BIOSYSTEMATICS OF
PANDANACEAE
IN JAVA
SRI ENDARTI RAHAYU
THE GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
STATEMENT OF RESEARCH ORIGINALITY AND
INFORMATION SOURCE
This is to certify that my dissertation entitled Biosystematics of Pandanaceaein Java is my own work and never been submitted to any institution before. All the incorporated data and information are valid and stated clearly in the text and listed in the references.
Bogor, Januari 2011
BIOSYSTEMATICS OF
PANDANACEAE
IN JAVA
SRI ENDARTI RAHAYU
THE GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
STATEMENT OF RESEARCH ORIGINALITY AND
INFORMATION SOURCE
This is to certify that my dissertation entitled Biosystematics of Pandanaceaein Java is my own work and never been submitted to any institution before. All the incorporated data and information are valid and stated clearly in the text and listed in the references.
Bogor, Januari 2011
ABSTRAK
SRI ENDARTI RAHAYU. Biosystematics of Pandanaceae in Java. Di bawah bimbingan ALEX HARTANA, TATIK CHIKMAWATI, KUSWATA KARTAWINATA dan MIEN A. RIFAI.
Pandanaceae di Jawa diwakili oleh dua marga, yaitu Freycinetia Gaud. danPandanusParkins. Sejak penelitian oleh Backer dan Bakhuizen van den Brink pada tahun 1968 dan Stone pada tahun 1972, belum pernah dilakukan eksplorasi lebih jauh tentang flora pandan di Jawa sehingga sampai saat ini banyak hal yang belum diketahui tentang flora pandan tersebut. Masalah taksonomi tentang Pandanaceae yang ada di Jawa, tidak hanya tentang status spesies dari P. odoratissimus L.f dan P. tectorius var. littoralis yang dianggap sinonim, tetapi juga status spesies yang merupakan sinonim dari P. furcatus Roxb, yaitu P. bantamensisKoord.,P. oviger Martelli,P. pseudolais Warb., dan P. scabrifolius Martelli. Backer dan Bakhuizen van den Brink memasukkan P. bantamensis Koord., P. oviger Martelli, P. pseudolais Warb., dan P. scabrifolius Martelli ke dalam satu spesies, yaituP. furcatusRoxb., sedangkan menurut Stone ke empat spesies tersebut adalah empat spesies yang berbeda. Berdasarkan latar belakang permasalahan tersebut perlu dilakukan penelitian untuk dapat mendeskripsikan kembali spesies-spesies tersebut dengan menggunakan data morfologi, anatomi dan molekular, seperti sekuen antar genatpB-rbcL, karena suatu klasifikasi yang paling memuaskan tergantung pada interpretasi dari sebanyak mungkin karakter. Dalam penelitian ini hanya ditemukan tiga dari empat spesies tersebut di atas, yaitu P. bantamensisKoord., P. pseudolais Warb., dan P. scabrifolius Martelli. Hasil penelitian menunjukkan bahwa P. bantamensis Koord., P. pseudolais Warb., dan P. scabrifolius Martelli diperlakukan sebagai tiga spesies yang berbeda, dan P. odoratissimus L.f dan P. tecrorius var. littoralis diperlakukan sebagai dua spesies yang berbeda. Penelitian ISSR menunjukkan bahwa ke enam spesies Freycinetia dan 13 spesies Pandanus di Jawa memiliki keanekaragaman genetik yang tinggi, walaupun keanekaragaman genetik Freycinetia sedikit lebih rendah bila dibandingkan denganPandanus.Hasil penelitian menunjukkan bahwa di Jawa dapat dikenali tujuh spesies Freycinetia dan 16 spesies Pandanus. Ketujuh spesiesFreycinetia tersebut mencakupF. angustifoliaBl.,F. funicularis Merr., F. imbricata Bl., F. insignis Bl., F. javanicaBl., F. scandensGaud., dan F. sumatrana Hemsl. Keenambelas spesies Pandanus tersebut terdiri dari P. amaryllifolius Roxb.,P. bantamensis Koord.,P. bidur Jungh.,P. dubius Spreng, P. favigerBacker,P. kurziiMerr.,P. labyrinthicusKurz, P. multifurcatusFagerl., P. nitidus Kurz, P. odoratissimus L.f., P. polycephalus Lam., P. pseudolais Warb., P. scabrifoliusMartelli, P. spinistigmaticus Fagerl.,P. tectorius Parkins, dengan dua varietas, yakniP. tectoriusvar.littoralis, P. tectoriusvar.samak, satu kultivar, yaitu Pandanus tectoriuscv. Sanderi, P. utilisBory; satu varietas, P. leramJones var.andamanensium( Kurz) Stone, dan satu kultivar,P. spuriusMiq. cv. Putat. Di Jawa, keanekaragaman Pandanaceae yang paling tinggi terdapat di hutan pegunungan bawah dan bukit pegunungan.
ABSTRACT
SRI ENDARTI RAHAYU. Biosystematics of Pandanaceae in Java. Supervised by ALEX HARTANA, TATIK CHIKMAWATI, KUSWATA KARTAWINATA, and MIEN A. RIFAI.
Pandan family (Pandanaceae) is represented in Java by two genera: Freycinetia Gaud. and Pandanus Parkins. Since the studies by Backer and Bakhuizen van den Brink in 1968 and Stone in 1972, there were no further exploration on the pandan flora of the island have been made, thus the pandan flora remains largely unknown. Taxonomical problem as far as the Java pandans concerned are centered not only on species status ofP. odoratissimus L.f and P. tecroriusvar.littoraliswhich are regarded as synonym, but also the species status which are given as synonym ofP. furcatus Roxb. by Backer and Bakhuizen van den Brink. Backer and Bakhuizen van den Brink included of P. bantamensis Koord., P. oviger Martelli, P. pseudolais Warb. and P. scabrifolius Martelli as synonym to P. furcatus Roxb. This Backer and Bakhuizen van den Brink’s classification is in contrast with Stone who stated that these species were regarded as four different species. For the reason of this taxonomical problem, therefore an effort has been made to redescribe these species in detail, using morphological, anatomical and molecular data such as sequence data of atpB-rbcL IGS, since a satisfactory classification depends upon the interpretation of many characters as possible. In this study we only found three of four species mentioned above, viz. P. bantamensis Koord., P. pseudolais Warb., and P. scabrifolius Martelli. The result showed that P. bantamensis Koord., P. pseudolais Warb, and P. scabrifolius Martelli are treated as three different species, and P. odoratissimus L.f and P. tectorius var.littoralis are treated as two different species. The ISSR marker showed that six species of Freycinetia and thirteen species of Pandanus from Java have high genetic diversity, although Freycinetia has a bit lower than Pandanus. This research showed seven species of Freycinetia could be recognized, viz. F. angustifolia Bl., F. funicularis Merr., .F. imbricata Bl., F. insignisBl.,F. javanicaBl.,F. scandensGaud., andF. sumatranaHemsl.; sixteen species of Pandanus, viz. P. amaryllifolius Roxb., P. bantamensis Koord., P. bidur Jungh., P. dubius Spreng., P. faviger Backer, P. kurzii Merr., P. labyrinthicus Kurz, P. multifurcatus Fagerl., P. nitidus Kurz, P. odoratissimus L.f., P. polycephalus Lam., P. pseudolais Warb., P. scabrifolius Martelli, P. spinistigmaticusFagerl., P. tectorius Parkins. with two varieties, viz. P. tectorius var. littoralis, P. tectorius var. samak, one cultivar, i.e Pandanus tectorius cv. Sanderi; P. utilis Bory; one variety P. leram Jones var. andamanensium (Kurz) Stone, and one cultivar,P. spuriusMiq. cv. Putat.Pandanaceaein Java are most diverse in lowland rain forest and hill forest.
SUMMARY
SRI ENDARTI RAHAYU. Biosystematics ofPandanaceaein Java. Supervised by ALEX HARTANA, TATIK CHIKMAWATI, KUSWATA KARTAWINATA, and MIEN A. RIFAI.
Pandan family (Pandanaceae) is represented in Java by two genera, Freycinetia Gaud. and Pandanus Parkins. Since the studies by Backer and Bakhuizen van den Brink in 1968 and Stone in 1972, there were no further exploration on the pandan flora of the island have been made, thus the pandan flora remains largely unknown. Taxonomical problem as far as the Java pandans concerned, are centered not only on the species status ofPandanus odoratissimus L.f andP. tecrorius var.littoralis which are considered as synonym, but also the species status which are given as synonym ofPandanus furcatusRoxb. by Backer and Bakhuizen van den Brink. Under the latter they included of P. bantamensis Koord.,P. oviger Martelli,P. pseudolaisWarb., and P. scabrifoliusMartelli into one species ofP. furcatusRoxb. This classification is in contrast with Stone who stated that these species were regarded as four different species. Therefore an effort has been made to redescribe these species in detail, using morphological, anatomical and molecular data such as sequence data ofatpB-rbcL IGS to provide better understanding of morphological, anatomical and molecular characters in supporting taxa delimitation and its distribution in Java.
This study was based mainly on available specimens at the Herbarium Bogoriemse (BO), National Herbarium Netherlands, Leiden (L) and Herbarium of the Royal Botanical Gardens Kew (K) and new collection specimens obtained from field work in different location in Java. In addition, living plants grown in botanical garden were also studied. Five species that was planted in Bogor Botanical Garden, viz. Pandanus kurzii, P. labyrinthicus, P. multifurcatus, P. polycephalusandP. spinistigmaticuswere also examined.
Characters of leaf shape, leaf apex, the morphology of leaf auricles and type of pistillate inflorecence were found useful in delimitation and identification of Javanese Freycinetia, while character of habit, the surface of stem, present or absent of prop root, the surface of prop root, the leaf shape, leaf apex, the armature of leaf margins and midrib, the colour of leaf margin and midrib teeth, the distinctness or indistinctness of tertiary cross vein, present or absent of apical ventral pleats, the texture of leaves in dry state, phalange shapes, the position of infructescence, position of seed chamber and stigma shape are proved useful for distinguishing species of JavanesePandanus.
the epidermal tissue including the stomata proves to be a great value in identification ofPandanusspecies in Java.
Morphological, anatomical and comparative sequence data of atpB-rbcL IGS were able to solve the taxonomical problem of Pandanus furcatus complex and P. tectorius complex and as a result P. bantamensis Koord, P. pseudolais Warb. and P. scabrifolius Martelli treated as three different species, and P. odoratissimusL.f andP. tectoriusvar.littoralis treated as two different species.
ISSR (Inter Simple Sequence Repeat) study showed that six species of Freycinetia and thirteen species of Pandanus from Java have high genetic diversity, although Freycinetia has a bit lower of genetic diversity than Pandanus, while Principal component analysis (PCA) for Javanese thirteen Pandanusand six JavaneseFreycinetiashowed a bit different in clustering pattern and species relationships compared to the cluster analysis.
This study showed that in Java there are seven species of Freycinetia, viz. F. angustifolia Bl., F. funicularis Merr., F. imbricata Bl., F. insignis Bl., F. javanica Bl.,F. scandensGaud., andF. sumatranaHemsl.; and sixteen species of Pandanus, viz.P. amaryllifoliusRoxb., P. bantamensisKoord., P. bidur Jungh., P. dubius Spreng., P. faviger Backer, P. kurzii Merr., P. labyrinthicusKurz, P. multifurcatus Fagerl., P. nitidus Kurz, P. odoratissimus L.f., P. polycephalus Lam., P. pseudolais Warb., P. scabrifolius Martelli., P. spinistigmaticus Fagerl., P. tectoriusParkins, with two varieties, viz.P.tectoriusvar.littoralis,P. tectorius var. samak, one cultivar, i.e Pandanus tectorius cv. Sanderi, P. utilis Bory; one variety P. leram Jones var. andamanensium (Kurz) Stone, and one cultivar, P. spuriusMiq. cv. Putat.
GENERAL INTRODUCTION
Taxonomical Aspects ofPandanaceaein Java and Its Systematic Problems
Pandanaceae was first recognized by Robert Brown in 1810. The
Pandanaceae, the sole representative of the Pandanales, is arborescent or
scandent monocotyledons confined to the Old World tropics and subtropics (Cox
1990). Pandanaceae is a large family, consist of 4 genera: Freycinetia Gaud.,
Pandanus Parkins., Sararanga Hemsl., and Martellidendron Callm. & Chassot
(Callmanderet al. 2003).Pandanaceaecommonly known as screw pine or screw
palm has a unique growing form. The large serrated leaves up the trunk are
formed in a circular motion, giving it the screw like look. The family is important
in several regions, wherein it has contributed to the fundamental structure and
physiognomy of the vegetation (Stone 1983b).
Pandan family (Pandanaceae) is represented in Java by two genera:
Freycinetia Gaud. andPandanus Parkins. (Backer and Bakhuizen van den Brink
1968; Stone 1972). The genus Freycinetia was described in 1824 by the French
botanist Gaudichaudii (Stone 1970), and may be called climbing pandans. This
genus has been known to occur in Java for a long time, since most of the oldest
taxa nomenclaturally speaking are typified by specimens from Java (Stone
1968), and according to Stone (1970) the real home ofFreycinetiaamong others
is Java. The name Pandanus originates from the Malay word pandan, it was
latinized and first published by George Eberhard Rumpf (Rumphius) whose
Herbarium Amboinensis of 1743 contains the description of eleven species of
which eight are illustrated (Stone 1965). Java harbours a large number of species
of Pandanus,not less than fifteen species of Pandanus are mentioned in the key
given by Backer and Bakhuizen van den Brink (1968). It becomes evident that
Pandanusin Java is rich in species, considering the widely variable morphology
The last attempt at a comprehensive treatment of Pandanaceae of Java
was Backer and Bakhuizen van den Brink (1968) in their “Flora of Java” in which
they recognized seven species of Freycinetia and fifteen species of Pandanus.
Since a short visit to Hortus Bogorienses by Stone (1972), there were no further
exploration on the pandan flora of the island have been made, thus the pandan
flora remains largely unknown.
Since a large number of morphological characters are now known for
Freycinetia and Pandanus species, it appears useful to consider their use in
identifyingPandanaceaeof Java, because morphological data are regarded as the
most appropriate and the most rapid mean for identification and for constructing
map of diversity of plant (Davis & Heywood 1963). Morphological characters
have the great advantage over other characters that we can see the plant variability
easier. Unfortunately complete material is not always available for the various
numbers of a group being studied, and sometimes we need to identify incomplete
material or small fragment of material. In this case we use anatomical characters.
It is now realized that anatomical characters is as valuable as morphological ones
(Stace 1989). Some of these anatomical characters are so diagnostic that they are
now commonly used in identification or identification of fragment of plants
(incomplete material). Valuable taxonomic evidence has been obtained from the
study of leaf epidermis and stomata ofPandanus, and the appearance of particular
anatomical characters seems sufficiently constant. In this study we are able to
propose anatomical key to species forPandanusin Java. Taxonomical problem as
far as the Java pandans concerned, are centered not only on the species status ofP.
odoratissimusandP. tecroriusvar.littoralis, which are considered as a synonym,
but also the species status which are given as synonyms of Pandanus furcatus
Roxb. Backer and Bakhuizen van den Brink (1968) included P. bantamensis
Koord.,P. oviger Martelli,P. pseudolaisWarb., and P. scabrifoliusMartelli into
one species ofP. furcatusRob. This classification is in contrast with Stone (1972)
who stated that these species were regarded as four different species. In this study
P. pseudolaisWarb, P. scabrifoliusMartelli. Therefore an effort has been made
to redescribe these species in detail, using morphological, anatomical and
molecular data, i.e sequence data of atpB-rbcL IGS since a satisfactory
classification depends upon the interpretation of as many characters as possible
(Davis & Heywood 1963). Molecular data can be useful in solving different kinds
of taxonomic problem, and choloroplast marker such as atpB-rbcL IGS can be
particularly valuable at lower taxonomical level (Soltis & Soltis 1998).
The pandans in general tend to be horticulturally interesting and often
curious or unusually elegant, and tend to cause considerable public interest. The
species that are already completely well known in cultivation are few, viz. P.
dubius, P. spurius cv. Putat, P. tectorius cv. Sanderi and P. utilis. In view of its
importance and considering the large number of wild species germsplasm
available for Freycinetia and Pandanus in Java, the genetic analysis through
molecular marker is a prerequisite to have a deep insight of the genome
organization in the wild species. It is imperative, threfore to establish strategies
for the preservation of Freycinetia and Pandanus germplasm. In this study,
genetic diversity have been assessed using ISSR marker. This analysis is a
preliminary step to ensure the conservation and the development of genetic
resources, and to know the most important location to conserve Pandanaceae in
Java, we studied the distribution and ecology ofPandanaceaein Java.
Every chapter was written in different style, following the format of the
journal we are going to publish these articles, e,g. the article of Biological Flora of
Java:Pandanus tectoriusParkinson is in preparation for publication in Journal of
Ecology.
The objectives of the study
Our objective were to unravel species which is having taxonomical
problem. A combined morphological, anatomical and molecular marker was
undertaken to evaluate the suitability of the species concept; to determine genetic
A TAXONOMIC STUDY OF
PANDANUS FURCATUS
AND
PANDANUS TECTORIUS
COMPLEXES WITH SPECIAL
EMPHASIS ON PLANTS FROM JAVA
Introduction
The term species complex is used to describe species aggregation sharing
the specific morphological and molecular features (Juddet al. 1999). Within this
complex a complicated morphological overlap, without any discontinuities, has
led to taxonomic difficulty (Pak and Kawano 1990). Although their taxonomic
affinity may be difficult to determine, some form of taxonomic resolution is
desirable.
According to Stone (1972) Pandanus in Java contains many rather
problematical species. He suggested that detailed studies were required to get
more refined taxonomic scheme. The main problem as far as the Java plant are
concerned appears to be the status species which are given as synonyms of P.
furcatus Roxb. by Backer and Bakhuizen van den Brink (1968). According to
Backer and Bakhuizen van den Brink,P. bantamensisKoord.,P. ovigerMartelli,
P. pseudolais Warb., and P. scabrifolius Martelli; and P. odoratissimus L.f are
regarded as synonymous with P. tectorius var. littoralis. Stone (1972) did short
revision on Pandanaceae in Java based on specimen herbarium kept in BO and
living plant cultivated in Hortus Bogoriensis and tried to develop a more stable
species concept forPandanus furcatusRoxb. In Stone’s opinion, Backer’s species
concept for Pandanus furcatus is far too comphrehensive that required certain
readjusment because some species omitted or reduced to be lumped toPandanus
furcatus Roxb., and by Stone these species were regarded as four different
species, viz.P. bantamensisKoord.,P. pseudolaisWarb.,P. scabrifoliusMartelli,
and P. oviger Martell, while the status of P. tectorius var. littoralis and P.
odoratissimus L.f. is still more or less in question. Therefore those
circumscription should now be reviewed in light of our recent fieldwork in Java
with these taxa. A practical difficulty here is that almost all morphological
characters and/or character states used for evaluating species complex are only
slightly differentiated from one another and usually show considerable overlap.
combination of several characters. The aims of the study was to provide
taxonomic resolution of the P. furcatus and P. tectorius complexes based on
morphology, anatomy and molecular approach, i.e comparative sequence ofatp
B-rbcL IGS.
Materials and Methods
Studies of herbarium specimens were conducted in Herbarium Bogoriemse
(BO), Herbarium of the Royal Botanical Gardens Kew (K) and National
Herbarium Netherlands, Leiden (L). Observations on living plants and anatomical
were undertaken in Herbarium Bogoriense, while the molecular data was analyses
in Van der Klauw Laboratory, Leiden.
Morphology— Data on morphology were collected from specimens and
field collections. The procedure for morphological variation followed those
described by Rifai (1976) and Vogel (1987). Measurement were taken from
spirit-preserved material and dried herbarium specimen and from living collection.
Floral parts were measured from spirit-preserved material, dried specimens and
rehydrated by boiling.
Anatomy — Leaf anatomy was investigated by first fixing the leaves
(small part of the middle base) in FAA. Paradermal sections were taken from the
upper and lower surfaces of leaves, then stained with safranin 1% in water and
then mounted in glycerin (Johansen 1940).
atpB-rbcL IGS sequences — Genomic DNA of the silica gel dried leaf
were extracted according to the protocol described by Doyle and Doyle (1987).
Double stranded DNA was directly amplified by PCR. Reaction volumes were 25
µl and contained 2.5 µl PCR buffer, 2.5 µl dNTPs, 1 µl each of the 5 mM primers,
0.3 µl Taq Pol and 12.7 µl ddH2O. Approximately 5 µl genomic DNAs were
added to the PCR mixture. PCR was performed 3 min at 94oC for the activation of
the polymerase, followed by 35 cycles of 49 sec at 94oC, 45 sec at 55oC, 2 min at
72oC, with a final extension period of 10 min at 72oC. The primers used in this
study for atpB-rbcL intergenic spacers are forward
5’-GAAGTAGTAGGATTGATTCTC- 3’ and reverse
5’-TACAGTTGTCCATGTACC AG-3’. The PCR product was checked on 1%
clean up system (PROMEGA) following the manufacturer’s protocol prior to
sequencing. The DNA concentration was measured with the nanodrop. Cycle
sequencing was performed by Macrogen Korea. The sequences were edited using
sequencher 4.6 and MEGA 3.0 (Kumaret al. 2004).
Result and Discussion Result
Morphological Characters ofPandanus furcatuscomplex
In this study, we only found three of four species that recognized withinP.
furcatuscomplex. i.e P. bantamensis,P. pseudolaisand P. scabrifolius.
Concerning the prop root characters of Pandanus furcatus complex,
prickles in prop root did not display consistence differentiation among the taxa
analysed, except for P. scabrifolius where it was no prickles on the prop root.
Leaf base colour divided the taxa into two groups which one of it was overlapping
group. P. bantamensisandP. pseudolaiswere taxa with reddish brown leaf base,
andP. scabrifolius displayed yellowish white leaf base. The leaf dimensions of
Pandanus furcatus complex were highly variable. Pandanus pseudolais were
characterized by bigger and longer leaf, whereas the leaf of P. scabrifolius were
clearly smaller and shorter.P. bantamensiswas characterized by intermediate leaf
dimension. Number of drupe per cephalium of P. pseudolais were higher
compared with the other taxa. Drupe length divided the taxa into two group which
one of it was overlapping group. P. scabrifolius showed longer drupe compare
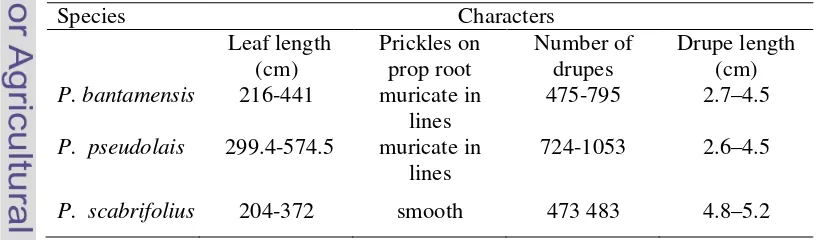
with the other taxa (Table 4.1).
Table 4.1 Morphological characters of species recognized within Pandanus furcatuscomplex
Among all morphological characters of species recognized within P.
furcatus complex, there were four characters, viz. leaf base colour, peduncle
shapes, fruit shapes and style shapes could be used to discriminate among species
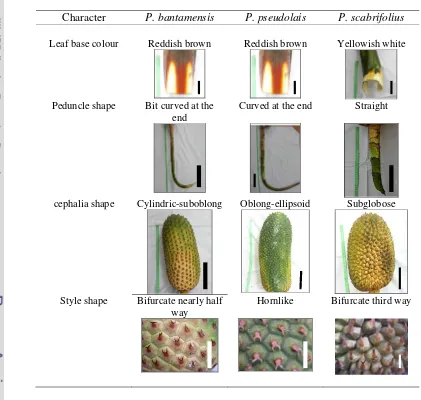
recognized withinP. furcatuscomplex. The summary of contrasting characters of
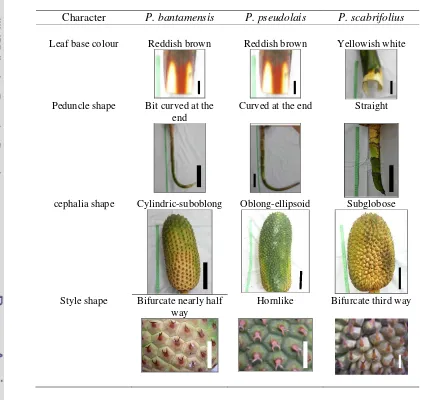
species recognized withinPandanus furcatuscomplex is shown in Table 4.2.
Table 4.2 Summary of contrasting characters of species recognized within Pandanus furcatuscomplex. Leaf base colour, scale bar = 5 cm; Peduncle, scale bar = 13 cm; Cephalia shape, scale bar = 10 cm; Style shape, scale bar = 1 cm
Character P. bantamensis P. pseudolais P. scabrifolius
Leaf base colour Reddish brown Reddish brown Yellowish white
Peduncle shape Bit curved at the end
Curved at the end Straight
cephalia shape Cylindric-suboblong Oblong-ellipsoid Subglobose
Style shape Bifurcate nearly half way
Morphological Characters ofPandanus tectoriuscomplex
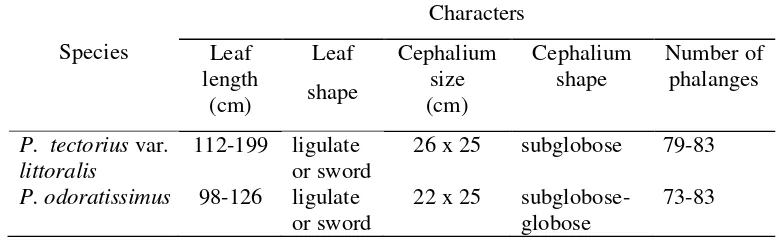
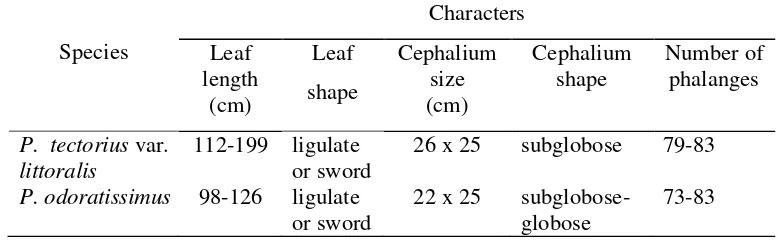
Leaf dimension, leaf shape, cephalium size, cephalium shape and number
of phalanges per cephalium did not display consistence differentiation between
the taxa analysed, but united taxa into overlapping group (Table 4.3). Among all
morphological characters of species recognized within P. tectorius complex,
there were three characters, viz. prickles on leaf apex, phalange shapes, and
phalange apexes could be used to discriminate among species recognized within
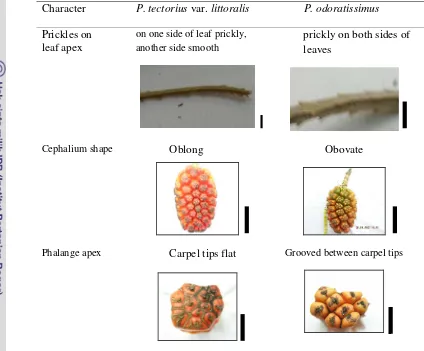
P. tectorius complex. The summary of contrasting characters of species
recognized withinPandanus tectoriuscomplex is shown in Table 4.4.
Table 4.3 Morphological characters of species recognized within Pandanus tectoriuscomplex
Species
Characters
Leaf length
(cm)
Leaf
shape
Cephalium size (cm)
Cephalium shape
Number of phalanges
P. tectoriusvar. littoralis
112-199 ligulate or sword
26 x 25 subglobose 79-83
P. odoratissimus 98-126 ligulate or sword
22 x 25 subglobose-globose
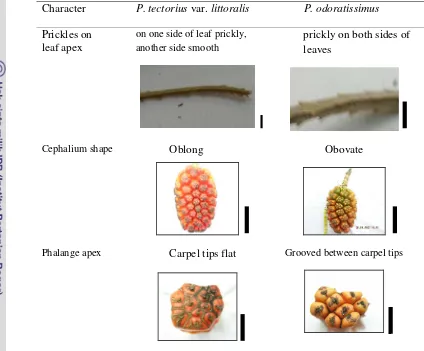
Table 4.4 Summary of contrasting characters of species recognized within Pandanus tectoriuscomplex ; Prickles on leaf apex, scale bar = 2 cm; Cephalium shape, scale bar = 10 cm; Phalange apex, scale bar = 1 cm
Character P. tectoriusvar.littoralis P. odoratissimus
Prickles on leaf apex
on one side of leaf prickly, another side smooth
prickly on both sides of leaves
Cephalium shape Oblong Obovate
Phalange apex Carpel tips flat Grooved between carpel tips
Anatomical Characters
Anatomical characters are just as valuable as morphological characters and
could be used to delimit species. These characters, qualitative characters were
observed directly on paradermal surfaces, viz. occurence of papillae on stomata.
Four types of stomata were found in Pandanus tectoriusand Pandanus furcatus
complexes. The variation on stomatal structures in Pandanus depends on the
number of papillae that develops on the subsidiary and neighbouring cells
(Tomlinson 1965; Kam 1971).
The species recognized within Pandanus furcatus complex had different
type 2, type 1 and type 3 respectively; while the species recognized within
Pandanus tectorius complex had also different stomatal type. P. tectorius var.
littoralisandP. odoratissimushad stomatal type 4 and type 2 respectively.
Molecular Characters
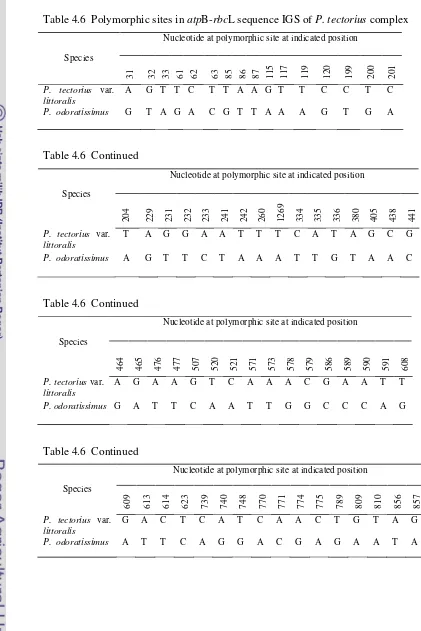
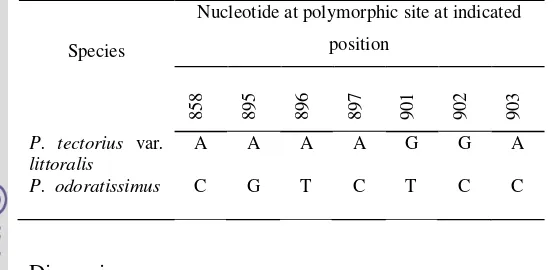
atpB-rbcL IGS sequence was a good marker for delimiting the different
species in Pandanus furcatus complex and Pandanus tectorius complex.
Polymorphic site in atpB-rbcL IGS sequence of Pandanus furcatus complex is
shown in Table 4.5.
Table 4.5 Polymorphic sites inatpB-rbcL IGS sequence ofP. furcatuscomplex
Species
Nucleotide at polymorphic site at indicated position
4 6 16 17 18 12
Polymorphic site inatpB-rbcL sequence ofPandanus tectoriuscomplex is shown
Table 4.6 Polymorphic sites inatpB-rbcL sequence IGS ofP. tectoriuscomplex
Species
Nucleotide at polymorphic site at indicated position
3
Nucleotide at polymorphic site at indicated position
2
Nucleotide at polymorphic site at indicated position
4
Nucleotide at polymorphic site at indicated position
Table 4.6 Continued
In the species recognized within Pandanus furcatus complex, they were
sharing some morphological features, viz. leaf length, prickles on prop root,
number of drupes, and drupe length. Beside those characters, these species also
had four contrasting morphological characters that could be used to separate each
species, viz. leaf base colour, peduncle shape, fruit shape and style shape. P.
bantamensishad reddish brown leaf base, peduncle with a bit curved at the end,
cylindric-suboblong fruit shape and style that bifurcate nearly half way. P.
pseudolaiswere characterized by reddish brown leaf base, peduncle that curved at
the end, oblong-ellipsoid fruit shape and hornlike style; whereas P. scabrifolius
had yellowish white leaf base, straight peduncle, subglobose fruit shape and style
that was bifurcate third way.
These species also had different anatomical characters especially on
stomatal type. The difference was on the occurence of papillae, whether occured
in lateral subsidiary cells as in P. bantamensis, in terminal and lateral subsidiary
cells as in P. scabrifolius, or papillae absent in stomata that was called
unspecilaized stomata as inP. pseudolais.
Molecular variation of the chloroplast noncoding region between atp
B-rbcL genes amongP. bantamensis,P. pseudolais, andP. scabrifoliusshowed that
most variation were contributed by nucleotide substitution, 19, 24 and 22
nucleotide substitution were found betweenP. bantamensis andP. pseudolais,P.
pseudolais and P. scabrifolius, and P. bantamensis and P. scabrifolius
Pandanus tectoriuscomplex
AlthoughPandanus tectoriusvar.littoralis andP. odoratissimusare very
closely related, they could be distinguished (Stone 1994). Stone have proposed
delimitingP. odoratissimusby just two characters, fleshy shoulders on phalanges
and large white spines on the leaves (Stone 1967). In our study we found that P.
odoratissimushas large white spines, the same asP. tectoriusvar.littoralis,but it
does not have fleshy shoulders on the phalanges. It is supported by John (1979)
who stated that P.odoratissimus has large white spines, but does not have fleshy
shoulders. Morphological observation revealed that large white spine on the leave
character are not good characters for separating the two species because the two
species have those characters on their leaves.
In this study, we found three contrasting morphological characters that
could be used to discriminate each species recognized within P. tectorius
complex, i.e prickles on leaf apex, phalange shape and phalange apex.P. tectorius
var.littoraliswas characterized by leaf apex that had not prickly on both sides of
leaves, oblong phalange shape, and carpels tip flat; whereas P. odoratissimushad
leaf apex that is prickly on both sides of leaves with obovate phalange shape, and
grooved between carpel tips.
These species also had different anatomical characters, especially on
stomatal type. The stomata was differed on the occurence of papillae on stomata.
InP. odoratissimusthe papillae only occured on lateral subsidiary cells, while in
P. tectorius var. littoralis papillae was occured also in neighbouring subsidiary
cells.
Molecular variation of atpB-rbcL intergenic spacer in both P. tectorius
var. littoralis and P. odoratissimus showed that most variation were contributed
by 71 substitution.
Conclusion
Pandanus furcatuscomplex
Based on morphological, anatomical and comparative sequence of atp
B-rbcL IGS, we concluded that Pandanus bantamensis Koord., Pandanus
different species. Our study confirm the opinion of Stone (1972) that three
species should recognized in Java, viz. Pandanus bantamensisKoord.,Pandanus
pseudolaisWarb., andPandanus scabrifoliusMartelli.
Key to species of thePandanus furcatuscomplex
1a. Leaf base reddish brown ... 2
1b. Leaf base yellowish white ... P. scabrifolius
2a. Peduncle a bit curved at the end, fruit shape cylindric–suboblong, style bifurcate nearly halfway, stomata with papillae on lateral
subsidiary cells ... P. bantamensis
2b. Peduncle curved at the end, fruit shape oblong–ellipsoid, style hornlike, stomata without papillae ... P. pseudolais
Pandanus tectoriuscomplex
Based on morphological, anatomical and comparative sequence of atp
B-rbcL IGS, P. tectorius var.littoralis should be recognized as a separate species
and distinct from P. odoratissimusL.f.
Key to species of thePandanus tectoriuscomplex
One side of the leaf apex smooth, phalange oblong, phalange apex flat, stomata with papillae on neighbouring epidermal and subsidiary cells
... P. tectorius var.littoralis
Both sides of the leaf apex prickly, phalange obovate, phalange apex grooved between carpel tips, stomata with papillae on lateral
A TAXONOMIC STUDY OF
PANDANUS FURCATUS
AND
PANDANUS TECTORIUS
COMPLEXES WITH SPECIAL
EMPHASIS ON PLANTS FROM JAVA
Introduction
The term species complex is used to describe species aggregation sharing
the specific morphological and molecular features (Juddet al. 1999). Within this
complex a complicated morphological overlap, without any discontinuities, has
led to taxonomic difficulty (Pak and Kawano 1990). Although their taxonomic
affinity may be difficult to determine, some form of taxonomic resolution is
desirable.
According to Stone (1972) Pandanus in Java contains many rather
problematical species. He suggested that detailed studies were required to get
more refined taxonomic scheme. The main problem as far as the Java plant are
concerned appears to be the status species which are given as synonyms of P.
furcatus Roxb. by Backer and Bakhuizen van den Brink (1968). According to
Backer and Bakhuizen van den Brink,P. bantamensisKoord.,P. ovigerMartelli,
P. pseudolais Warb., and P. scabrifolius Martelli; and P. odoratissimus L.f are
regarded as synonymous with P. tectorius var. littoralis. Stone (1972) did short
revision on Pandanaceae in Java based on specimen herbarium kept in BO and
living plant cultivated in Hortus Bogoriensis and tried to develop a more stable
species concept forPandanus furcatusRoxb. In Stone’s opinion, Backer’s species
concept for Pandanus furcatus is far too comphrehensive that required certain
readjusment because some species omitted or reduced to be lumped toPandanus
furcatus Roxb., and by Stone these species were regarded as four different
species, viz.P. bantamensisKoord.,P. pseudolaisWarb.,P. scabrifoliusMartelli,
and P. oviger Martell, while the status of P. tectorius var. littoralis and P.
odoratissimus L.f. is still more or less in question. Therefore those
circumscription should now be reviewed in light of our recent fieldwork in Java
with these taxa. A practical difficulty here is that almost all morphological
characters and/or character states used for evaluating species complex are only
slightly differentiated from one another and usually show considerable overlap.
combination of several characters. The aims of the study was to provide
taxonomic resolution of the P. furcatus and P. tectorius complexes based on
morphology, anatomy and molecular approach, i.e comparative sequence ofatp
B-rbcL IGS.
Materials and Methods
Studies of herbarium specimens were conducted in Herbarium Bogoriemse
(BO), Herbarium of the Royal Botanical Gardens Kew (K) and National
Herbarium Netherlands, Leiden (L). Observations on living plants and anatomical
were undertaken in Herbarium Bogoriense, while the molecular data was analyses
in Van der Klauw Laboratory, Leiden.
Morphology— Data on morphology were collected from specimens and
field collections. The procedure for morphological variation followed those
described by Rifai (1976) and Vogel (1987). Measurement were taken from
spirit-preserved material and dried herbarium specimen and from living collection.
Floral parts were measured from spirit-preserved material, dried specimens and
rehydrated by boiling.
Anatomy — Leaf anatomy was investigated by first fixing the leaves
(small part of the middle base) in FAA. Paradermal sections were taken from the
upper and lower surfaces of leaves, then stained with safranin 1% in water and
then mounted in glycerin (Johansen 1940).
atpB-rbcL IGS sequences — Genomic DNA of the silica gel dried leaf
were extracted according to the protocol described by Doyle and Doyle (1987).
Double stranded DNA was directly amplified by PCR. Reaction volumes were 25
µl and contained 2.5 µl PCR buffer, 2.5 µl dNTPs, 1 µl each of the 5 mM primers,
0.3 µl Taq Pol and 12.7 µl ddH2O. Approximately 5 µl genomic DNAs were
added to the PCR mixture. PCR was performed 3 min at 94oC for the activation of
the polymerase, followed by 35 cycles of 49 sec at 94oC, 45 sec at 55oC, 2 min at
72oC, with a final extension period of 10 min at 72oC. The primers used in this
study for atpB-rbcL intergenic spacers are forward
5’-GAAGTAGTAGGATTGATTCTC- 3’ and reverse
5’-TACAGTTGTCCATGTACC AG-3’. The PCR product was checked on 1%
clean up system (PROMEGA) following the manufacturer’s protocol prior to
sequencing. The DNA concentration was measured with the nanodrop. Cycle
sequencing was performed by Macrogen Korea. The sequences were edited using
sequencher 4.6 and MEGA 3.0 (Kumaret al. 2004).
Result and Discussion Result
Morphological Characters ofPandanus furcatuscomplex
In this study, we only found three of four species that recognized withinP.
furcatuscomplex. i.e P. bantamensis,P. pseudolaisand P. scabrifolius.
Concerning the prop root characters of Pandanus furcatus complex,
prickles in prop root did not display consistence differentiation among the taxa
analysed, except for P. scabrifolius where it was no prickles on the prop root.
Leaf base colour divided the taxa into two groups which one of it was overlapping
group. P. bantamensisandP. pseudolaiswere taxa with reddish brown leaf base,
andP. scabrifolius displayed yellowish white leaf base. The leaf dimensions of
Pandanus furcatus complex were highly variable. Pandanus pseudolais were
characterized by bigger and longer leaf, whereas the leaf of P. scabrifolius were
clearly smaller and shorter.P. bantamensiswas characterized by intermediate leaf
dimension. Number of drupe per cephalium of P. pseudolais were higher
compared with the other taxa. Drupe length divided the taxa into two group which
one of it was overlapping group. P. scabrifolius showed longer drupe compare
with the other taxa (Table 4.1).
Table 4.1 Morphological characters of species recognized within Pandanus furcatuscomplex
Among all morphological characters of species recognized within P.
furcatus complex, there were four characters, viz. leaf base colour, peduncle
shapes, fruit shapes and style shapes could be used to discriminate among species
recognized withinP. furcatuscomplex. The summary of contrasting characters of
species recognized withinPandanus furcatuscomplex is shown in Table 4.2.
Table 4.2 Summary of contrasting characters of species recognized within Pandanus furcatuscomplex. Leaf base colour, scale bar = 5 cm; Peduncle, scale bar = 13 cm; Cephalia shape, scale bar = 10 cm; Style shape, scale bar = 1 cm
Character P. bantamensis P. pseudolais P. scabrifolius
Leaf base colour Reddish brown Reddish brown Yellowish white
Peduncle shape Bit curved at the end
Curved at the end Straight
cephalia shape Cylindric-suboblong Oblong-ellipsoid Subglobose
Style shape Bifurcate nearly half way
Morphological Characters ofPandanus tectoriuscomplex
Leaf dimension, leaf shape, cephalium size, cephalium shape and number
of phalanges per cephalium did not display consistence differentiation between
the taxa analysed, but united taxa into overlapping group (Table 4.3). Among all
morphological characters of species recognized within P. tectorius complex,
there were three characters, viz. prickles on leaf apex, phalange shapes, and
phalange apexes could be used to discriminate among species recognized within
P. tectorius complex. The summary of contrasting characters of species
recognized withinPandanus tectoriuscomplex is shown in Table 4.4.
Table 4.3 Morphological characters of species recognized within Pandanus tectoriuscomplex
Species
Characters
Leaf length
(cm)
Leaf
shape
Cephalium size (cm)
Cephalium shape
Number of phalanges
P. tectoriusvar. littoralis
112-199 ligulate or sword
26 x 25 subglobose 79-83
P. odoratissimus 98-126 ligulate or sword
22 x 25 subglobose-globose
Table 4.4 Summary of contrasting characters of species recognized within Pandanus tectoriuscomplex ; Prickles on leaf apex, scale bar = 2 cm; Cephalium shape, scale bar = 10 cm; Phalange apex, scale bar = 1 cm
Character P. tectoriusvar.littoralis P. odoratissimus
Prickles on leaf apex
on one side of leaf prickly, another side smooth
prickly on both sides of leaves
Cephalium shape Oblong Obovate
Phalange apex Carpel tips flat Grooved between carpel tips
Anatomical Characters
Anatomical characters are just as valuable as morphological characters and
could be used to delimit species. These characters, qualitative characters were
observed directly on paradermal surfaces, viz. occurence of papillae on stomata.
Four types of stomata were found in Pandanus tectoriusand Pandanus furcatus
complexes. The variation on stomatal structures in Pandanus depends on the
number of papillae that develops on the subsidiary and neighbouring cells
(Tomlinson 1965; Kam 1971).
The species recognized within Pandanus furcatus complex had different
type 2, type 1 and type 3 respectively; while the species recognized within
Pandanus tectorius complex had also different stomatal type. P. tectorius var.
littoralisandP. odoratissimushad stomatal type 4 and type 2 respectively.
Molecular Characters
atpB-rbcL IGS sequence was a good marker for delimiting the different
species in Pandanus furcatus complex and Pandanus tectorius complex.
Polymorphic site in atpB-rbcL IGS sequence of Pandanus furcatus complex is
shown in Table 4.5.
Table 4.5 Polymorphic sites inatpB-rbcL IGS sequence ofP. furcatuscomplex
Species
Nucleotide at polymorphic site at indicated position
4 6 16 17 18 12
Polymorphic site inatpB-rbcL sequence ofPandanus tectoriuscomplex is shown
Table 4.6 Polymorphic sites inatpB-rbcL sequence IGS ofP. tectoriuscomplex
Species
Nucleotide at polymorphic site at indicated position
3
Nucleotide at polymorphic site at indicated position
2
Nucleotide at polymorphic site at indicated position
4
Nucleotide at polymorphic site at indicated position
Table 4.6 Continued
In the species recognized within Pandanus furcatus complex, they were
sharing some morphological features, viz. leaf length, prickles on prop root,
number of drupes, and drupe length. Beside those characters, these species also
had four contrasting morphological characters that could be used to separate each
species, viz. leaf base colour, peduncle shape, fruit shape and style shape. P.
bantamensishad reddish brown leaf base, peduncle with a bit curved at the end,
cylindric-suboblong fruit shape and style that bifurcate nearly half way. P.
pseudolaiswere characterized by reddish brown leaf base, peduncle that curved at
the end, oblong-ellipsoid fruit shape and hornlike style; whereas P. scabrifolius
had yellowish white leaf base, straight peduncle, subglobose fruit shape and style
that was bifurcate third way.
These species also had different anatomical characters especially on
stomatal type. The difference was on the occurence of papillae, whether occured
in lateral subsidiary cells as in P. bantamensis, in terminal and lateral subsidiary
cells as in P. scabrifolius, or papillae absent in stomata that was called
unspecilaized stomata as inP. pseudolais.
Molecular variation of the chloroplast noncoding region between atp
B-rbcL genes amongP. bantamensis,P. pseudolais, andP. scabrifoliusshowed that
most variation were contributed by nucleotide substitution, 19, 24 and 22
nucleotide substitution were found betweenP. bantamensis andP. pseudolais,P.
pseudolais and P. scabrifolius, and P. bantamensis and P. scabrifolius
Pandanus tectoriuscomplex
AlthoughPandanus tectoriusvar.littoralis andP. odoratissimusare very
closely related, they could be distinguished (Stone 1994). Stone have proposed
delimitingP. odoratissimusby just two characters, fleshy shoulders on phalanges
and large white spines on the leaves (Stone 1967). In our study we found that P.
odoratissimushas large white spines, the same asP. tectoriusvar.littoralis,but it
does not have fleshy shoulders on the phalanges. It is supported by John (1979)
who stated that P.odoratissimus has large white spines, but does not have fleshy
shoulders. Morphological observation revealed that large white spine on the leave
character are not good characters for separating the two species because the two
species have those characters on their leaves.
In this study, we found three contrasting morphological characters that
could be used to discriminate each species recognized within P. tectorius
complex, i.e prickles on leaf apex, phalange shape and phalange apex.P. tectorius
var.littoraliswas characterized by leaf apex that had not prickly on both sides of
leaves, oblong phalange shape, and carpels tip flat; whereas P. odoratissimushad
leaf apex that is prickly on both sides of leaves with obovate phalange shape, and
grooved between carpel tips.
These species also had different anatomical characters, especially on
stomatal type. The stomata was differed on the occurence of papillae on stomata.
InP. odoratissimusthe papillae only occured on lateral subsidiary cells, while in
P. tectorius var. littoralis papillae was occured also in neighbouring subsidiary
cells.
Molecular variation of atpB-rbcL intergenic spacer in both P. tectorius
var. littoralis and P. odoratissimus showed that most variation were contributed
by 71 substitution.
Conclusion
Pandanus furcatuscomplex
Based on morphological, anatomical and comparative sequence of atp
B-rbcL IGS, we concluded that Pandanus bantamensis Koord., Pandanus
different species. Our study confirm the opinion of Stone (1972) that three
species should recognized in Java, viz. Pandanus bantamensisKoord.,Pandanus
pseudolaisWarb., andPandanus scabrifoliusMartelli.
Key to species of thePandanus furcatuscomplex
1a. Leaf base reddish brown ... 2
1b. Leaf base yellowish white ... P. scabrifolius
2a. Peduncle a bit curved at the end, fruit shape cylindric–suboblong, style bifurcate nearly halfway, stomata with papillae on lateral
subsidiary cells ... P. bantamensis
2b. Peduncle curved at the end, fruit shape oblong–ellipsoid, style hornlike, stomata without papillae ... P. pseudolais
Pandanus tectoriuscomplex
Based on morphological, anatomical and comparative sequence of atp
B-rbcL IGS, P. tectorius var.littoralis should be recognized as a separate species
and distinct from P. odoratissimusL.f.
Key to species of thePandanus tectoriuscomplex
One side of the leaf apex smooth, phalange oblong, phalange apex flat, stomata with papillae on neighbouring epidermal and subsidiary cells
... P. tectorius var.littoralis
Both sides of the leaf apex prickly, phalange obovate, phalange apex grooved between carpel tips, stomata with papillae on lateral
GENETIC DIVERSITY OF JAVANESE
PANDANUS
AND
FREYCINETIA
BASED ON ISSR MARKER
Introduction
The pandan family (Pandanaceae) of the monocotyledonous Angiosperm
or flowering plants is represented in Java by two genera, Pandanus and
Freycinetia(Stone 1970). The genusPandanuscontain about 500-600 species of
trees and shrubs of the Old World tropics, distributed from East Africa westward
through Indomalaysia to remote island of Polynesia, it extends south to tropical
Australia (but not to New Zealand), north to Ryukyus, Bonins, Taiwan, and to
Hawaiian island (Stone 1965), whileFreycinetiacontains about 180-200 species
of root climbing lianas or low growing shrubs occur from Sri Lanka throughout
South-Eastern Asia to Northern Australia, Polynesia and New Zealand (Coxet al.
1995). The habitat of Pandanus include nearly all possible habitats available to
plants of comparable size, from sea level to the highest peaks (Stone 1966),
whereas Freycinetia are usually lacking in open environment because they are
dependent on forest environment (Stone 1982).
Pandanus species specialize in wind pollination. This anemophily,
coupled with syncarpus dispersal unit (the pistillate phalange) and also the
presence of facultative apomixis (Cox 1990) allowing Pandanus species with
these features have considerable ability to colonize new areas. Immediate genetic
isolation as well as exposuring to new habitats without regard to pollinator
availability may account for the extreme richness of species inPandanusas well
its very wide distribution (Cox et al. 1995), whereas Freycinetia has been
believed to be strictly dioecious (Dahlgren et al. 1985), but recent fieldwork has
indicated that a variety of breeding systems exist inFreycinetia(Coxet al. 1984).
Some individuals of Freycinetia imbricata in Sumatra produce staminate and
pistillate shoots on the same plant, such divergences from a dioecious breeding
system may be important in island colonization (Baker & Cox 1984) particularly
since monoecious individuals of Freycinetia scandens in Philippine have been
apomixis and water dispersal. However, their attractiveness to a wide variety of
vertebrate pollination and disperses, as well as infrequent leaky dioecy would
assure them a large range and high speciation rate (Cox 1990).
Many species ofPandanushave been used by Indonesian people for daily
purposes, i.e. as the raw materials for mats, and other handicraft such as hat and
bag, but not all species of Pandanus are suitable as raw materials for mat, the
most suitable species are P. dubius, P. furcatus,and P. tectorius. All along the
leaves border of the three species are thorny, but their texture are flexible,
unbreakable (Purwanto 2007). Pandanus amaryllifolius is rare in the wild, but
cultivated and widely used as flavouring in cooking (Ng & Yap 2003). Some
species are cultivated as ornamentals, i.e. P. dubius, P. spurius cv. Putat, P.
tectorius cv. Sanderi, andP. utilis, (Thomsonet al. 2006), whereas some species
ofFreycinetiaare important plants in the Pacific as an emergency food and for the
construction of fish traps (Brown 1931). In Indonesia, utilization of the
Freycinetiais still minimum, but looking at its elegant figure, Freycinetiahas the
potential as an ornamental plant (Purwanto 2007).
In view of its importance and considering the large number of wild species
germplasm available for Pandanus andFreycinetia in Java, the genetic analysis
through molecular marker is a prerequisite to have a deep insight of the genome
organization in the wild species. Therefore it is imperative to establish strategies
for the preservation of Pandanus and Freycinetia germplasm. This analysis is a
preliminary step to ensure the conservation and the development of genetic
resources.
In the past, genetic diversity in species has typically been assessed using
morphological, physiological and biochemical traits. Since morphological and
physiological traits are subject to environmental influences, emphasis has shifted
to biochemical studies (Moodieet al. 1997). In particular, allozyme analysis has
been used to document genetic diversity in a range of different species. However,
allozymes may underestimate genetic diversity (Esselmanet al. 1999). Recently
more sensitive DNA based-techniques have been developed to detect the genetic
diversity in different plant groups. The commonly used polymerase chain
DNA (RAPD), amplified fragment length polymorphism (AFLP), and more
recently simple sequence repeat (SSRs) or microsatelites (Staubet al. 1996). The
major limitation of these methods are low reproducibility of RAPD, high cost of
AFLP and the need to know the flanking sequence to develop species specific
primers for SSR polymorphism. ISSR-PCR is a technique that overcome most of
these limitations (Zietkiewiczet al. 1994).
Inter simple sequence repeats (ISSR) exhibit a few advantages over other
markers. ISSR primer anneal to simple sequence repeats that are abundant
through the eukaryotic genome and evolve rapidly, and hence may reveal a high
level of polymorphism (Zietkiewiczet al.1994). In addition, ISSR may produce
more reliable and reproducible bands than RAPD because of the higher annealing
temperature and longer primer sequences (Wei et al. 2001), have proved their
usefulness to detect genetic diversity in Ficusspecies (Rout & Asparajita 2009)
andMorusspecies (Awasthiet al. 2004).
There is no information regarding the genetic diversity of Pandanus and
Freycinetia from Java. Our objective was to obtain information based on ISSR
marker ofPandanusandFreycinetiaspecies sampled.
Materials and Methods Plant materials
Totally 19 samples of species ofPandanusandFreycinetiawere collected
from various places in Java. They consisted of 13 species of Pandanus and 6
species of Freycinetia. All samples were identified to species based on
morphological characteristic following the method of Stone (1983b) (Table 5.1
and Table 5.2). One sample represented a species. Two or three leaves were
collected from each species and stored in silica gel in the field. In the laboratory,
samples were maintained at–5oC until DNA extraction could be performed.
The voucher specimens are deposited in the Herbarium of the Biology
DNA extraction
Genomic DNA of the silica gel dried leaf samples were extracted
according to the protocol described by Doyle and Doyle (1987) with minor
modification mainly aimed to minimize the presence of phenolic compounds. For
each sample, 100 mg of leaf were ground to fine powder, followed by the addition
of 1 ml preheated (65oC) extraction buffer constitute 3% (w/v) CTAB, 1.4 MnCl,
0.2% (v/v) β-mercaptoethanol, 20mM EDTA, 100mM Tris-HCl (pH 8.0) and 1%
(w/v) PVP-40. The homogenous was incubated at 65oC for 30 min and extracted
two times with a phenol chloroform: isoamyl alcohol (25:24:1) solution, and
phenol was eliminated by chloroform: isoamyl alcohol (24: 1) solution. Then
DNA was precipitated in cold isopropanol and treated with Rnase A (37oC) for 60
min. After electrophoresis with a standard DNA on 1% agarose gels, stained with
ethidium bromide, DNA concentration were determined by comparison against
the standard of DNA with known concentration. The DNA was suspended to a
final concentration of 10 ng/µl in 0.5 x TE and stored at 4oC.
Table 5.1 Thirteen species ofPandanusof Java used in this study
Table 5.2 Six species ofFreycinetiaof Java used in this study
screening the amplification of unambiguously visible and polymorphic ISSR
bands. A final set of 6 ISSR primers (Table 5.3) which produced unambiguously
visible and polymorphic bands across the 19 samples were chosen for further
analysis.
PCR Conditions
PCR was performed in a total volume of 25 µl containing 1X reaction
buffer, 50 ng genomic DNA, 3.0 mM of MgCl2, Taq polymerase (2.5 units), 0.4
mMdNTPs and 10 µM primer ISSRs were amplified using GeneAmp PCR
ethidium bromide (10 µg/ml) and visualized under UV light and photograph with
a digital camera.
Data Analyses
The numerical taxonomy and Multivariate Analyses System (NTSYS-pc)
were used in this study. The presence of band was scored from the photograph.
a polymorphic when they were absent in some samples in frequency a greater than
1% (Jorde 1995). Change in band intensity was not considered as a
polymorphism. Clear band were scored as present (1) or absent (0) at particular
position or distance migrate on the gel. The data matrix of 1’s and 0’s has been
prepared from the scorable bands and entered into the data analysis package
(Amstronget al. 1994). The indices similarity was calculated across all possible
pair wise comparisons of species following the method of Nei and Li (1979).
Cluster analysis of the data was carried out using UPGMA (Sneath and Sokal
1973) method. The bootstrap values were calculated using WINBOOT (Yap and
Nelson 1996). Principal component analysis (PCA) was performed in order to
make more effective view of the clustering pattern of the taxa included in this
study.
Results and Discussion Result
Primer Selection
Six primers were applied on thirteen species of Pandanusand six species
of Freycinetia from Java. The results showed that different primers generated
various numbers of fragments with different length of amplified products as
shown in Table 5.3 and Table 5.4. The six primers amplified 50 band position in
Pandanus, and 32 band position in Freycinetia. The number of amplification
bands per primer ranged from 6 to10 inPandanusand from 2 to 8 inFreycinetia.
The primer ISSR4 produced more bands in Pandanus and primer ISSR7
produced more bands in PandanusandFreycinetiathan the other primers (Table
5.3 and Table 5.4), probably because ISSR4 and ISSR7 are greater abundance in
PandanusandFreycinetiagenome. The repeats (GA)n were most abundant in rice
(Nagarajuet al. 2002) and date palm (Trifiet al. 2000) genome. This data might
indicate that dinucleotide-based ISSR-PCR marker could provide potential
markers in thePandanusandFreycinetiagenome. A representative amplification
pattern obtained by using primer ISSR4 and ISSR7 are shown in Figure 5.1 and
M s1 s2 s5 s6 s7 s14 s15 s20 s22 s24
1500 1250 1000 750
500
250
ISSR4
Figure 5.1 ISSR profile ofPandanusspp and
Freycinetiaspp of Java using primer ISSR4. M : Marker, S1:P. bantamensis,
S2:P. tectoriusvar.littoralis,; S5:P. odoratissimus S6:F. javanica; S7:F. angustifolia, S14:P. nitidus, S15:F. angustifolia, S20:F. sumatrana,
S22:F. imbricata, S24:P. kurzii,
Kb s3 s4 s8 s13 s14 s21 s23 s24 s25 s26
.
1000 750 500 250
ISSR7
Figure 5.2 ISSR profile of Pandanusspp and
Freycinetiaspp of Java using primer ISSR7. M: Marker, S3:P. bidur; S4:P. spinistigmaticus S8:P. pseudolais, S13:P. multifurcatus
Table 5.3 ISSR primers sequence and amplified results on thirteen species of
Table 5.4. ISSR primers sequence and amplified results on six species of Freycinetiaof Java
A total of 50 ISSR fragments were generated by six primers from thirteen
species of Pandanus, and 32 ISSR fragments were generated in six species of
Freycinetia. The highest number of fragments in Pandanus was detected in
Pandanus amaryllifolius (34), P. spinistigmaticus (29) and P. pseudolais (28)
respectively, whereas the least number was found in P. multifurcatus containing
six fragments. In other species the number of fragments ranged from 12 in
P.bantamensis and P. tectorius var. littoralis to 22 in P. bidur, while within
Freycinetia, the highest number of fragments was detected inF. javanica andF.
imbricatacontaining 21 fragments respectively, while the least number was found
ranged from 15 in F. angustifoliato 18 in F. sumatrana. The average number of
fragments generated by all analyzed primer was 8.3 in Pandanus and 5.3 in
Freycinetia.The choice of primers used in amplification is critical to demonstrate
high polymorphism. The results showed that samples had different banding
pattern. Primers had several characteristic that can affect the number and quality
of DNA fragment amplified during PCR (Hassel and Gunnarsson 2003). Among
all the species subjected in this research, no species specific band was detected.
Genetic similarity and species relationships
Genetic similarity can reflect the difference among species in certain
extend. The average similarity index for Pandanus was 0.250 to 0.889. The
similarity indices among species ofPandanusare represented in Table 5.5. High
genetic similarity was observed between P. nitidus and P. scabrifolius (0.889),
whileP.amaryllifoliuswere distantly related toP. multifurcatus(0.250). Genetic
similarity in other species varied from 0.333 (P. bantamensis and P.
multifurcatus) to 0.789 (P. kurziiandP.odoratissimus).
Table 5.5.Nei and Li’s similaritymatrix of thirteenPandanusspecies of Java by using ISSR primers
S1 S2 S3 S4 S5 S8 S13 S14 S21 S23 S24 S25 S26
S1 1.000
S2 0.750 1.000
S3 0.529 0.412 1.000
S4 0.488 0.488 0.745 1.000
S5 0.667 0.733 0.450 0.511 1.000
S8 0.550 0.500 0.480 0.702 0.565 1.000
S13 0.333 0.444 0.286 0.286 0.333 0.353 1.000
S14 0.600 0.733 0.350 0.426 0.778 0.565 0.333 1.000
S21 0.522 0.478 0.464 0.635 0.538 0.774 0.250 0.577 1.000
S23 0.563 0.563 0.714 0.694 0.526 0.625 0.462 0.526 0.519 1.000
S24 0.625 0.688 0.429 0.490 0.789 0.667 0.308 0.842 0.593 0.550 1.000
S25 0.667 0.733 0.350 0.468 0.833 0.565 0.333 0.889 0.577 0.526 0.842 1.000
S26 0.545 0.545 0.558 0.760 0.513 0.735 0.444 0.462 0.618 0.732 0.537 0.513 1.000
In Freycinetia, the average similarity index was 0.396 to 0.923. The