. Atanassova R, Favet N, Martz F, Chabbert B, Tollier M-T, Monties B, Fritig B, Legrand M:Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequences in sense and antisense orientation.Plant J1995,8:465-477.
48. Van Doorsselaere JD, Baucher M, Chognot E, Chabbert B, Tollier M-T, Petit-Conil M, Lepl `e J-C, Pilate G, Cornu D, Monties Bet al.: A novel lignin in poplar trees with a reduced caffeic acid/5-hydroxyferulic acidO-methyltransferase activity.Plant J1995, 8:855-864.
49. Vignols F, Rigau J, Torres MA, Capellades M, Puigdom ¨enech P: Thebrown midrib3(bm3) mutation in maize occurs in the gene encoding caffeic acidO-methyltransferase.Plant Cell
1995,7:407-416.
50. Chapple CCS, Vogt T, Ellis BE, Somerville CR:AnArabidopsis
mutant defective in the general phenylpropanoid pathway.
Plant Cell1992,4:1413-1424.
51. Meyer K, Cusumano JC, Somerville C, Chapple CCS: Ferulate-5-hydroxylase fromArabidopsis thalianadefines a new family of cytochrome P450-dependent monooxygenases.Proc Natl Acad Sci USA1996,93:6869-6874.
52. Bell-Lelong DA, Cusumano JC, Meyer K, Chapple C: Cinnamate-4-hydroxylase expression inArabidopsis. Regulation in response to development and the environment.Plant Physiol