www.elsevier.com / locate / bres
Research report
Expression and conditioned inhibition of fear-potentiated startle after
stimulation and blockade of AMPA / Kainate and GABA receptors in
Athe dorsal periaqueductal gray
*
Markus Fendt
¨ ¨ ¨
Tierphysiologie, Universitat Tubingen, Auf der Morgenstelle 28, D-72076 Tubingen, Germany Accepted 20 June 2000
Abstract
Previous work showed that the dorsal periaqueductal gray is involved in the inhibition of fear-potentiated startle. The present study investigated the effects of blockade and stimulation of Kainate /AMPA and GABA receptors within the dorsal periaqueductal gray onA expression and conditioned inhibition of fear-potentiated startle. Blockade of the Kainate /AMPA receptors enhanced whereas stimulation of the Kainate /AMPA receptors decreased expression of fear-potentiated startle. These effects do not reflect conditioned inhibition since this modulation was not changed by injections of Kainate /AMPA receptor agonists or antagonists into the dorsal periaqueductal gray. Stimulation and blockade of GABA receptors within the dorsal periaqueductal gray neither affected expression of fear-potentiated startleA nor its conditioned inhibition. The present results together with findings from the literature indicate that glutamate in the dorsal periaqueductal gray is a critical substrate for the expression and modulation of fear-related behaviours. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behavior
Topic: Motivation and emotion
Keywords: Acoustic startle response; Anxiety; Conditioned fear; Conditioned inhibition; GABA; Glutamate; Periaqueductal gray
1. Introduction 18,22,42,43]). One possibility to inhibit fear-potentiated startle is conditioned inhibition which was established by The inhibition of fear is important in situations where it Falls and co-workers [16,19]. In their procedure, the fear would lead to inappropriate behaviour, e.g. when an conditioning training (pairings of a light CS with a organism recognises that a threat is over. The knowledge footshock US) is followed by a further training in which of the neurochemical and neuroanatomical basis of this the rats learn that a combination of a conditioned inhibitor mechanism might help to develop strategies to treat (CI: e.g. a noise) with the CS is not associated with an US, pathological fear in humans. One of the most valuable but the CS alone further predicts a US. After these training models to investigate the neural basis of fear in humans procedures, the ASR amplitude is still potentiated in the and rats is the fear-potentiated startle paradigm presence of the CS alone, whereas the combination of the [8,12,24,32]. Here, the acoustic startle response (ASR) is CI and the CS inhibits fear-potentiated startle. The CI potentiated in the presence of a conditioned stimulus (CS: alone does not affect the ASR amplitude.
e.g. a light) which has previously been paired with an A series of studies were carried out to investigate the aversive, unconditioned stimulus (US: e.g. a footshock). neural basis of conditioned inhibition of fear-potentiated During the last years, the investigation of inhibition of startle. A c-fos study [9] pointed to several brain regions fear has received increasing interest (e.g., [9,16– possibly being involved in the inhibition of fear. Most of these and other regions were lesioned during the last years without effects on conditioned inhibition of
fear-poten-*Tel.:149-7071-2975347; fax:149-7071-292618.
E-mail address: [email protected] (M. Fendt). tiated startle (e.g., [17,18,23,26]). Two recent studies gave
first hints to brain structures possibly involved in con- available. The experiments were done in accordance with ditioned inhibition of fear-potentiated startle: (1) Fendt ethical guidelines for the care and use of experimental [22] showed that a blockade of GABAA receptors within animals and were approved by the local council of animal
¨ ¨
the dorsal region of the periaqueductal gray (dPAG) care (Regierungsprasidium Tubingen, ZP 4 / 96). attenuates expression of conditioned inhibition of
fear-potentiated startle; (2) Heldt and Falls [29] demonstrated 2.2. Surgery that electrolytic lesions of the brachium of the colliculus
inferior block the expression of conditioned inhibition of The animals were anaesthetised with ketamine / xylazine fear-potentiated startle. (9:1, 100 mg / kg, i.p.). Two stainless guide cannulae Whereas the brachium of the colliculus inferior is a part (diameter: 0.7 mm) were implanted bilaterally into the of the auditory pathway mediating the noise CI [37,50], the brain using following stereotaxic coordinates of Paxinos dPAG is a brain structure which is not directly involved in and Watson [39]: rostrocaudal: 26.3 mm, mediolateral: the processing of the auditory CI, but is well known for 60.6 mm, dorsoventral: 25.0 mm (relative to Bregma). modulation of fear and anxiety [5,24,48]. Beside its The guide cannulae were fixed to the skull with dental possible role in conditioned inhibition of fear-potentiated cement and three anchoring screws. After the surgery and startle [22], the dPAG plays a role in the expression of between the experiments, stainless steel stylets (diameter: fear-potentiated startle. Walker and colleagues [49] demon- 0.4 mm) were inserted into the guide cannulae to maintain strated that dPAG stimulation by injections of Kainic acid patency. All subjects were allowed 4 days recovery before block fear-potentiated startle whereas dPAG lesions pre- training and testing.
vent the loss of fear-potentiated startle seen after training
with high intensity USs [11]. Furthermore, we previously 2.3. Apparatus demonstrated [25] a projection from the dPAG to the
caudal pontine reticular nucleus, a crucial part of the The rats were trained in two identical, acoustically
3
primary startle circuit [32]. A number of other studies isolated dark boxes (38360328 cm ). The floor of the using different animal models to investigate fear-related boxes was composed of stainless steel bars spaced |15
behaviours showed that the dPAG is strongly involved in mm apart, through which the unconditioned stimulus (US), the expression and modulation of fear [20,28,30,31,34– a 0.5 s, 0.6 mA footshock could be administered. The 36,44]. It is important to note that with the methods used footshocks were produced by a shock generator
(custom-¨
in the studies cited above it is not possible to distinguish made at the University of Tubingen) located outside the between the dorsomedial and the dorsolateral part of the chamber. The conditioned stimulus (CS) for fear-con-PAG, two areas which can be clearly differentiated [2]. ditioning (paired with the US) was a 3.7 s white light
1
The aim of the present study was to investigate the role produced by a 15 W bulb (light trials), the conditioned of AMPA / Kainate and GABA receptors within the dPAGA inhibitor (CI), predicting that the light is not followed by a in the expression and conditioned inhibition of fear-poten- footshock, was a 3.7 s, 70 dB white noise produced by a
2
tiated startle. First, we tested whether the blockade of loudspeaker (noise→light trials). The bulbs and the fear-potentiated startle which were reported after injections loudspeakers were located on the top of the boxes. The of the AMPA / Kainate receptor agonist Kainic acid into the presentation of the US, CS and CI were controlled by a dPAG reflects conditioned inhibition. In addition, the microcomputer and an appropriate interface (Hortmann effects of injections of the AMPA / Kainate receptor an- universal function synthesiser, Hortmann, Neckartenzling-tagonist NBQX were investigated. Second, we attempted en, Germany).
to replicate the blockade of conditioned inhibition of fear- To measure fear-potentiated startle and conditioned potentiated startle reported after GABA receptor blockadeA inhibition, the animals were tested in two identical test by Picrotoxin within the dPAG and to confirm these results chambers. The rats were placed in wire mesh cages (203
3
by an opposite effect after GABA receptor stimulation byA 10312 cm ) with a steel floor, which were put up on a Piperidine. piezoelectric accelerometer (custom-made at the University
¨
of Tubingen). The accelerometer was located inside a
3
sound-attenuated test chamber (100380360 cm ).
Neckartenzling-en, Germany). The whole body startle amplitude was 2.5. Drugs calculated from the difference between the peak-to-peak
voltage output of the accelerometer within time-windows The following drugs were injected into the dPAG of the of 80 ms after and 80 ms before the startle stimulus onset. rats: 0, 60, and 120 pmol Kainic acid ([2S-(2a,3b,4b 9]-2-The spontaneous motor activity was calculated as the root Carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid; mean square value of the accelerometer output, measured AMPA / Kainate receptor agonist; Sigma, Deisenhofen, in a time window of 28 s before the presentation of each of Germany); 0, 50, and 100 nmol NBQX (2,3-Dioxo-6-nitro-the tone alone startle trials (see below). 1,2,3,4 - tetrahydrobenzo[f]quinoxaline - 7 - sulphonamide; AMPA / Kainate receptor antagonist; RBI, distributed by
¨
2.4. Behavioural procedures Biotrend, Koln, Germany); 0, 4.2, and 8.3 nmol Picrotoxin
2
(GABA -receptor-Cl -channel blocker; RBI, distributedA
¨
On the first day, animals were placed into the training by Biotrend, Koln, Germany); 0, 25, and 50 nmol Piper-boxes and after an acclimatisation time of 5 min, they idine (Piperidine-4-sulphonic acid; GABA -receptor agon-A
1
¨
received a fear-conditioning training with 10 light trials. ist; RBI, distributed by Biotrend, Koln, Germany). All The US was presented during the last 0.5 s of the 3.7 s drugs were dissolved in saline.
light CS at a mean intertrial interval (ITI) of 2 min (range
1.5–2.5 min). On the second day, animals were trained for 2.6. Histology conditioned inhibition (using the procedure developed by
2 1
[19]): the rats received 15 noise→light trials and 5 light After the tests, the rats were killed by an overdose of trials in a pseudorandomized order (mean ITI: 2 min, range Nembutal. The animals were decapitated, and their brains
2
1.5–2.5 min). A noise→light trial consisted of a 3.7 s were removed and immersion-fixed with 8% paraformal-noise following by a 3.7 s light (without footshock). dehyde in PBS with 20% sucrose. Coronal sections of 60 Four hours after this training session, the first test of mm were taken on a freezing microtome and stained with fear-potentiated startle and conditioned inhibition occurred. thionin. The injection sites were drawn onto plates from Two injection cannulae with a diameter of 0.4 mm were the atlas of Paxinos and Watson [39].
inserted into the guide cannulae of the rats. The injection cannulae were connected via a liquid swivel (Instech Labs,
2.7. Statistical analysis Plymouth Meeting, PA, USA) and by a 50 cm flexible PVC
tubing to two 1ml syringes (Scientific Glass Engineering,
Statistical analysis of the data was accomplished by Weiterstadt, Germany). This method allowed us to inject
analysis of variance (ANOVA), followed by post hoc drugs during the test without handling the rats. For testing,
Tukey-tests. Trial type and treatment were used as within the animals were placed into the test cage and after an
subjects factors and injection sites were used as between acclimatisation time of 5 min, 10 initial startle stimuli (10
subjects factors. For all statistical comparisons P,0.05 kHz, 20 ms duration including 0.4 ms rise and fall times,
was taken as the criterion for statistical significance. ASR 100 dB SPL, 30 s interstimulus interval) were presented to
difference scores were calculated for each rat by subtract-obtain a baseline ASR amplitude. Injections of 0.5ml drug
ing the mean ASR amplitude on tone alone trials from the solution were given at a rate of 0.1ml / 10 s after the fifth
mean ASR amplitude on light tone or noise-light-tone startle stimulus. The injection cannulae remained in the
trials. brain during the whole test. After the ten initial startle
stimuli, each animal received 15 further startle stimuli, one third presented alone, one third presented 3.2 s after the
onset of the light, and one third presented after a light 3. Results
preceded by a 3.7 s noise. All trial types were presented in
a pseudo-random order (30 s interstimulus interval) with 3.1. Histology the constraint that each trial type occurred only once in
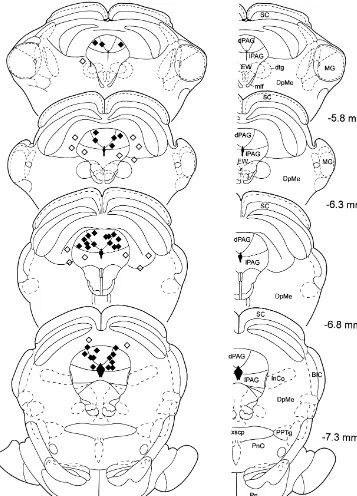
each consecutive three-trial block. Histological analysis revealed that injection sites of 22 The rats received maximal five injections of drug animals were located in the dPAG (Fig. 1). Sixteen rats solutions across five subsequent days. All rats received received injections of Kainic acid into the dPAG, 12 rats injections of saline and two different drugs in a pseudo- received injections of NBQX, 14 rats received injections of randomized order into the dPAG. In order to avoid Picrotoxin, and 16 rats received injections of Piperidine. extinction of fear-conditioning and conditioned inhibition The injection sites of the remaining eight animals were during the test days, the animals were retrained once daily located in the superior colliculus, in the deep mesence-4 h before testing. The retraining sessions (days 3–6) phalic nucleus, or in the lateral region of the PAG and were
2 1
Fig. 1. Drawings of frontal sections through the midbrain of the rat depicting the injection sites into the dPAG (X), as well as the misplaced injections (x). The plates are adapted from Paxinos and Watson [39], the numerals indicate the distance from Bregma. Abbreviations: 3, oculomotor nucleus; BIC, nucleus of the brachium of the inferior colliculus; dPAG, dorsal PAG; DpMe, deep mesencephalic nucleus; dtg, dorsal tegmental bundle; EW, Edinger–Westphal nucleus; InCo, intercollicular nucleus; MG, medial geniculate nucleus; mlf, medial longitudinal fasciculus; Pn, pontine nuclei; PnO, oral pontine reticular nucleus; PPTg, pedunculopontine tegmental nucleus; SC, superior colliculus; xscp, decussation of the superior cerebellar peduncle.
3.2. Baseline startle of the previous day were detected (ANOVA: F ’s,1, data not shown).
Neither injections of Kainic acid (ANOVA: F(2,11)5
0.92, P50.41), NBQX (ANOVA: F(2,16)52.26, P50.14), 3.3. Expression of fear-potentiated startle Picrotoxin (ANOVA: F(2,20)50.49, P50.62), nor
Fig. 2. Bar diagrams showing the effects of injections of Kainic acid
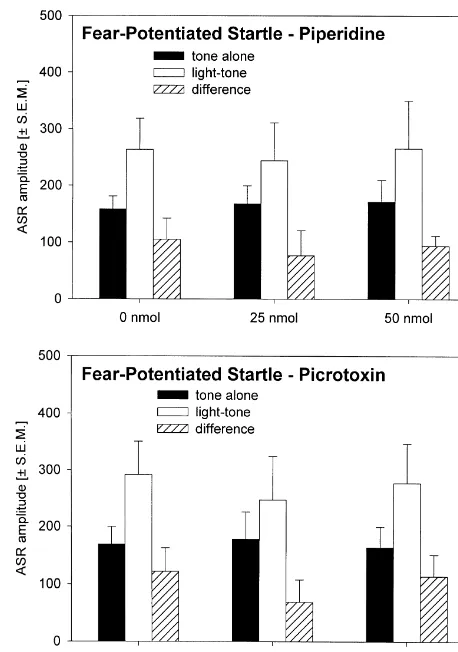
(upper panel) and NBQX (lower panel) into the dPAG on the expression Fig. 3. Bar diagrams showing the effects of injections of Piperidine of fear-potentiated startle. The bars represent the mean ASR amplitudes (upper panel) and Picrotoxin (lower panel) into the dPAG on expression after tone alone and light-tone trials, as well as the difference scores of fear-potentiation of the ASR. The bars represent the mean ASR (6S.E.M.). A significant effect of injections on expression of fear- amplitudes after tone alone and light-tone trials, as well as the difference potentiated startle (difference scores) is indicated by ** P,0.01 and * scores (6S.E.M.).
P,0.05 (repeated-measure ANOVA followed by Tukey-test).
attenuated fear-potentiated startle (ANOVA: F(2,11)53.73, difference scores of noise-light-tone trials (ANOVA:
P50.03), NBQX strongly increased fear-potentiated startle F(2,16)51.68, P50.22), whereas the difference between (ANOVA: F(2,16)56.83, P50.007). Post-hoc Tukey tests light-tone and noise-light-tone trials was strongly at-showed a P,0.05 for the comparison between the effects tenuated (ANOVA: F(2,16)55.53, P50.02). But this of injections of 0 and 120 nmol Kainic acid and a P,0.01 effect was caused by the increase in fear-potentiated startle for the comparison between the effects of injections of 0 (light-tone trials), since the analysis of the percent inhibi-and 100 nmol NBQX into the dPAG. In contrast, injections tion by the noise CI revealed no significant differences of both, Picrotoxin and Piperidine (Fig. 3), had no effect (data not shown; ANOVA: F(2,16)52.21, P50.14). After on the expression of fear-potentiated startle (ANOVAs: injections of Kainic acid, Picrotoxin, and Piperidine into
F ’s,0.69, P’s.0.52). No effects of test day or treatment the dPAG (Fig. 4 and 5), both difference scores were not of the previous day were detected (ANOVA: F ’s,1, data changed (ANOVA’s: F ’s,2.40, P’s.0.11). No effects of not shown). test day or treatment of the previous day were detected
(ANOVA: F ’s,1, data not shown). 3.4. Expression of conditioned inhibition of
fear-potentiated startle 3.5. Motor activity
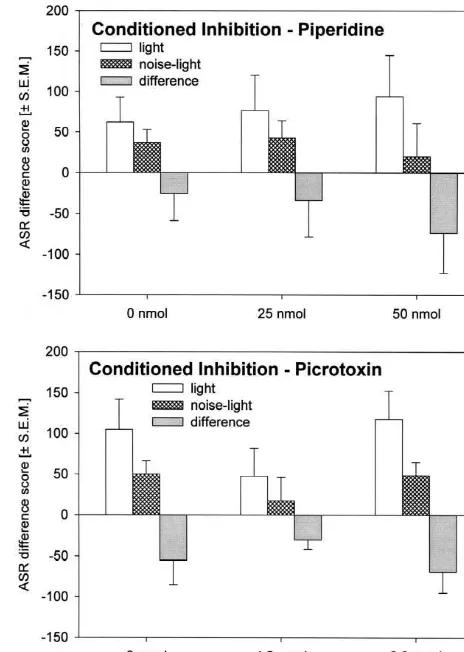
Fig. 4. Bar diagrams showing the effects of injections of Kainic acid Fig. 5. Bar diagrams showing the effects of injections of Piperidine (upper panel) and NBQX (lower panel) into the dPAG on conditioned (upper panel) and Picrotoxin (lower panel) into the dPAG on conditioned inhibition of fear-potentiated startle. The bars represent the mean ASR inhibition of fear-potentiated startle. The bars represent the mean ASR difference scores after light and noise-light trials, as well as their difference scores after light and noise-light trials, as well as their
differences (6S.E.M.). differences (6S.E.M.).
GABA receptor antagonist [10,15,41] were not observed in of fear-potentiated startle; likewise, blockade of the the present study. AMPA / Kainate receptors enhanced fear-potentiated startle. It is important to note that this inhibition of fear-poten-tiated startle by stimulation of the AMPA / Kainate
re-4. Discussion ceptors did not reflect the inhibition by an conditioned inhibitor. Earlier results from our laboratory indicating a The present study tested the hypothesis that AMPA / role of the GABAA receptors within the dPAG in the Kainate receptors and GABAA receptors in the dPAG are expression of conditioned inhibition could not be con-involved in the expression of fear-potentiated startle and / firmed in the present study. Both, injections of a GABAA
or the expression of conditioned inhibition of fear-poten- receptor agonist into the dPAG as well as of a GABAA
tiated startle. This hypothesis was based on the observa- receptors antagonist neither affected expression nor con-tions that injeccon-tions of Kainic acid into the dPAG totally ditioned inhibition of fear-potentiated startle.
block the expression of fear-potentiated startle [48,49] and Since excitation of the PAG using stimulation electrodes that injections of the GABA receptor antagonist Picrotox-A or injections of excitatory amino acids or GABA receptorA
4.1. The injection sites within the dPAG much more caudal and ventral parts of the PAG (compared with the injection sites of the present study).
All injection sites of the present study were located into
the dPAG, i.e. into the dorsomedial and the dorsolateral 4.3. Effects of NBQX injections into the dPAG region of the PAG. Due to the small number of misplaced
injections, it was not possible to statistically analyse their Injections of NBQX into the dPAG strongly increased effects, but none of the individual animals (except those the expression of fear-potentiated startle. Conditioned with injections into the lateral PAG) showed overt be- inhibition was not affected. Since NBQX had the contrary havioural changes after injections of the different drugs. As effects of Kainic acid, the important role of the AMPA / we previously demonstrated in our laboratory [21,22], Kainate receptors within the dPAG in the expression of injections of Picrotoxin (and also Kainic acid) into the fear-potentiated startle was fully confirmed.
lateral PAG (2 animals) resulted in an increase in fear- To the best of our knowledge, this was the first time that potentiated startle, whereas injections of Picrotoxin into behavioural effects of NBQX injections into the dPAG the deep layers of the superior colliculus (4 animals) has were tested. Furthermore, this is one of the few studies no effects on fear-potentiated startle or conditioned inhibi- showing an increase in fear-potentiated startle. For exam-tion. Furthermore, injections of Kainic acid into the deep ple, a previous study of our laboratory reported an increase layers of the superior colliculus did not affect fear-poten- of fear-potentiated startle after Picrotoxin injection into the tiated startle. Hence, we are confident that the effects lateral region of the PAG [22].
described in the present experiments are mediated by the
dPAG. 4.4. The role of glutamate within the dPAG
4.2. Effects of Kainic acid injections into the dPAG All types of glutamatergic receptors are abundant in the dPAG [1,47] and a lot of glutamate-immunopositive termi-Injections of Kainic acid into the dPAG decreased nals are present in the dPAG [4]. The rostral hypothalamus expression of fear-potentiated startle. This was earlier seems to be the main source of glutamatergic input into the described by Walker and colleagues [48,49] and confirmed dPAG [38,46] and it has been suggested that this projection by the present experiments. Since there was a hint that the is important for the mediation of defensive behaviours. dPAG is involved in conditioned inhibition of fear-poten- Beitz [6] counted a high number of different brain areas tiated startle [22], the idea came up that the reduction of projecting with glutamate to the dorsal and lateral parts of fear-potentiated startle by stimulation of the dPAG might the PAG, but because of the sizes of the injections sites of reflect conditioned inhibition. However, the present study the retrograde tracer in this study, it was impossible to showed that after injections of Kainic acid into the dPAG, differentiate between projections to the dPAG and to the conditioned inhibition of fear-potentiated startle was not other regions of the PAG. The most neurones projecting affected. This was found after infusion of a concentration with glutamate to the PAG were found in the zona incerta, of Kainic acid which attenuated expression of fear-poten- hypothalamus, cingulate, perirhinal and frontal cortex, tiated startle, as well as after infusion of a concentration of cuneiform nucleus, spinal trigeminal nucleus, deep Kainic acid which did not affect expression of fear-poten- mesencephalic nucleus, amygdala, cerebellar nuclei and in tiated startle. the PAG itself (see also [7]).
gluta-mate receptor subtypes mediate different aspects of fear 4.6. Effects of Piperidine injections into the dPAG (which are measured in the different animal models of fear
and anxiety). Since NMDA receptors have a low affinity Piperidine injections into the dPAG neither affected for glutamate if the neurone is not depolarised, this would expression nor conditioned inhibition of fear-potentiated suggest that the first result of glutamate release in the startle. Since previous work of our laboratory [22] showed dPAG would be an inhibition of fear via Kainate /AMPA a reduction of conditioned inhibition after blockade of receptors. Different glutamate concentrations within the GABAA receptors within the dPAG, we expected that dPAG could have different effects, e.g. low concentrations Piperidine injections into the dPAG elicits an enhancement mainly activate the AMPA / Kainate receptor, whereas high in conditioned inhibition. To the best of our knowledge, concentrations mainly activate the NMDA receptor. The there are no previous reports on the behavioural effects of different glutamate receptors could be located on different Piperidine injections into the dPAG.
neurones of the dPAG and theses neurones could modulate different fear-related behaviours. Consistent with this
considerations, Walker and colleagues [48,49] hypothesised 4.7. The role of GABA within the dPAG that only high levels of fear activate the dPAG.
The present study showed that NMDA receptors within The present results suggest that the GABAergic trans-the dPAG mediate a reduction of fear-potentiated startle mission within the dPAG does not affect the expression but are not involved in conditioned inhibition of fear- and modulation of fear measured by the fear-potentiated potentiated startle. It is important to note that after startle paradigm. Results of previous studies showing that injection of the highest dose of NBQX the absolute but not GABA within the dPAG is involved in conditioned inhibi-the percent conditioned inhibition is increased, i.e. that inhibi-the tion [22] were not confirmed. Since GABA release within ‘increase’ in the absolute value of conditioned inhibition is the PAG is decreased during pain [33], it can not be due to the enhancement of fear-potentiated startle. These excluded that GABA within the dPAG plays a role in the results let suggest that there are different neuronal systems modulation of fear-related behaviours. So, further work mediating the dPAG inhibition of fera-potentiated startle using higher concentrations of GABA drugs into the and conditioned inhibition of fear-potentiated startle. different regions of the PAG and using other animal
models of fear and anxiety is necessary.
4.5. Effects of Picrotoxin injections into the dPAG
Picrotoxin injections into the dPAG neither affected 5. Conclusion
expression nor conditioned inhibition of fear-potentiated
startle. This is partly in contrast with previous work of our Manipulation of the glutamatergic transmission within laboratory [22] showing a slight reduction of conditioned the dPAG leads to one of the most effective modulation of inhibition after blockade of GABAA receptors within the the expression of fear-potentiated startle. Blockade of the dPAG. The reason for this discrepancy could be procedural AMPA / Kainate receptors increases and stimulation de-differences between the two studies. For example, the rats creases or blocks expression of fear-potentiated startle in the study of 1998 showed a much higher mean without affecting conditioned inhibition of fear-potentiated amplitude of both, baseline startle response and fear- startle. These effects on fear-potentiated startle can be potentiation of startle, so one might suggest that the effect mediated via a direct [25] or an indirect (e.g. via the of Picrotoxin injections on conditioned inhibition can only ventrolateral PAG [5]) projection from the dPAG to the be observed in highly fearful rats. Another difference is primary startle pathway. The GABAergic transmission that the injection sites of the present study are a little more within the dPAG is not critical for expression of fear-caudal (ca. 0.5 mm) than in the study of 1998. The data of potentiated startle. Likewise, conditioned inhibition of the present study were also analysed with respect to the fear-potentiated startle was also not affected by GABAA
rostrocaudal coordinates and ASR amplitude, respectively, receptor agonists and antagonists. So, the neural basis of but no correlation between these factors and the Picrotoxin conditioned inhibition of fear-potentiated startle is still effect were found (data not shown). Nevertheless, it should unclear.
be noted that ten of the fourteen rats receiving Picrotoxin The present results together with several findings from injections into the dPAG showed a reduction of con- the literature [20,28,30,31,34–36,44,48,49] showed that ditioned inhibition at one of the two doses of Picrotoxin, the different transmitter systems of the dPAG as well as but only one rat showed this reduction at both doses. So, their different receptors are involved differently in the the present results do not totally exclude a role of GABAA expression of fear-related behaviours. The dPAG is a brain receptors within the dPAG in the expression of conditioned nucleus where a fine tuning of several fear responses is
cortex interfere with conditioned excitation but not with conditioned
Acknowledgements
inhibition of fear, Behav. Neurosci. 111 (1997) 476–486. [18] W.A. Falls, M. Davis, Lesions of the central nucleus of the
This work was supported by the Deutsche Forschungs- amygdala block conditioned excitation, but not conditioned inhibi-gemeinschaft (SFB 307 / C2, Fe 483 / 1-1 & Schn 138 / 26- tion of fear as measured with the fear-potentiated startle effect, 1). I am grateful to Professor Michael Koch for helpful Behav. Neurosci. 109 (1995) 379–387.
[19] W.A. Falls, M. Davis, Inhibition of fear-potentiated startle can be
comments to the manuscript and Helga Zillus for excellent
detected after the offset of a feature trained in a serial
feature-technical assistance.
negative discrimination, J. Exp. Psychol. 23 (1997) 3–14. [20] M.S. Fanselow, J.P. DeCola, B. De Oca, J. Landeira-Fernandez,
Ventral and dorsolateral regions of the midbrain periaqueductal gray (PAG) control different stages of defensive behavior: Dorsolateral
References
PAG lesions enhance the defensive freezing produced by massed and immediate shock, Aggress. Behav. 21 (1995) 61–77. [1] R.L. Albin, R.L. Makowiec, Z. Hollingsworth, L.S. Dure, J.B.
[21] M. Fendt, Stimulation of startle modulating midbrain areas has Penney, A.B. Young, Excitatory amino acid binding sites in the
different effects on the acoustic startle response, Soc. Neurosci. periaqueductal gray of the rat, Neurosci. Lett. 118 (1990) 112–115.
Abstr. 23 (1997) 627. [2] R. Bandler, P. Carrive, A. Depaulis, Emerging principles of
organi-[22] M. Fendt, Different regions of the periaqueductal grey are involved zation of the midbrain periaqueductal gray matter, in: A. Depaulis,
differently in the expression and conditioned inhibition of fear-R. Bandler (Eds.), The Periaqueductal Gray Matter, Plenum Press,
potentiated startle, Eur. J. Neurosci. 10 (1998) 3876–3884. New York, 1991, pp. 1–8.
[23] M. Fendt, M. Davis, Lesions of the red nucleus and the sep-[3] R. Bandler, K.A. Keay, Columnar organization in the midbrain
tohypothalamic nucleus do not affect conditioned inhibition of the periaqueductal gray and the integration of emotional expression, in:
acoustic startle response in rats, unpublished observations, (1997) G. Holstege, R. Bandler, C.B. Saper (Eds.), Progress in Brain
[24] M. Fendt, M.S. Fanselow, The neuroanatomical and neurochemical Research, Elsevier Science, B.V, 1996.
basis of conditioned fear, Neurosci. Biobehav. Rev. 23 (1999) [4] P. Barbaresi, G. Gazzanelli, M. Malatesta, Glutamate-positive
743–760. neurons and terminals in the cat periaqueductal gray matter (PAG): a
[25] M. Fendt, M. Koch, H.-U. Schnitzler, Lesions of the central gray light and electron microscopic immunocytochemical study, J. Comp.
block the sensitization of the acoustic startle response in rats, Brain Neurol. 383 (1997) 381–396.
Res. 661 (1994) 163–173. [5] M.M. Behbehani, Functional characteristics of the midbrain
[26] J.C. Gewirtz, W.A. Falls, M. Davis, Normal conditioned inhibition periaqueductal gray, Prog. Neurobiol. 46 (1995) 575–605.
and extinction of freezing and fear-potentiated stratle following [6] A.J. Beitz, Possible origin of glutamatergic projections to the
electrolytic lesions of medial prefrontal cortex in rats, Behav. midbrain periaqueductal gray and deep layer of the superior
Neurosci. 111 (1997) 712–726. colliculus of the rat, Brain Res. Bull. 23 (1989) 25–35.
[27] F.S. Guimaraes, A.P. Carobrez, J.C. de Aguiar, F.G. Graeff, [7] A.J. Beitz, Periaqueductal gray, in: G. Paxinos (Ed.), The Rat
Anxiolytic effect in the elevated pluz-maze of the NMDA receptor Nervous System, Academic Press, San Diego, 1995, pp. 173–182.
antagonist AP7 microinjected into the dorsal periaqueductal grey, [8] J.S. Brown, H.I. Kalish, I.E. Farber, Conditioned fear as revealed by
Psychopharmacology 103 (1991) 91–94. magnitude of startle response to an auditory stimulus, J. Exp.
[28] F.S. Guimaraes, J.C. de Aguiar, E.A. Del Bel, G. Ballejo, Anxiolytic Psychol. 41 (1951) 317–328.
effect of nitric oxide synthase inhibitors microinjected into the [9] S. Campeau, W.A. Falls, W.E. Cullinan, D.L. Helmreich, M. Davis,
dorsal central grey, NeuroReport 5 (1994) 1929–1932. S.J. Watson, Elicitation and reduction of fear: behavioural and
neuroendocrine indices and brain induction of the immediate-early [29] S.A. Heldt, W.A. Falls, Electrolytic lesions of the brachium of the gene c-fos, Neuroscience 78 (1997) 1087–1104. inferior colliculus block the expression of conditioned inhibition of [10] P. Carrive, The periaqueductal gray and defensive behavior: func- fear-potentiated startle, Soc. Neurosci. Abstr. 24 (1998) 365.
¨
tional representation and neuronal organization, Behav. Brain Res. [30] A. Kask, L. Rago, J. Harro, Anxiogenic-like effect of the NPY Y1
58 (1993) 27–47. receptor antagonist BIBP3226 administered into the dorsal
[11] M. Davis, D.I. Astrachan, Conditioned fear and startle magnitude: periaqueductal gray matter in rats, Regulatory Peptides 75–76 effects of different footshock or backshock intensities used in (1998) 255–262.
¨
training, J. Exp. Psychol. 4 (1978) 95–103. [31] A. Kask, L. Rago, J. Harro, NPY Y1 receptors in the dorsal [12] M. Davis, W.A. Falls, S. Campeau, M. Kim, Fear-potentiated startle: periaqueductal gray matter regulate anxiety in the social interaction
a neural and pharmacological analysis, Behav. Brain Res. 58 (1993) test, NeuroReport 9 (1998) 2713–2716.
175–198. [32] M. Koch, The neurobiology of startle, Prog. Neurobiol. 59 (1999) [13] A. Depaulis, R. Bandler, M. Vergnis, Characterization of pretentorial 107–128.
periaqueductal gray matter neurons mediating intraspezific defensive [33] S. Maione, I. Marabese, P. Olivia, V. de Novellis, L. Stella, F. Rossi, behaviors in the rat by microinjections of kainic acid, Brain Res. A. Filippelli, Periaqueductal gray matter glutamate and GABA 486 (1989) 121–132. decrease following subcutaneous formalin injection in rat, NeuroRe-[14] A. Depaulis, K.A. Keay, R. Bandler, Longitudinal neuronal organi- port 10 (1999) 1403–1407.
zation of defensive reactions in the midbrain periaqueductal gray [34] A.P. Martins, R.A. Marras, F.S. Guimaraes, Anxiogenic effects of region of the rat, Exp. Brain Res. 90 (1992) 307–318. corticotropin-releasing hormone in the dorsal periaqueductal grey, [15] A. Depaulis, M. Vergnes, Elicitation of intraspecific defensive NeuroReport 8 (1997) 3601–3604.
behaviors in the rat by microinjection of picrotoxin, a gamma- [35] M.G. Matheus, R.L. Nogueira, A.P. Carobrez, F.G. Graeff, F.S. aminobutyric acid antagonist, into the midbrain periaqueductal gray Guimaraes, Anxiolytic effect of glycine antagonists microinjected matter, Brain Res. 367 (1986) 87–95. into the dorsal periaqueductal grey, Psychopharmacology 113 [16] W.A. Falls, Conditioned inhibition of fear-potentiated startle, 1993, (1994) 565–569.
˜
Ph.D. Thesis, Yale University. [36] V. Motta, K. Penha, M.L. Brandao, Effects of microinjections ofm
submitted to the plus maze test, Psychopharmacology 120 (1995) [45] M.L. Schmitt, F.G. Graeff, A.P. Carobrez, Anxiolytic effect of
470–474. kynurenic acid microinjected into the dorsal periaqueductal gray
[37] K.N. O’Connors, T.L. Allison, M.E. Rosenfield, J.W. Moore, Neural matter of rats placed in the elevated plus-maze test, Braz. J. Med. activity in the medial geniculate nucleus during auditory trace Biol. Res. 23 (1990) 677–679.
conditioning, Exp. Brain Res. 113 (1997) 534–556. [46] K. Schubert, M.B. Shaikh, A. Siegel, NMDA receptors in the [38] D.M. Parry, N. Johns, F.M. Semenenko, R.K. Snowball, P.M. midbrain periaqueductal gray mediate hypothalamically evoked
Hudson, B.M. Lumb, Glutamatergic projections from the rostral hissing behavior in the cat, Brain Res. 726 (1996) 80–90. hypothalamus to the periaqueductal grey, NeuroReport 7 (1996) [47] T.R. Tolle, A. Berthele, W. Zieglgansberger, P.H. Seeburg, W.
1536–1540. Wisden, The differential expression of 16 NMDA and non-NMDA
[39] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, receptor subunits in the rat spinal cord and in the periaqueductal Academic Press, San Diego, 1997. gray, J. Neurosci. 13 (1993) 5009–5028.
[40] C.F. Plappert, P.K.D. Pilz, H.-U. Schnitzler, Acoustic startle re- [48] D.L. Walker, J.V. Cassella, Y. Lee, T.C.M. de Lima, M. Davis, sponse and habituation in freezing and nonfreezing rats, Behav. Opposing roles of the amygdala and dorsolateral periaqueductal gray Neurosci. 107 (1993) 981–987. in fear-potentiated startle, Neurosci. Biobehav. Rev. 21 (1997) [41] P. Redgrave, P. Dean, W. Souki, G. Lewis, Gnawing and changes in 743–753.
reactivity produced by microinjections of picrotoxin into the su- [49] D.L. Walker, M. Davis, Involvement of the dorsal periaqueductal perior colliculus of rats, Psychopharmacology 75 (1981) 198–203. gray in the loss of fear-potentiated startle accompanying high [42] C. Schauz, M. Koch, Latent inhibition of fear-potentiated startle in footshock training, Behav. Neurosci. 111 (1997) 692–702.
rats, Behav. Pharmacol. 9 (1998) 175–178. [50] T.J. Webber, E.J. Green, R.W. Winters, N. Schneiderman, P.M. [43] C. Schauz, M. Koch, Lesions of the nucleus basalis magnocellularis McCabe, Contribution of NMDA and non-NMDA receptors to do not impair prepulse inhibition and latent inhibition of fear- synaptic transmission from the brachium of the inferior colliculus to potentiated startle in the rat, Brain Res. 815 (1999) 98–105. the medial subdivision of the medial geniculate nucleus in the rabbit, [44] M.L. Schmitt, W. Coelho, A.S. Lopes-de-Souza, F.S. Guimaraes, Exp. Brain Res. 124 (1999) 295–303.
A.P. Carobrez, Anxiogenic-like effect of glycine and D-serine [51] J.R. Wecker, J.R. Ison, Effects of motor activity on the elicitation microinjected into dorsal periaqueductal gray matter of rats, Neuro- and modification of the startle reflex in rats, Anim. Learn. Behav. 14