www.elsevier.com / locate / bres
Research report
Protective effect of green tea extract on ischemia / reperfusion-induced
brain injury in Mongolian gerbils
National Institute of Toxicological Research, Korea Food and Drug Administration, 5, Nokbun-dong, Eunpyung-gu, Seoul, 122-704, South Korea
b
College of Pharmacy, Chungbuk National University, 48, Gaesin-Dong, Heungduk-Gu, Cheongju, Chungbuk, 361-763, South Korea
c
Division of Toxicology, College of Pharmacy, SengKyunKwan University, Chunchun-dong 300, Suwon, Kyunggido, 440-746, South Korea
Accepted 29 August 2000
Abstract
Free radical-induced oxidative damages of macromolecules and cell death are important factors in the pathogenesis of ischemia / reperfusion brain injury. In the present study, an investigation as to whether green tea extract reduces ischemia / reperfusion-induced brain injury in Mongolian gerbils was conducted. The effect of green tea on the ischemia / reperfusion-induced production of hydrogen peroxide, lipid peroxidation and oxidative DNA damage (formation of 8-hydroxydeoxyguanosine), and cell death in addition to locomotor activity was studied. Two doses (0.5 or 2%) of green tea extract were added into the drinking water and to be accessed by animals ad libitum for 3 weeks prior to the induction of ischemia. A global ischemia was induced by the bilateral occlusion of the common carotid arteries for 5 min. Reperfusion was achieved by releasing the occlusion and restoring blood circulation for 48 h. The infarction volumes were 112631
3 3 3
mm and 76611 mm in the 0.5 and 2% green tea pretreated animals compared to 189612 mm in the ischemia / reperfusion animals. Green tea extract also reduced the levels of ischemia / reperfusion-induced hydrogen peroxide (from 14706170 to 1034646 and 555630 nmole / mg protein), lipid peroxidation products (from 14106210 to 930640 and 330620 nmole / mg protein) and 8-oxodG (from 3.960.1
22
to 2.860.3 and1.960.3 ng /mg DNA,310 ) by pretreatment of 0.5 or 2% green tea for 3 weeks, respectively. Moreover, green tea also reduced the number of ischemia / reperfusion-induced apoptotic cells (from 59612 to 3768, 15611 apoptotic cells / high power field in the striatum region) and locomotor activity (from 1514062940 to 39006600 and 410061200). This study therefore suggests that green tea may be a useful agent for the prevention of cerebral ischemia damage. 2001 Elsevier Science B.V. All rights reserved.
Keywords: Green Tea extract; Ischemia / reperfusion; Oxidative damage; Gerbil
1. Introduction The formation of these oxidation products in the human
brain increases with age and ischemia / reperfusion animal It is generally believed that ischemia / reperfusion-me- brain [15,24]. It is therefore of interest to study the effects diated brain injury results, at least in part, from the of antioxidants, free radical scavengers or trapping agents oxidation of cellular macromolecules [21]. Because of the for use as potential cerebroprotective agents of various brain’s high consumption of oxygen, high concentration of brain injuries including ischemia / reperfusion-mediated polyunsaturated fatty acid and transition metals, and low brain injury.
concentration of antioxidants, it is vulnerable to ischemia / Green tea has been reported to have antioxidant prop-reperfusion-induced reactive oxygen species which cause erties [19]. Green tea reduces iron-induced lipid peroxida-oxidative damage to lipids and brain DNA. The formation tion in brain homogenates as well as in cultured C6 of lipids and DNA oxidation products e.g. malondial- astrocytes and lung cells [13,14,27,11]. In addition, green dehyde and 8-hydroxydeoxyguanosine have been found to tea has also been shown to reduce the formation of the cause cellular dysfunction or / and cell death, which leads spin-adducts of hydroxyl radicals and hydroxyl radical-to further impairment of the central nervous system [7]. induced DNA strand breakage in vitro [6] in addition to UV radiation-induced oxidative DNA damage in calf thymus DNA [23]. Moreover, green tea has been found to *Corresponding author. Tel.:182-2-380-1783; fax:182-2-380-1786.
E-mail address: [email protected] (J.T. Hong). have inhibitory effects on the UVB light-induced skin [20]
and chemical-induced lung tumorigenesis [25]. There is each brain slice was defined by the 2,3,5-triphenyltet-also considerable epidemiological evidence suggesting that razolium chloride (TTC) staining method. After 48 h the consumption of green tea lowers the risk of heart reperfusion, the gerbils received an intracardiac perfusion disease as well as several types of cancer incidences as a of 0.9% buffered saline. The brain was then removed, and result of these antioxidant mechanisms [1]. cut into 2-mm serial slices starting 1 mm from frontal pole. In the present study, the protective effect that green tea The coronal slices were then immersed in a 2% phosphate-extract has on ischemia / reperfusion-induced brain injury buffered solution for 50 min at 378C. After TTC staining, was examined. In particular, the ischemia / reperfusion- the slices were fixed in 10% phosphate-buffered formalin induced production of hydrogen peroxide, lipid peroxida- and the infarction area was then determined by an image tion and oxidative DNA damage (formation of 8-hydroxy- analyzer using the leicaQwin programme (Leica Mi-deoxyguanosine), and cell death as well as locomotor crosystem Image Solution Ltd., Cambridge, UK). The
2
activity on the Mongolian gerbil were focused. infarct area (mm ) from each thicken-brain slice was
3
determined, and the infarct volume (mm ) was calculated from sum of the slice areas (7 slices in all)3thickness (2
2. Materials and methods mm).
2.1. Animals 2.4. Determination of hydrogen peroxide level, lipid
peroxidation and8-hydroxydeoxyguanosine (8-oxodG) The female Mongolian gerbils used were maintained in
accordance with the National Institute of Toxicological The level of hydrogen peroxide in the whole brain tissue Research of the Korea Food and Drug Administration was measured using the BIOXYTECH H O -560 assay kit2 2
guideline for the care and use of laboratory animals. The (OXIS International Inc., USA). The formation of malon-female Mongolian gerbils (body weight 50–60 g) were aldehyde and 4-hydroxynonenal, as lipid peroxidation housed 4–5 per cage and maintained at 22628C with a products in the whole brain homogenate was also de-constant humidity for at least 1 week prior to the com- termined using a lipid peroxidation assay kit (OXIS mencement of the experiments. All the animals were International Inc., Ohio, USA) according to the methods allowed free access to food and water before and after described in the manufacturer’s protocol. For the determi-ischemia surgery. The 2 doses (0.5 or 2%) of green tea nation of 8-oxodG, isolated DNA from whole brain extract were added into the drinking water and were homogenate was purified and digested as described previ-accessed by animals for 3 weeks ad libitum before the ously [5]. The digested DNA was then analyzed by the induction of ischemia. The method of green tea extraction ELISA assay kit (Japan Institute for the Control of Aging, and compounds in the extracts are described elsewhere [8]. Nikken Foods Co., Japan) to detect 8-oxodG formation. Briefly, dried green tea leaves were extracted with 70%
ethanol at 90–958C for 6 h. The extract was then fraction- 2.5. Detection of apoptotic cells ated by column chromatography using Amberite XAD-7
(mean pore diameter, 90 A). The total catechin content of The terminal deoxynucleotidyltransferase (TdT)-medi-the extract was determined by UV spectroscopy, and (TdT)-medi-the ated dUTP-biotin nick end-labeling (TUNEL) method was composition was determined using HPLC. used for the detection of apoptotic bodies. The brains were fixed with 4% phosphate-buffered paraformaldehyde (pH
2.2. Ischemic surgery 7.4), and stored in 4% phosphate-buffered
paraformal-dehyde (pH 7.4). After 24 h, they were transferred into a The acclimatized Mongolian gerbils were anesthetized 20% phosphate-buffered sucrose solution and cut into 10 with a gas mixture of 2% isoflurane, 75% N O and 25%2 mm cryostat coronal slices starting between 7 and 9 mm O . The bilateral common carotid arteries were occluded2 from the frontal pole. The tissue sections were fixed with using sugita aneurysm clips for 5 min. During the occlu- 4% phosphate-buffered paraformaldehyde (pH 7.4) for 30 sion and postoperative period, the gerbils were kept on min at room temperature, and then incubated with a thermostat-control warming plates in order to maintain blocking solution (3% H O in water) for 5 min at room2 2
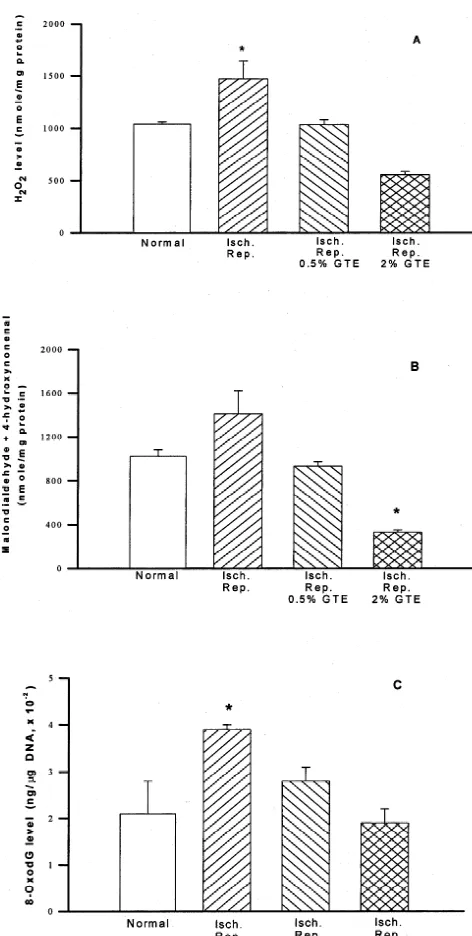
tetrachloride) substrate solution was added onto tissue tein in brain of animals pretreated by 0.5 and 2% green tea sections for 10 min at room temperature. After each step, for 3 weeks, respectively. Consistent with the hydrogen the tissue sections were rinsed twice with a phosphate- peroxide level, the level of lipid peroxidation products buffered saline solution (PBS, pH 7.4). The apoptotic (malonaldehyde and 4-hydroxynonenal) in the animals had bodies were identified under microscope (3200) as con- also increased as a result of ischemia / reperfusion (from taining brown colored nuclei. The quantity of apoptotic 1020660 to 14106210 nmole / mg protein). The animals bodies were expressed as the average number of apoptotic pretreated with green tea had substantially reduced lipid cells per high power field (visible apoptotic cells / HPF). peroxidation products especially the ones that were ad-ministered the 2% extract. The malonaldehyde and 4-2.6. Measurement of locomotor activity hydroxynonenal levels were 930640 and 330620 nmole / mg protein in brains of animals pretreated with 0.5 and 2% The locomotor activity was determined by the distances green tea for 3 weeks, respectively (Fig. 2B). The 8-oxodG traveled by the animals for 1 h (counts / cm) in the level had also increased in brains of ischemia / reperfusion
22
locomotor activity apparatus (OPTI-Varimex , Columbus animals (3.960.1 ng /mg DNA, 310 ) compared to the
22
Instrument, Ohio, USA). Spontaneous locomotor activity control animals (2.160.7 ng /mg DNA, 310 ). These
22
was monitored by a single break (defined as an activity) in values were reduced to 2.860.3 ng /mg DNA, 310 ,
22
the photocell light beam emitted from 15 sites in the 1.960.3 ng /mg DNA, 310 as a result of pretreatment
photocell chamber. with 0.5 or 2% green tea for 3 weeks, respectively (Fig.
2C). 2.7. Statistics
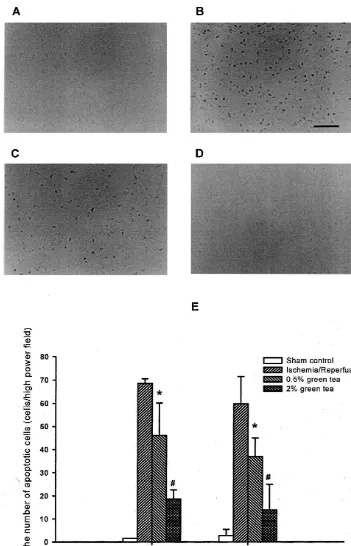
3.3. Formation of apoptotic cells The data was expressed as the mean6standard error.
The data was analyzed with a one-way analysis of the In order to determine whether green tea ingestion had variance followed by either Dunnett’s or Bonferroni’s the effect of reducing the level of oxidative damage to method as a post hoc test. Differences were considered macromolecules and hence prevent neuronal death, the significant at P,0.05. level of apoptotic cell formation was measured. In the preliminary study, it was found that the number of surviving neurons was greatly reduced in the striatum and
3. Results cortex as well as the hippocampus [16]. Therefore,
neuro-nal cell death in striatum and cortex regions was detected 3.1. Ischemia /reperfusion-induced brain infarction to observe whether cell death could influence neuronal cell loss. In the ischemia / reperfusion group, there was a clear The effect of the occlusion and reperfusion time on increase in the number of apoptotic cells in the striatum of brain infarct was examined initially. Different degrees of the brain. In this region, only occasional apoptotic cells ischemia infarction volume were created by varying dura- (2–3 cells / HPF) were detected in the sham-operated group tion of occlusion and reperfusion (data not shown). The (Fig. 3). In the striatum region of the brains of animals highest infarct volume (total infarction volume was pretreated by 0.5 and 2% green tea, the number of
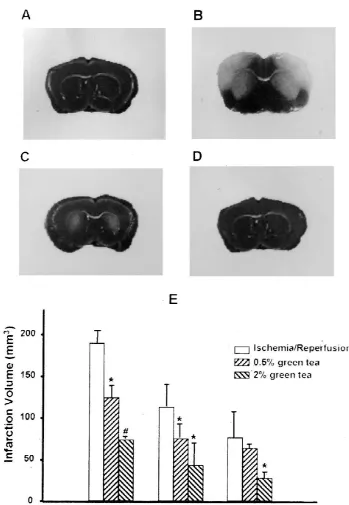
3
189612 mm ) was induced in the animals after 5 min- apoptotic cells (59611) by ischemia / reperfusion was ischemia / 48 h-reperfusion. Treatment with green tea for 3 reduced to 3768 and 15611, respectively. A similar weeks prior to the induction of ischemia attenuated the increase in the number of apoptotic cells by ischemia / ischemia / reperfusion-induced brain infarction (112631 reperfusion and inhibitory effect of green tea was also
3
mm in the 0.5% green tea pretreated animals and 76611 found in the cortex region (Fig. 3).
3
mm in the 2% green tea pretreated animals, Fig. 1).
3.4. Locomotor activity 3.2. Hydrogen peroxide, lipid peroxidation product and
8-oxodG levels The value of locomotor activity (traveled distance, cm)
Fig. 1. Brain infarction after 5 min-ischemia / 48 h-reperfusion caused by the occlusion of the bilateral common carotid artery in Mongolian gerbils. The brain infarction was detected in brain slices cut at 6 mm away from the frontal pole by the 2,3,5-triphenyltetrazolium chloride staining methods described in Materials and methods. 0.5 and 2% green tea extracts (GTE) were given to the animals for 3 weeks before induction of ischemia. A; sham control, B; 5 min-ischemia / 48 h-reperfusion, C; 5 min-ischemia / 48 h-reperfusion after 0.5% GTE pretreatment for 3 weeks, D; 5 min-ischemia / 48 h-reperfusion after 2% GTE treatment for 3 weeks. E; Infarction volumes (n56). *; significant difference from ischemia / reperfusion group (P,0.05),[significant difference
from 0.5% green tea group.
of cerebral ischemia has been widely studied [16,4]. In this investigation, it was found that pretreatment with green tea extract had inhibitory effects on the ischemia / reperfusion-induced increase of hydrogen peroxide, lipid peroxidation products and 8-oxodG (a form of oxidative DNA damage) levels. Moreover, pretreatment with green tea attenuated ischemia / reperfusion-induced brain infarction and cell death.
Both the modification of enzymes involving the free radical generating procedure and treatment of free radical scavengers have been used by many investigators to ameliorate brain injury in animals following focal or global cerebral ischemia. Chan et al. [3] reported that increased endogenous superoxide dismutase activity in transgenic mice resulted in the alteration of the antioxidant system that favored cytoprotection of the brain against ischemia injury. Dimethyl sulfoxide (DMSO) and ethanol, having an ability to scavenge free radicals were also found to reduce 5-min ischemia / 5-day reperfusion-evoked neuronal cell death in the gerbil hippocampus [18]. Melatonin protected ischemia / reperfusion-induced oxidative lipid and DNA damage by scavenging free radicals [22]. The effect of green tea on the hydrogen peroxide level in the brain was first examined since hydrogen peroxide is one of the major reactive oxygen species present after ischemia / reper-fusion-induced oxidative brain injury. The hydrogen peroxide level had increased about 30% after ischemia / reperfusion. This increased level was prevented in green tea extract pretreated animals. This suggests that green tea extract acts as a scavenger of existing hydrogen peroxide, as seen in the other in vitro and in vivo systems [12,6,23]. The hydrogen peroxide levels found in the 2% green tea extract pretreated group was far lower than that found in the sham control group. This inhibitory effect of green tea extract on the hydrogen peroxide level partly results from the preventive effect on hydrogen peroxide production via increasing enzyme activity or expression of the enzyme. In fact, it was reported that pretreatment with green tea extract increased the level of catalase activity in the liver, lung and kidney [12,9,26].
Fig. 2. Level of hydrogen peroxide (A), lipid peroxidation products
(malondialdehyde plus 4-hydroxynonenal, B) and 8-oxodeoxyguanosine The next stage was to determine whether the production (8-OxodG, C) in the 5 min-ischemia / 48 h-reperfusion brain caused by of hydrogen peroxide subsequently resulted in oxidative the bilateral common carotid artery occlusion of Mongolian gerbils. 0.5
damage to the lipids and DNA (lipid peroxidation and and 2% green tea extracts (GTE) were treated for 3 weeks before
formation of 8-oxodG) and whether green tea pretreatment induction of ischemia (n56). *; significant difference from normal group
could inhibit such damage. Consistent with the hydrogen (P,0.05).
peroxide level, the lipid peroxidation products (malon-aldehyde and 4-hydroxynonenal) level had increased as a result of ischemia / reperfusion. However, this increase was
4. Discussion inhibited by pretreatment with 0.5 or 2% green tea extract
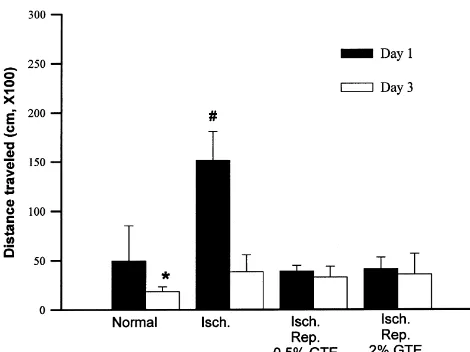
tea has on brain injury is related to the inhibitory effect on macromolecule oxidative damage, which leads to a protec-tive effect on the ischemia / reperfusion-induced cell death. Since ischemia generally results in significant changes in behavior due to brain damage, the effect that green tea extract has on the locomotor activity after an ischemia / reperfusion-induced change was examined. The locomotor activity had increased significantly in the ischemia / re-perfusion animals. Pretreatment with green tea extract inhibited the ischemia / reperfusion-induced increase in locomotor activity measured 1 day after ischemia / reperfu-sion. After 3 days, the increased locomotor activity was not inhibited by green tea extract, and was maintained at a level 2 times that of the control. It has been reported that antioxidants protect ischemia / reperfusion-induced brain damage, and consequently protect behavioral deficits Fig. 4. Changes in the locomotor activity after 5 min-ischemia / 48
h-reperfusion brain injury caused by the bilateral common artery occlusion [2,17]. It is possible that the protective effect of green tea of Mongolian gerbils. 0.5 and 2% green tea extracts were treated for 3 on brain injury might contribute to a beneficial effect on weeks before the induction of ischemia (n56). *; significant difference
the behavioral changes. from day 1 (P,0.05),[significant difference from control group of day
In conclusion, these findings clearly demonstrate that 1 (P,0.05).
green tea has a protective effect on ischemia / reperfusion-induced brain injury and behavior deficits that are at least 8-oxodG level. The reasons for the differential vulnerabili- in part the result of its antioxidant properties. This study ty of macromolecules to hydrogen peroxide are unclear. suggests that green tea may be a useful agent for the One possible explanation is that catalase (induced by green prevention of cerebral ischemia damage. Recently, it was tea extract) can detoxify cytosolic hydrogen peroxide, but also found that green tea has anti-thrombosis effects in not in the nucleus where the DNA resides. However, this mice and anti-platelet aggregation activity in the human causative correlation between hydrogen peroxide product- platelet. This may have an important influence on the ion and increase in oxidative damage of macromolecules, re-establishment and maintenance of cerebral blood flow in addition to the inhibitory effect of green tea extract, post ischemia [8]. The roles and mechanisms of the anti-further confirmed the free radical hypothesis in ischemia / platelet and thrombosis activities of green tea on ischemia / reperfusion-induced brain injury. reperfusion-induced brain injury, and other biological Evidence that lipid peroxidation product mediates oxida- functions of green tea, are currently under investigation. tive stress-induced neuronal cell death has been
demon-strated [10]. There was also a report showing the co-incidence of 8-oxodG formation and cell death during
Acknowledgements ischemia / reperfusion [24]. Therefore, the inhibitory effect
of green tea on cell death was examined. It was previously
This study was partially supported by RESEARCH found that the number of surviving neuronal cells in the
FUND (HMP-96-D-2-1033) from Korean Ministry of brain of ischemia / reperfusion animals significantly
de-Health and Welfare from 1996 to 1999. creased in the striatum and cortex region as well as
hippocampus [4,18]. In the present study, there was a clear increase in the number of apoptotic cells in the ischemia /
reperfusion brain. The number of apoptotic cells as a References consequence of ischemia / reperfusion was reduced by
green tea pretreatment (values reported in the Results [1] N. Ahmad, H. Mukhtar, Green tea polyphenols and cancer: biologic mechanisms and practical implications, Nutr. Rev. 57 (1999) 78–83. section). A similar protective effect of green tea extract on
[2] F. Block, W. Schmitt, M. Schwarz, The antioxidant LY 231617 the increase in the number of apoptotic cells was detected
ameliorates functional and morphological sequelae induced by in the cortex region. This attenuation pattern was corre- global ischemia in rats, Brain Res. 694 (1995) 308–311.
lated with the amelioration of the brain infarction (the [3] P.H. Chan, H. Kinouchi, C.J. Epstein, E. Carlson, S.F. Chen, S. correlation coefficient values were r50.995 in cortex and Imaizumi, G.Y. Yang, Role of superoxide dismutase in ischemic brain injury: reduction of edema and infarction in transgenic mice
r50.971 in striatum, respectively). The infarction volume
3 following focal cerebral ischemia, Prog. Brain Res. 96 (1993)
was 189612 mm in the 5 min-ischemia / 48 h-reperfusion
97–104.
3
sham-operated animals, whereas it was 112631 mm in [4] J. Chen, K. Jin, M. Chen, W. Pei, K. Kawaguchi, D.A. Greenberg,
3
apoptosis and neuronal cell death, J. Neurochem. 69 (1997) 232– glutamine synthetase activity, and production of free radicals during
245. ischemia / reperfusion-induced injury to gerbil brain, Proc. Natl.
[5] D.H. Cho, J.T. Hong, K. Chin, T.S. Cho, B.M. Lee, Organotropic Acad. Sci. 87 (1990) 5144–5147.
formation and disappearance of 8-hydroxydeoxyguanosine in the [17] J.W. Phillis, C. Clough-Helfman, Protection from cerebral ischemic kidney of Sprague–Dawley rats exposed to adriamycin and KbrO3, injury in gerbils with the spin trap agent N-tert-butyl-alpha-Cancer Lett. 74 (1993) 141–145. phenylnitrone (PBN), Neurosci. Lett. 116 (1990) 315–319. [6] K. Hiramoto, N. Ojima, K. Sako, K. Kikugawa, Effect of plant [18] J.W. Phillis, A.Y. Estevez, M.H. O’Regan, Protective effects of the
phenolics on the formation of the spin-adduct of hydroxyl radical free radical scavengers, dimethyl sulfoxide and ethanol, in cerebral and the DNA strand breaking by hydroxyl radical, Biol. Pharm. ischemia in gerbils, Neurosci. Lett. 244 (1998) 109–111. Bull. 19 (1996) 558–563. [19] R.L. Prior, G. Cao, Antioxidant capacity and polyphenolic com-[7] H. Islekel, S. Islekel, G. Guner, N. Ozdamar, Evaluation of lipid ponents of teas: implications for altering in vivo antioxidant status,
peroxidation, cathepsin L and acid phosphatase activities in ex- Proc. Soc. Exp. Biol. Med. 220 (1999) 255–261.
perimental brain ischemia-reperfusion, Brain. Res. 843 (1999) 18– [20] I.R. Record, I.E. Dreosti, Protection by black tea and green tea
24. against UVB and UVA1B induced skin cancer in hairless mice,
[8] W.S. Kang, I.H. Lim, D.Y. Yuk, K.H. Chung, J.B. Park, H.S. Yoo, Mutat. Res. 422 (1998) 191–199.
Y.P. Yun, Antithrombotic activities of green tea catechins and (2)- [21] B.K. Siesjo, A new perspective on ischemic brain damage?, Prog. epigallocatechin gallate, Thromb. Res. 96 (1999) 229–237. Brain. Res. 96 (1993) 1–9.
[9] S.G. Khan, S.K. Katiyar, R. Agarwal, H. Mukhtar, Enhancement of [22] A. Wakatsuki, Y. Okatani, C. Izumiya, N. Ikenoue, Melatonin antioxidant and phase II enzymes by oral feeding of green tea protects against ischemia and reperfusion-induced oxidative lipid polyphenols in drinking water to SKH-1 hairless mice: possible role and DNA damage in fetal rat brain, J. Pineal Res. 26 (1999) in cancer chemoprevention, Cancer Res. 52 (1992) 4050–4052. 147–152.
[10] I. Kruman, A.J. Bruce-Keller, D. Bredesen, G. Waeg, M.P. Mattson, [23] H. Wei, X. Zhang, J.F. Zhao, Z.Y. Wang, D. Bickers, M. Lebwohl, Evidence that 4-hydroxynonenal mediates oxidative stress-induced Scavenging of hydrogen peroxide and inhibition of ultraviolet light-neuronal apoptosis, J. Neurosci. 17 (1997) 5089–5100. induced oxidative DNA damage by aqueous extracts from green and [11] P. Leanderson, A.O. Faresjo, C. Tagesson, Green tea polyphenols black teas, Free Radic. Biol. Med. 26 (1999) 1427–1435.
inhibit oxidant-induced DNA strand breakage in cultured lung cells, [24] M.H. Won, T.C. Kang, G.S. Jeon, J.C. Lee, D.Y. Kim, E.M. Choi, Free Radic. Biol. Med. 23 (1997) 235–242. K.H. Lee, C.D. Choi, M.H. Chung, S.S. Cho, Immunohistochemical [12] S.F. Lee, Y.C. Liang, J.K. Lin, Inhibition of 1,2,4-benzenetriol- detection of oxidative DNA damage induced by ischemia-reperfu-generated active oxygen species and induction of phase II enzymes sion insults in gerbil hippocampus in vivo, Brain Res. 836 (1999) by green tea polyphenols, Chem. Biol. Interact. 98 (1995) 283–301. 70–78.
[13] A.M. Lin, B.Y. Chyi, L.Y. Wu, L.S. Hwang, L.T. Ho, The antioxida- [25] Y. Xu, C.T. Ho, S.G. Amin, C. Han, F.L. Chung, Inhibition of tive property of green tea against iron-induced oxidative stress in rat tobacco-specific nitrosamine-induced lung tumorigenesis in A / J brain, Chin. J. Physiol. 41 (1998) 189–194. mice by green tea and its major polyphenol as antioxidants, Cancer [14] E.A. Mazzio, N. Harris, K.F. Soliman, Food constituents attenuate Res. 52 (1992) 3875–3879.
monoamine oxidase activity and peroxide levels in C6 astrocyte [26] T. Yokozawa, T. Nakagawa, K.I. Lee, E.J. Cho, K. Terasawa, S. cells, Planta Med. 64 (1998) 603–606. Takeuchi, Effects of green tea tannin on cisplatin-induced nephro-[15] P. Mecocci, U. MacGarvey, A.E. Kaufman, D. Koontz, J.M. pathy in LLC-PK1 cells and rats, J. Pharm. Pharmacol. 51 (1999)
Shoffner, D.C. Wallace, M.F. Beal, Oxidative damage to mito- 1325–1331.
chondrial DNA shows marked age-dependent increases in human [27] T. Yoneda, M. Hiramatsu, M. Sakamoto, K. Togasaki, M. Komatsu, brain, Ann. Neurol. 34 (1993) 609–616. K. Yamaguchi, Antioxidant effects of ‘beta catechin’, Biochem. Mol. [16] C.N. Oliver, P.E. Starke-Reed, E.R. Stadtman, G.J. Liu, J.M. Biol. Int. 35 (1995) 995–1008.