B R Doshi School of Biosciences, Sardar Patel University, Vallabh Vidyanagar, Gujarat, India
Hydrothermically processedProsopis juliflora(PJ) seed meal as a supplementary diet forLabeo rohitais found to be rich in protein (330 g kg)1) having antinutritional factors in permis-sible limits and containing essential amino acids adequately except lysine, methionine and cysteine. Ten iso-nitrogenous and iso-energetic diets with crude, soaked and autoclaved seed meal at 20%, 35% and 50% replacement of fish meal were tested (D1–D9, respectively). The growth of fish (weight gain, specific growth rate, feed conversion ratio and protein efficiency ratio) fed diet D4 (soaked seed meal at 20% replacement) was higher among the test diets, but lower than reference diet (RD). Diets with 50% seed meal resulted in lowering of growth, carcass composition, digestive enzyme activity and digestibility compared to test diets at 20% and 35% inclusion levels in the respective groups. Hydrothermi-cally processed seed meal improved the growth compared to unprocessed one, though not up to RD level. This could be because of amino acid imbalance and presence of non-starch polysaccharides in seed meal. Looking to the easy availability and its nutritional quality, processed PJ seed meal can be incorporated in carp diet at lower inclusion level.
KEY WORDS
KEY WORDS: antinutritional factors, diets, growth,
hydroth-ermically processed,Labeo rohita,Prosopis julifloraseed meal
Received 7 May 2009, accepted 23 October 2009
Correspondence: Dr Sujata S. Bhatt, B R Doshi School of Biosciences, Sardar Patel University, Vallabh Vidyanar 388120, Gujarat, India. E-mail: [email protected]
The formulation of feed with high nutritional quality and its availability as an alternative to fish meal are known to be very
important aspects of intensive aquaculture. Emphasis has been given for the development of cost-effective feed using plant proteins. Oil seed by-products and legume seeds as plant protein sources in aqua feed have shown promising results, but the presence of antinutritional factors (ANFs) and defi-ciency of essential amino acids (EAAs) are known to adversely affect their use in complete replacement of fish meal (Tacon 1993, 1997). However, processing technologies have helped in removal of ANFs and improvement in the nutritional quality of feed to some extent (Wee 1991; Fagbenro 1999).
Legume seeds can form an important component of fish feed because they are rich in protein, lipid and carbohy-drates. Soybeans have been widely used as plant protein source in feed because of their high protein content in spite of the presence of ANFs and low level of certain EAAs (Wilson & Poe 1985; Robaina et al. 1995). Prosopis juli-flora(PJ) (mesquite) is a leguminous tree and grows in arid and semi-arid regions of the world (Batista et al.2002). In Gujarat (India), PJ trees grow extensively in semi-dried regions (Shukla et al. 1990). Mesquite pods form an important feed source for livestock in many areas of world (Riveros 1992). Because of their palatability and nutritional value, pods of PJ are largely used for feeding dairy and beef cattle with good nutritional and economic results (Silvaet al.2007). Products from this plant have also been used for human consumption in bread, biscuits, sweeties, syrup and liquors (Van Den Eynden et al. 2003). Infor-mation available on PJ mainly deals with PJ pods and leaves (Lyon et al. 1988), whereas not much is reported about use and nutritive value of PJ seeds. Recently, effect of prosopis seed meal on the growth performance of broiler chicken has been reported (Yusuf et al. 2008). Looking to the large-scale availability of PJ pods in Gujarat and comparatively high protein content (306–373 g kg)1) in the seeds (Shukla et al. 1990), we evaluate PJ seed meal as a plant protein source in the supplementary
201117;e164–e173
. . . .
doi: 10.1111/j.1365-2095.2009.00745.x
feed for Labeo rohita (rohu) fingerlings by partial replac-ement of fish meal. Garg et al. (2002) and Hossain et al. (2001) have reported improvement in the nutritional quality of leguminous seeds by hydrothermical processing, and their incorporation in the feed have improved the growth of the carps. In the present investigation, to elim-inate/reduce the levels of ANFs like tannin, trypsin inhibitor (TI) and phytic acid as well as to improve the nutritional quality of the PJ seed meal, we have processed the seed meal by water soaking and by soaking + auto-claving. Test diets have been formulated by replacing the fish meal at 20%, 35% and 50% levels by each of unprocessed as well as processed PJ seed meal in fish meal–based supplementary feed (D1–D9), and their effects on the growth of carp are compared with the fish meal– based reference diet (RD).
The present study seems to be the first attempt to investi-gate the use of PJ seed meal as a plant protein source in the diet for cultivable fish species including Indian major carps (IMCs) and is aimed at evaluating the nutritional potential of raw and processed PJ seed meal. An attempt is also made to determine proper processing techniques to eliminate/reduce the levels of ANFs.
PJ seeds were obtained from Khodiyar Seed Agency, Bhavnagar, Gujarat, India. PJ seeds were soaked in water
(1 : 3, w v)1) for 6, 12 and 24 h. Another batch of seeds was soaked in the same way and autoclaved for 20 min at 120C at 15 lb cm)2. Water soaked and autoclaved seeds were oven dried at 50–60C for 24 h and grinded
separately along with unprocessed PJ seeds into powder to pass through 0.5-mm sieve. Because TI, tannin and phytic acid contents of the seed meal were minimum in 24-h water soaked (PJ24) and 24-h water soaked followed by autoclaved samples (PJ24+) (data not shown), they have been used in the formulation of experimental feed along with unprocessed seed meal (PJN). The fish meal of Indian origin was prepared from javla (small shrimps from the cod end of dol net, from Jafarabad, Gujarat), oven dried at 50–60C for 24 h and grinded into powder. Before diet
formulation, the proximate composition of feed ingredi-ents (Table 1) and amino acid analysis of unprocessed, water soaked and autoclaved PJ seed meals along with fish meal were performed (Table 2). Ten iso-nitro-genous (360 g kg)1 crude protein) and iso-energetic (15 564 kJ kg)1 metabolic energy content) diets were formulated (Table 3). Each of crude, water soaked and autoclaved PJ seed meals was included in the experimental diets at 200, 350 and 500 g kg)1 replacement of fish meal and designated as D1–D9, respectively; for the control diet, fish meal was used as a main protein source and designated as RD (Table 3). To make all the feed iso-nitrogenous, corn gluten (obtained from the local market) was used to adjust total nitrogen content. Chromic oxide was used as an external marker for the nutrient digestibility study, and bentonite was used as a binder. The
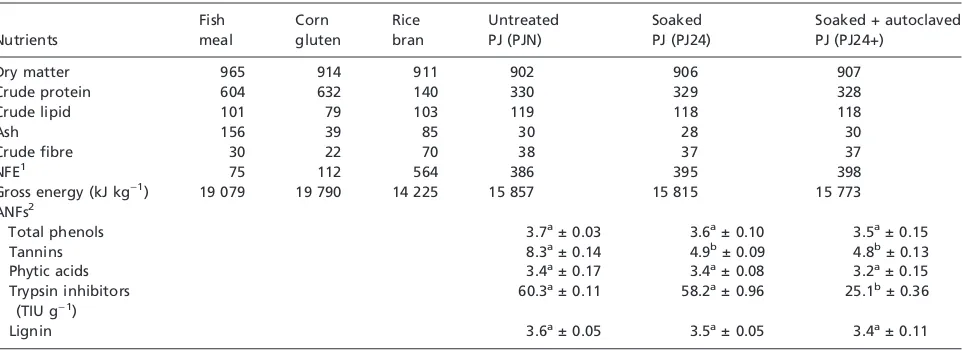
Table 1 Proximate composition of diet ingredients and antinutritional factors (ANFs) of treated and untreatedProsopis juliflora(PJ) seed meal
(as g kg)1dry matter unless otherwise stated)
Nutrients
Fish meal
Corn gluten
Rice bran
Untreated PJ (PJN)
Soaked PJ (PJ24)
Soaked + autoclaved PJ (PJ24+)
Dry matter 965 914 911 902 906 907
Crude protein 604 632 140 330 329 328
Crude lipid 101 79 103 119 118 118
Ash 156 39 85 30 28 30
Crude fibre 30 22 70 38 37 37
NFE1
75 112 564 386 395 398
Gross energy (kJ kg)1) 19 079 19 790 14 225 15 857 15 815 15 773
ANFs2
Total phenols 3.7a± 0.03 3.6a± 0.10 3.5a± 0.15
Tannins 8.3a± 0.14 4.9b± 0.09 4.8b± 0.13
Phytic acids 3.4a± 0.17 3.4a± 0.08 3.2a± 0.15
Trypsin inhibitors (TIU g)1)
60.3a± 0.11 58.2a± 0.96 25.1b± 0.36
Lignin 3.6a± 0.05 3.5a± 0.05 3.4a± 0.11
1
Nitrogen-free extract (NFE) calculated as 100)% (moisture)crude protein)crude lipid)ash)crude fibre).
2Data are mean values ± SE. Values with the same superscript letters in the same row are not significantly different (P>0.05) from each
other.
feed ingredients were mixed and made into moist pellets of 3 mm in diameter with hand pelletizer, oven dried at 55–60C for 24 h and stored at 4C.
Rohu fingerlings were obtained from Gujarat Govt. Fish Seed Centre, Navali (Dist. Anand), acclimatized to
Table 2 Amino acid composition of feed ingredients
Amino acid (g per 16 g N2)
Fish meal (javla)
PJ normal
PJ 24 water soaked
PJ 24+ water soaked + autoclaved
Requirement of carp1
Aspartic acid 9.43 8.73 10.49 10.75
Threonine 3.32 3.49 2.98 3.22 1.3
Serine 5.33 4.98 4.32 4.56
Glutamic acid 12.0 28.39 27.03 26.51
Proline 0.27 5.02 4.41 3.81
Glycine 8.0 7.77 7.33 7.38
Alanine 9.62 7.39 7.26 6.93
Cysteine 0.47 0.31 0.42 0.29
Valine 8.01 5.02 5.05 4.87 1.2
Methionine 8.60 0.35 0.65 0.38 0.6
Isoleucine 6.73 2.70 2.90 2.65 0.9
Leucine 8.02 8.60 9.09 8.22 1.6
Tyrosine 2.03 1.93 2.25 1.83 2.1
Phenylalanine 3.24 4.68 4.92 4.84 1.2
Histidine 1.28 3.43 3.28 3.22 0.6
Lysine 9.32 0.74 0.75 0.95
Arginine 1.13 0.93 1.31 1.77 1.5
Tryptophan 2.09 2.48 2.55 2.45 0.2
PJ,Prosopis juliflora.
1Data for essential amino acids requirement of carp from Ogino (1980).
Table 3 Ingredient and composition of the diets used in the study
Diets
Reference diet
Prosopis juliflora (PJ) seed meal
Raw Soaking Soaking + autoclaving
D1 D2 D3 D4 D5 D6 D7 D8 D9
Ingredient (g kg)1dry weight)
Fish meal 400 294 244 170 294 244 170 294 244 170
PJ – 200 350 500 200 350 500 200 350 500
Corn gluten1 120 120 120 120 120 120 120 120 120 120
Rice bran 394 300 200 124 300 200 124 300 200 124
Premix2 25 25 25 25 25 25 25 25 25 25
Oil premix3 50 50 50 50 50 50 50 50 50 50
Bentonite4 01 01 01 01 01 01 01 01 01 01
Cr2O35 10 10 10 10 10 10 10 10 10 10
Proximate composition (g kg)1on dry matter basis)
Crude protein 372 361 366 360 361 366 360 360 366 359
Lipid 92 95 97 99 95 97 99 95 97 99
Ash 112 91 77 62 90 77 61 91 77 61
Crude fibre 44 41 38 36 41 38 35 41 37 35
Nitrogen-free extract 380 412 422 443 413 423 445 417 423 445
Gross energy (kJ kg)1) 15 899 15 606 15 564 15 355 15 564 15 522 15 313 15 564 15 480 15 271
1Corn gluten and Rice bran were purchased form Charotar Animal Feeds Pvt. Ltd., GIDC, V.V. Nagar, Gujarat, India.
2Vitamin and mineral mixture (Vitaminetes Forte; Roche Products Ltd., Mumbai, Maharashtra, India).
3Oil premix [2 corn oil (Tirupati active; N.K. Proteins Co., Mehsana, Gujarat, India) : 1 cod liver oil (Seacod; Universal Medicare Pvt. Ltd.,
Mumbai, Maharashtra, India)].
4Bentonite purchased from Gujarat Minechem, Bhavnagar, Gujarat, India.
5Chromic oxide (Cr
2O3): Qualigens
.
laboratory conditions for 15 days and fed with a 1 : 1 mixture of finely powdered rice bran and groundnut oil cake. The feeding trial was conducted in 150-L glass aquaria (0.91·0.38·0.45 m3). Fingerlings (mean weight
3.82 ± 0.06 g) were stocked at a density of 25 fishes per aquarium with three replicates for each treatment group. Unchlorinated tube well water was used for the experiment. The fishes were fed with the formulated feed twice a day at 9.00 and 15.00 h at the rate of 5% of the body weight per day for 60 days. The fishes were weighed every week and the feeding ration adjusted accordingly. During experimentation, continuous aeration was provided and temperature was maintained at 30C. The water quality parameters like pH, dissolved oxygen and total organic carbon were monitored weekly following the methods of American Public Health Association (APHA 1980) and are found to be 7.2–7.9, 6.2–7.2 and 107–115 mg L)1, respectively.
Faeces were collected once in the morning during the last 2 weeks of the experimentation by the method of Spyridakis et al. (1989). Uneaten feed was siphoned out from the aquaria after the last feeding in the evening. Faeces collected from triplicate treatment groups were pooled, oven dried at 60C and stored for digestibility study. Pooled faecal
samples for each treatment were analysed separately. At the termination of experiment, five fishes were killed from each aquarium and analysed for carcass composition and intestinal digestive enzyme activity.
Feed ingredients, experimental feed, faecal samples and fish carcass were analysed for their proximate composition fol-lowing Association of Official Analytical Chemists (AOAC 1990) as follows: moisture was determined by oven drying at 105C for 24 h, protein (N·6.25) by Micro-Kjeldahl
digestion and distillation after acid digestion, ash by ignition at 550C in a Muffle furnace to constant weight, crude fibre
by Sigma kit (Catalog no. TDF-100A; Sigma, St. Louis, Missouri, USA), lipid by Folchet al.(1957), total carbohy-drate by anthrone method (Hedge & Hofreiter 1962) and amino acids were analysed using HPLC method (Ishidaet al. 1981; Central Institute of Fisheries Technology, Cochin, Kerela, India). In processed and unprocessed test feed, total phenol, tannin, phytic acid, TI and lignin were determined by spectrophotometric methods (Stafford 1960; Kakade et al. 1969; Schanderi 1970; Wheeler & Ferrel 1971; Molick & Singh 1980, respectively). The energy contents of the diets were calculated using the average caloric conversion factors 9.45, 4.10 and 5.65 kcal g)1 for lipid, carbohydrate and
protein, respectively (Henken et al. 1986). Nitrogen-free extract was computed by taking the sum of values for crude protein, crude lipid, ash, crude fibre and moisture and sub-tracting this from 100 (Maynardet al.1979). Chromic oxide in the diets and faeces was estimated following the method of Furukawa & Tsukahara (1966).
Chemicals used for the analysis of earlier mentioned parameters were purchased from Qualigens
; Qualigen Fine Chemicals, Mumbai, Maharashtra, India.
The gut was rinsed with chilled distilled water, homogenized with tissue homogenizer in cold (4C) phosphate buffer pH 7.2 (1 : 10 w v)1), centrifuged (10 000g·10 min at 4C) and
supernatant stored at)20C for enzyme analysis. The con-centration of soluble protein in pooled samples was determined by the method of Lowry et al.(1951). In the supernatant, a-amylase, protease and lipase activities were assayed by using soluble starch (Qualigens), casein (Qualigens) and olive oil (Figaro, Madrid, Spain) as substrates, respectively (Bernfeld 1955; Kunitz 1974 and Rathelotet al.1975).
The apparent nutrient digestibility (AD) of the diets was calculated according to Choet al.(1982), AD = 100)100· (% Cr2O3in diet/% Cr2O3in faeces)·(% nutrient in faeces/ % nutrient in diet).
Growth parameters were calculated according to Steffens (1989). The data were subjected to aANOVAANOVA, and the
signif-icance of the difference between means was determined by TukeyÕs multiple range test (P<0.05) using the SPSS version 15 (Chicago, Illinois, USA). Values are expressed as means ± SE.
The proximate composition of the feed ingredients and test feed is shown in Table 1. There was not much variation in nutrient content among different processed and unprocessed PJ groups (PJN, PJ24 and PJ24+). The estimated ANF contents of different PJ seed meal groups are presented in
Table 1. Hydrothermical treatment of PJ seed meal resulted in significant decrease (P<0.05) in TI in all the auto-claved groups and in tannin (P<0.05) in PJ24 as well as PJ24+ groups (n= 3). Other ANFs like phytic acid, total phenol and lignin are also decreased in processed groups (Table 1). The amino acid content of fish meal and crude as well as processed PJ seed meal is presented in Table 2. None of the test feed was deficient in any of the EAAs except lysine when compared to the requirement of carp (Ogino 1980). However, the test feeds are found to be deficient in sulphur amino acids (cystine + methionine) and lysine as compared to the requirement of rohu (Singh 1987). In comparison to test feed, methionine, lysine, valine and isoleucine levels were observed to be higher in fish meal. Soaking of the seed meal (PJ24) improved the level of majority of EAAs, whereas they have been found decreased in autoclaved group (PJ24+) as compared to unprocessed group (PJN).
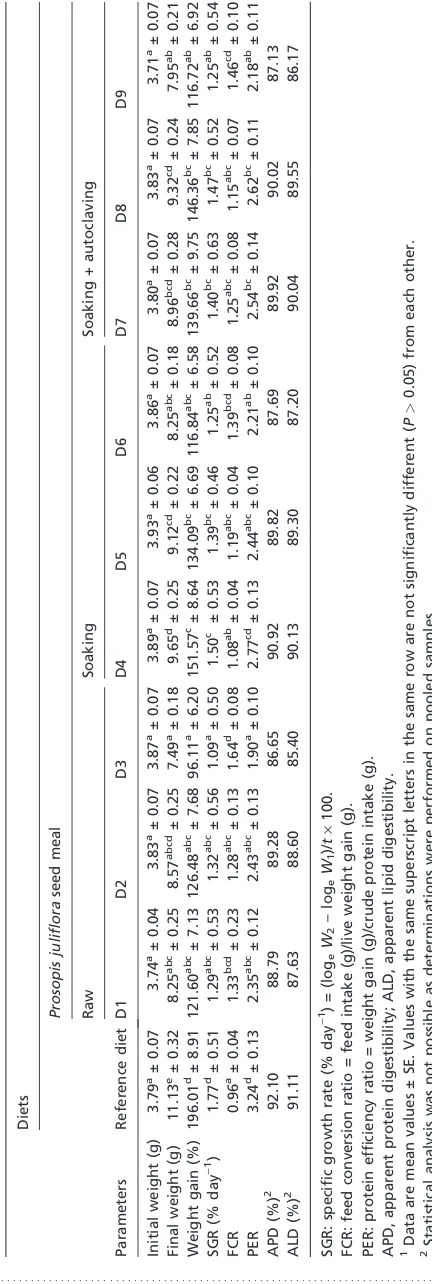
Rohu fingerlings readily accepted the experimental diets and were observed to feed aggressively during experimentation. No mortality was observed in any of the dietary groups during the present study. The growth performance in terms of per-centage weight gain, specific growth rate (SGR), feed con-version ratio (FCR) and protein efficiency ratio (PER) as well as apparent protein digestibility (APD) and apparent lipid digestibility (ALD) are presented in Table 4. Rohu fingerlings fed with the RD had higher level growth performance in terms of per cent weight gain, SGR, FCR and PER, whereas there are not much differences in APD and ALD among different diet groups (RD and D1–D9). Fishes exhibited higher growth rate with processed seed meal as compared to unprocessed seed meal at each individual inclusion level. Diet D4 (200 g kg)1 replacement of fish meal with PJ24) produced better growth in terms of per cent weight gain, SGR, FCR and PER among experimental groups. As compared to RD in D4, the values of FCR and PER are not significantly different, whereas the values of SGR and per cent weight gain are sig-nificantly lower. Inclusion of processed and unprocessed seed meal at the level of 500 g kg)1replacement of fish meal (D3, D6 and D9) depressed all the calculated parameters of growth in comparison to inclusion at 200 and 350 g kg)1level.
Proximate composition of fish carcass and gut digestive enzyme activity is presented in Table 5. Fishes fed RD, D4, Table
D7 and D8 had the higher carcass crude protein and crude lipid as well as higher gut a-amylase, protease and lipase activities. In the diet groups (D3, D6 and D9) with 500 g kg)1 replacement of fish meal with processed and unpro-cessed seed meal protein and lipid deposition in the carcass and gut digestive enzyme activities were lowest among all the diet groups. Carcass moisture and ash content were lowest in the fishes fed with RD.
The results show that the use of hydrothermically processed PJ seed meal as a plant protein source improves the growth of rohu fingerlings as compared to unprocessed seed meal at their respective inclusion levels. The diet with soaked PJ seed meal (D4) at 200 g kg)1replacement of fish meal exhibited higher growth of the fishes among the groups with different test diets, though it was found to be lower as compared to RD. Inclusion of processed seed meal at higher levels and unprocessed seed meal at all inclusion levels failed to achieve the growth of the fishes to a level of performance obtained with fish meal–based RD (Table 4). It is well documented that inclusion of plant protein sources in fish diets results in reduced growth, and this is likely to be caused by the pres-ence of ANFs and amino acid imbalance in plant-based feed (Hossainet al.2001).
Soaking and autoclaving of PJ seed meal resulted in decrease in ANFs like tannin (P<0.05), TI (P<0.05) (PJ24+), phytic acid, total phenol and lignin (Table 1). Hossainet al. (2001), Garg et al. (2002) and several other researchers have also reported the reduction in ANFs of legume seeds by hydrothermical treatment. The presence of tannin has been associated with lower nutritive value and lower biological availability of protein, carbohydrate, amino acids, vitamins and minerals (Makkaret al.1987). Common carp has been shown to tolerate addition of 2% quebracho tannin without any adverse effect on the growth (Francis et al.2001). In the present study, 4.9 and 4.8 g kg)1tannin content of soaked (PJ24) and autoclaved (PJ24+) groups as well as 8.3 g kg)1tannin content of unprocessed group (PJN) (Table 1) seem to be low to cause any adverse effect on the growth of rohu fingerlings. TI has been reported to inhibit growth of animals. Commercial soybean products are reported to show 2–6 mg g)1 TI activity (Synder & Kwon 1987). Carp has not exhibited negative effect on growth when fed with untreated and heat-treated jatropha meal with high TI activity (Makkar & Becker 1999). Hossain et al. (2001) have also ruled out the growth-inhibiting effect of TI
in common carp when fed with sesbania seeds with Ta
4.7–5.2 mg g)1 of TI activity. In the present experiment, significant reduction in TI activity (P<0.05) has been measured in autoclaved group (from 60.3 to 25.1 TIU g)1). Low TI activity in all the test feed groups is unlikely to have caused inhibition of growth in rohu (Table 1). Processing is also found to be effective in reducing the level of phytic acid in PJ seed meal. Phytic acid in feed is known to reduce the bioavailability of minerals and proteins; however, the growth in commonly cultivated fishes like carp, tilapia, trout and salmon is reported not to be affected by phytate-containing ingredients in the diet (Franciset al.2001). Low phytic acid content in processed and crude PJ seed meal (3.2–3.4 g kg)1) (Table 1), mineral supplementation in diet and availability of phosphorous in fish meal suggest that presently observed phytic acid content of different diets should not have inhibited the growth of rohu. Pro-cessing is also found to be effective in reducing the level of total phenol and lignin (Table 1). In different PJ meals, total phenols and lignin were found to be 3.5–3.7 and 3.4– 3.6 g kg)1, respectively (Table 1). Results suggest that soaking and autoclaving of PJ seed meal resulted in decrease in TI, phytic acid, tannin, total phenol and lignin; they do not seem to be responsible for inhibiting the growth of rohu fingerlings.
Test feeds are not found to be deficient in EAAs except cysteine, methionine and lysine as compared to the require-ment of carps (Ogino 1980; Singh 1987). In comparison to the amino acid level of fish meal, valine, methionine, iso-leucine and lysine are found to be lower in all the PJ groups (Table 2). Soaking (PJ24) of PJ seed meal improved the level of EAAs content as compared to unprocessed (PJN) and autoclaved (PJ24+) groups (Table 2). In all the PJ seed meals, EAAs are found to be higher as compared to mustard oil cake and linseed meal (Mukhopadhyay & Ray 2001). In the present study, autoclaving has resulted in some negative effect on the EAA composition of PJ meal except lysine and arginine as compared to unprocessed and soaked groups (Table 2). During autoclaving, extended heating and over processing may have denatured the protein and caused reduction in amino acid availability. Hossain & Jauncey (1990) have reported reduction in lysine in linseed and ses-ame seed after autoclaving. Autoclaving of sesbania meal has not lowered its EAAs content except histidine (Hossainet al. 2001). Calculated EAAs showed that except in cystine, methionine and lysine, none of the PJ groups were deficient in other EAAs.
The results of the present study show that hydrothermi-cally processed PJ seed meal in the feed of rohu found to be effective in improving the growth in terms of per cent weight
gain, SGR, FCR and PER as compared to unprocessed seed meal, but not to the level of growth performance achieved with fish meal–based RD (Table 4). Fishes grew poorly when fed with diet containing graded level of unprocessed seed meal (D1, D2 and D3). The growth of the D4 fishes was moderately higher as compared to fishes fed with other test diets, whereas it was lower than those fed with RD. Inclusion of prosopis seed meal at 20% replacement of soybean meal in the diet of broiler chicken has been reported to have an economic advantage (Yusuf et al. 2008). Inclusion of processed PJ seed meal at higher replacement level (D6 and D9) is found to depress the growth of the fishes (Table 4). Mohantyet al.(1995) also reported poor growth rate in rohu fry fed diets containing graded levels of oil cakes. Deficiency of sulphur amino acids and lysine could be responsible for presently observed lower growth rate of experimental fishes. Another factor appears to be responsible for the reduced growth could be PJ gum (galactomannans), a non-starch polysaccharide. PJ seeds are reported to be rich in PJ seed gum (galactomannans), the main storage carbohydrate lying between the seed coat and cotyledons (Azero & Andrade 2006; Gallao et al. 2007). Guar gum galactomannan is reported to be responsible for reducing the availability of nutrients in the diets of salmonid and is found to be one of the factors responsible for hampering the growth of carp (Storebakken 1985; Garget al.2002). Thus, galactomannan content of PJ seed could be responsible for reduced growth of rohu. Among the test diets, higher growth of fishes with soaked seed meal at 20% replacement level (D4) may be attributed to the availability of EAAs because of the partial replacement of fish meal and presence of ANFs in permissi-ble limit in the formulated feed. The results suggest that hydrothermically processed PJ seed meal appear to be a better plant protein source for increasing the growth of IMCs as compared to raw and processed soybean, moong, cowpea and guar reported by Garget al.(2002) in the feed for rohu. Our results are in agreement with the findings of Garget al. (2002) and Hossain et al. (2001) who have reported improvement of nutritional quality of leguminous seeds by hydrothermical treatment. Fishes fed with RD exhibited highest per cent weight gain (P<0.05), SGR (P<0.05), FCR and PER. Not much difference is observed in the values of FCR among the groups with different test diets and it was found to be lower in RD. The value of PER in D4 is higher among the groups with different test diets, whereas its value in RD is greater. This can be attributed to the availability of good quality protein to the fishes and presence of ANFs in permissible limit in the test diets. The FCR and PER values obtained in the present studies were better than those
obtained by Mukhopadhyay & Ray (2001) and Garget al. (2002) for plant protein–based diet.
Carcass composition and intestinal enzyme activities of fishes were influenced by the processing of seed meal. Fishes fed with soaked and autoclaved seed meal at lower inclusion level (D4, D5, D7 and D8) had higher crude protein and lipid content as compared to their higher inclusion levels (D3, D6, D9) (Table 5). Intestinala-amylase, protease and lipase activities were found to be higher in the fishes fed with soaked (D4) and autoclaved groups (D7, D8) as compared to crude seed meal (D1–D3). Moisture and ash content of carcass are found to be similar with different test diets, though they have increased with higher levels of inclusion of test feed (D3, D6 and D9) and are also found to be higher as compared to RD. Highest crude protein and lipid content as well as lowest moisture and ash content of carcass with highest intestinal enzyme activity have been observed in fishes fed with the RD (Table 5). Similar trend in muscle protein and fat levels were reported in Oreochr-omis niloticus (Keembiyehetty & De Silva 1993) and rain-bow trout (Gomeset al.1993) fed with higher amounts of cowpea and black gram meal as well as pea and rapeseed meals, respectively. This observation is in agreement with Hossain et al. (2001) who reported significantly higher moisture and lowest lipid content of whole body with higher level of sesbania meal in the feed of common carp. Saha & Ray (1998) also observed decrease in muscle protein and fat content as well as significantly low digestive enzyme activ-ities with increasing level of Chuni in the diet of rohu. Deficiency of some of the EAAs and probably presence of non-starch polysaccharides (NSPs) could be responsible for reduced deposition of protein and lipid as well as increased deposition of moisture and ash with increasing concentra-tion of test feed. The muscle protein and lipid content and digestive enzyme activities correlate with the growth pattern of fish fed on different experimental diets.
The APD and ALD of processed PJ seed meal at lower inclusion level (20% and 35%) were found to be quite similar to fish meal–based RD, whereas they were lower with higher inclusion (50%) of seed meal (Table 4). Mukhopadhyay & Ray (2001) observed decreased APD with increased level of incorporated linseed meal protein in rohu fingerlings. Gomes et al.(1993) reported that inclusion of coextruded pea and rapeseed meal in the diet of rainbow trout at 16–24% inclusion level improved digestibility; whereas at higher inclusion level, digestibility was found to be hampered. The pattern of presently observed nutrient digestibility of the diets corresponds to the growth trend of fishes and nutri-tional quality of test diet.
The results of the present study show that processing of PJ seed meal improved the nutritional quality of test diet and growth performance of fishes as compared to unprocessed seed meal, although not to the level of performance obtained with fish meal–based RD. However, processed seed meal can be used in the diet at a lower level replacement of fish meal looking to its easy availability and nutrient utilization in fish. Reduced growth performance of fishes at higher inclusion level of PJ seed meal might be related to amino acid imbal-ance and presence of non-starch polysaccharides (galacto-mannans) of PJ seed meal.
The authors thank the University Grant Commission (UGC) for the research grant [UGC Major Research Project # No.F.31-228/2005 (SR)] provided for this investigation. The authors are very much grateful to the reviewers for very many constructive suggestions that led to thorough revision substantially improving the manuscript.
American Public Health Association (1980) Standard Methods for the Examination of Water and Wastewater, 15th edn. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington, DC, USA.
Association of Official Analytical Chemists (1990)Official Methods of Analysis, 15th edn. AOAC, Washington, DC, USA.
Azero, E.G. & Andrade, C.T. (2006) Characterisation ofProsopis Julifloraseed gum and the effect of its addition toj-carrageenan
systems.J. Braz. Chem. Soc.,17,844–850.
Batista, A., Mutstafa, A., McKinnon, J.J. & Kermasha, S. (2002) Ruminal and intestinal nutrient digestibilities of mesquite ( Proso-pis juliflora) pods.Anim. Feed Sci. Technol.,100,107–112. Bernfeld, P. (1955) Amylaseaandb. In:Methods in Enzymology,
Vol. I (Colowick, S.P. & Kaplan, N.O. eds), pp. 149–158. Academic Press, New York, NY.
Cho, C.Y., Slinger, S.J. & Bayley, H.S. (1982) Bioenergetics of salmonid fishes: energy intake, expenditure and productivity. Comp. Biochem. Physiol.,73B,25–41.
Fagbenro, O.A. (1999) Comparative evaluation of heat-processed Winged bean (Psophocarpus tetragonolobus) meals as partial replacement for fishmeal in diets for African catfish (Clarias gariepinus).Aquaculture,170,297–305.
Folch, J., Lees, M. & Sloane-Stanley, G.H. (1957) A simple method for the isolation and purification of total lipids from animal tissues.J. Biol. Chem.,226,407–509.
Francis, G., Makkar, H.P.S. & Becker, K. (2001) Antinutritional factors present in plant-derived alternate fish feed ingredients and their effect in fish.Aquaculture,199,197–227.
Furukawa, A. & Tsukahara, H. (1966) On the acid digestion for the determination of chromic oxide as an index substance in the study of digestibility of fish feed. Bull. Jpn. Soc. Sci. Fish., 32, 502–506.
Gallao, M.I., Vieira, G.P.I., Medes, N.P.F., Souza, de S.N.A. & Brito, de S.E. (2007) Reserve mobilisation in mesquite (Prosopis juliflora) seed (Leguminosae). J. Sci. Food Agric., 87, 2012– 2018.
Garg, S.K., Kalla, A. & Bhatnagar, A. (2002) Evaluation of raw and hydrothermically processed leguminous seeds as supplementary feed for the growth of two Indian major carp species.Aquac. Res., 33,151–163.
Gomes, E.F., Corraze, G. & Kaushik, S. (1993) Effects of dietary incorporation of a co-extruded plant protein (rapeseed and pea) on growth, nutrient utilisation and muscle fatty acid composi-tion of rainbow trout (Oncorhynchus mykiss). Aquaculture, 113, 339–353.
Hedge, J.E. & Hofreiter, B.T. (1962) Estimation of carbohydrates. In:Carbohydrate Chemistry 17(Whistler, R.L. & Be Miller, J.N. eds), pp. 17–22. Academic Press, New York, NY.
Henken, A.M., Lucas, H., Tijssen, P.A.T. & Machiels, M.A.M. (1986) A comparison between methods used to determine the energy content of feed, fish and faeces samples.Aquaculture,58, 195–201.
Hossain, M.A. & Jauncey, K. (1990) Detoxification of linseed and sesame meal and evaluation of their nutritive value in the diet of carp (Cyprinus carpioL.).Asian Fish Sci.,3,169–183.
Hossain, M.A., Focken, U. & Klaus, B. (2001) Evaluation of an unconventional legume seed,Sesbania aculeata, as a dietary pro-tein source for common carp,Cyprinus carpioL.Aquaculture,198, 129–140.
Ishida, Y., Fujita, T. & Asai, K. (1981) New detection and sep-aration method for amino acids by HPLC.J. Chromatogr.,204, 143–148.
Kakade, M.L., Simons, N. & Liener, I.E. (1969) An evaluation of natural versus synthetic substrates for measuring the antitryptic activity of soybean samples. Cereal Chem., 46, 518– 526.
Keembiyehetty, C.N. & De Silva, S.S. (1993) Performance of juvenile Oreochromis niloticus (L.) reared on diets containing cowpea, Vigna caring,and black gramPhaseolus mungo,seeds. Aquacul-ture,112,207–215.
Kunitz, M. (1974) Crystalline trypsin inhibitor II: general properties. J. Gen. Physiol.,30,297–310.
Lowry, O.H., Rosebrough, J.A.L. & Randall, R.J. (1951) Protein measurement with the folin–phenol reagent.J. Biol. Chem.,193, 265–275.
Lyon, C.K., Gumbmann, M.R. & Bocker, R. (1988) Value of Mesquite leaves and forage.J. Sci. Food Agric.,44,111–117. Makkar, H.P.S. & Becker, K. (1999) Nutritional studies on rats and
fish carp, (Cyprinus carpio.) fed diets containing unheated and heatedJatropha curcas meal of a non-toxic provenance. Plant Foods Hum. Nutr.,53,183–192.
Makkar, H.P.S., Singh, B. & Dawra, K.K. (1987) Tannin–nutrient interactions – a review.Int. J. Anim. Sci.,2,127–139.
Maynard, L., Loosli, J., Hintz, H. & Warner, R. (1979) In:Animal Nutrition, 7th edn, pp. 13–14. McGraw-Hill, New York, NY. Mohanty, S.N., Das, K.M. & Sarkar, S. (1995) Effects of feeding
varying dietary formulations on body composition of rohu fry. J. Aquac.,3,23–28.
Molick, C.P. & Singh, M.B. (1980) Plant Enzymology and Histo Enzymology. pp. 286. Kalyani Publishers, New Delhi.
Mukhopadhyay, N. & Ray, A.K. (2001) Effects of amino acid sup-plementation on the nutritive quality of fermented linseed meal protein in the diets for rohu,Labeo rohita, fingerlings. J. Appl. Ichthyol.,17,220–226.
Ogino, C. (1980) Requirement of carp and rainbow trout for essen-tial amino acids.Bull. Jpn. Soc. Sci. Fish.,46,171–174.
Rathelot, J., Julien, R., Canioni, P., Coeroli, C. & Sarda, L. (1975) Studies on the effect of bile salt and colipase on enzymatic lipol-ysis. Improved method for the determination of pancreatic lipase and colipase.Biochimie,57,1117–1122.
Riveros, F. (1992) The genusProsopisand its potential to improve livestock production in arid and semi arid regions. In: Legume Trees and Other Fodder Trees as Protein Sources for Livestock (Speedy, A. & Pugliese, P. eds), pp. 257–276. FAO Animal Production and Health Paper 102, FAO, Rome, Italy.
Robaina, L., Izquierdos, M.S., Moyano, F.J., Socorro, J., Vergara, J.M., Montero, M. & Fernandez-Palacios, H. (1995) Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata).Aquaculture,130, 219–233.
Saha, A.K. & Ray, A.K. (1998) Evaluation ofÔChuniÕa commer-cially available low cost cattle fodder in the diet for rohu,Labeo rohita(Ham.) fingerlings.Aquac. Res.,29,761–768.
Schanderi, S.H. (1970) Method in Food Analysis. p. 70. Academic Press, New York.
Shukla, P.C., Talpada, P.M. & Pande, M.B. (1990)Prosopis juliflora Pods, a New Cattle Feed Source. Technical Bulletin-1, pp. 1–16. I. C. A. R Animal Nutrition Department, Gujarat Agricultural University, Anand, Gujrat, India.
Silva, A.M.M., Silva, A.R., Pinheiro, A.M. et al. (2007) Alka-loids from Prosopis juliflora leaves induce glial activation, cytotoxicity and stimulate NO production. Toxicon, 49, 601– 614.
Singh, B.N. (1987) On the Sulphur Amino Acids (Methio-nine + Cystine) and Lysine Requirements of Rohu, Labeo rohita (Hamilton) and Essential Amino Acid Composition of Certain Promising Feeds Evolved for Carps. p. 67. The First Indian Fisheries Forum, Mangalore, India.
Spyridakis, P., Metailler, R., Gabaudan, J. & Riaza, A. (1989) Studies on nutrient digestibility in European sea bass ( Dicentrar-chus labrax) I. Methodological aspects concerning faeces collec-tion.Aquaculture,77,61–70.
Stafford, H.A. (1960) Differences between lignin-like polymers formed by peroxidation of eugenol and ferulic acid in leaf sections of phleum.Plant Physiol.,35,108–114.
Steffens, W. (1989) Principles of Fish Nutrition. Ellis Horwood, Chichester, UK.
Storebakken, T. (1985) Binders in fish feeds: I. Effect of alginate and guar gum on growth, digestibility, feed intake and passage through the gastrointestinal tract of rainbow trout.Aquaculture, 47, 11–26.
Synder, H.E. & Kwon, T.W. (1987) Soybean Utilization. Van Nostrand Reinhold, New York.
Tacon, A.G.J. (1993) Feed Ingredients for Warmwater Fish: Fish Meal and Other Processed FeedstuffsFAO Fisheries Circular. No. 856. FAO, Rome, Italy.
Tacon, A.G.J. (1997) Fishmeal replacers: review of antinutrients within oilseed and pulses – a limiting factor for the aquafeed green revolution? In: Feeding Tomorrow Fish. Cahiers Options Me´diterrane´ennes (Tacon, A. & Basurco, B. eds), 22, pp. 153– 182. Proceedings of the Workshop of the CHEAM Network on Technology of Aquaculture in the Mediterranean (TECAM) Zaragoza, Spain.
Van Den Eynden, V., Cueva, E. & Cabrera, O. (2003) Wild foods from Southern Ecuador.Econ. Bot.,57,576–603.
Wee, K.L. (1991) Use of non conventional feed stuffs of plant origin as fish feeds- is it practical and economically feasible? In: Fish Nutrition Research in Asia. Proc. 4th Asian Fish Nutrition Workshop(De Silva, S.S. ed.), pp. 13–32. Asian Fisheries society, Manila, The Philippines.
Wheeler, E.L. & Ferrel, R.E. (1971) A method for phytic acid determination in wheat and wheat fractions. Cereal Chem., 48, 312–320.
Wilson, R.P. & Poe, W.E. (1985) Effects of feeding soybean meal with varying trypsin inhibitor activities on growth of fingerling channel catfish.Aquaculture,46,19–25.
Yusuf, N.D., Ogah, D.M., Hassan, D.I., Musa, M.M. & Doma, U.D. (2008) Effect of decorticated fermented prosopis seed meal (Prosopis africana) on growth performance of broiler chicken. Int. J. Poult. Sci.,7,1054–1057.