A COMPLETE ANALYSIS ON PHA PRODUCTION AND THEIR
RATES USINGSUNFLOWER OIL AND MIXED CULTURES:
HYDRAULIC RETENTION TIMES (HRT) EFFECTS
Salim M R Md_Din, M. F1., Yunus, S.,1 Ujang, Z.1, Salim M. R1., van Loosdrecht, M.C.M2, Ahmad, A.3, Marpongahtun 4.
1
IPASA, Faculty of Civil Engineering, University Technology of Malaysia, 81310 Skudai Johor, Malaysia. 2
Delft University of Technology, Kluyver Laboratory for Biotechnology, Department of Biochemical Engineering, Julianalaan 67, NL-2628 BC Delft, The Netherlands.
3
Microbiology Department, Faculty of Science, University Technology of Malaysia, 81310 Skudai Johor, Malaysia. 4
Dept. of Chemistry, Faculty of Mathematic and Sciences, University North Sumatera, 20155 Medan, Indonesia
Abstract
Abilities of four HRT/SRT experiments were conducted to assess the optimal conditions for PHA production using commercial fatty acid; sunflower oil (SO). Since the systems were conducted in a fast regime (uptake rate), no idle or settling phase is adapted resulting HRT ≈ SRT (HRT/SRT). First, the effect of carbon source was investigated. The system was dependent on microbial activity and operating conditions, particularly HRT/SRT, COD/N, growth and accumulation conditions. The target was to maintan the bacterial growth, so the capability of storage polymer (polyhydroxyalkanoates, PHAs) will significant in the survival period (limiting condition of nutrient). The PHA constituents such as HV and HH were also examined in order to compare the polyester production in commercial oil compositions. The typical composition of SO mainly contains long-chain-fatty-acid (LCFA) under unsaturated fatty acid (C14:1 – C18:3) and therefore, the bacterial not much utilize it. Here also, the highest PHA productions can
reach up to 33.77% of dry weight because the capability of storage mechanisms. Substrate uptake rates were found not to be proportional to the cell growth, suggesting that only a small fraction of the cell biomass was responsible for the main part of the substrate uptake. Based on these experiments, new fabrication of experiment will usefulness for the development of renewable carbon sources (especially from oil manufacturing) and sufficient for PHA production.
Key words: sunflower oil (SO), polyhydroxybutyrate (PHB), feast-famine regimes, mixed cultures, fatty acids component.
INTRODUCTION
Mixed culture or co-culture systems have been recognized to be important for several fermentation processes. There are several researchers claimed the integrity and effectiveness system using mixed culture. Unfortunately, they only emphasize the mixed culture using two or three well-known bacterial. The mixed culture using unknown bacterial consortium is not quite established previous. The idea of PHA production using mixed culture was ignited owing to PHA role as an metabolic intermediate of wastewater treatment and as a biodegradable plastic. Activated sludge (from organic waste) as a well known mixed culture process is able to store PHA as carbon and energy storage material under transition
conditions due to discontinuous feeding regime and variation in electron acceptor presence. Microorganisms which are able to quickly store and consume substrate in a more balanced way have a strong competitive advantage over organisms without capacity of substrate storage (Md Din et al., 2004a; van Loosdrecht et al., 1997).
are typically different from each other. Palm oil contains more monounsaturated (40%) than polyunsaturated (10%) and saturated (50%) component. For comparison, SO contributes more polyunsaturated (77%) compared to monounsaturated (13%) and saturated (10%) fatty acids. Among the fatty acids, oleic acid is particularly stable to thermal-oxidation, due to the presence of only one unsaturation in its structure. Typically, SO compositions are constituted up to 24% of oleic acid, while linoleic acid is present up to 64.9%. Thus, this vegetable oil (SO) is a very interesting substrate for the synthesis of esters, which possess application in pre-determined PHB production under highly substrate compositions.
Depending on the substrate given the organisms can include a wide variety of 3-hydroxy fatty acids in PHA. Since the first finding of PHB by Lemoigne et al. (1926), more than 100 different monomer units have been identified as constituents of PHA in above 300 different microorganisms (Lee, 1996b) including 3-hydroxyalkanotes of 3-12 carbon atoms with large variety of R-pendant groups, 4-hydroxyalkanoates of 4-8 carbon atoms, 5-hydroxypentanoates, 5-hydroxyhexanoate and 6-hydroxydodecanoate. However, only a few of these PHAs have been produced in amounts sufficient to enable the characterization of their material properties and to develop potential applications. PHB has been known to be useful biodegradable polymer which can be used as a thermoplastic (Byrom, 1987; Holmes., 1985; Doi, 1990). PHB is also accumulates by numerous microorganisms and is the best-characterized PHA (Lee, 1996; Steinbüchel and Füchtenbusch, 1998).A major problem in commercialization of PHAs as substitutes for conventional petrochemical-based polymers is the high production cost of these compounds (Byrom, 1987; Choi and Lee, 1999).
Many carbon sources, in particular organic acid can be utilized by several bacterial (even consortium) and theoretical approach for the biosynthesis of PHB from various carbon sources has been proposed (Yamane, 1993). The importance of bacterial storage polymers in general and poly-β-hydroxybutyrate (PHB) in particular for carbon substrate conversion in activated sludge processes is well recognized (Chiesa et al., 1985; Kohno et al., 1991; van Loosdrecht et al., 1997; Krishna and van Loosdrecht, 1999). The presence of storage compounds such as PHB and glycogen in activated sludge bacteria and mostly in pure culture has been extensively repeated (Doi et al., 1990; Pagni et al., 1992; Beccari et al.,
1998). There are three kinds of polymer store intracellular, which is, glycogen, PHA and polyphosphate. However, only glycogen and PHA are the main reported bacterial storage polymers (van Loosdrecht et al., 1997). In this study, we predominantly assume that only PHA (generally refers to PHB as the main copolymer storage) occurs in all of the storage phases. PHB has been reported from several researchers to be the more common storage polymer under conditions of carbon sources excess (van den Eynde et al., 1984; Smolders et al., 1995).
In the present study, we considered such a mixed culture system, where SO as volatile fatty acids from oil manufacturing were converted to medium-chain-carbon (up to short-chain-carbon) by significant bacterial consortium and then stored as PHAs (or PHB/HV) in one fed-batch system. Very little attention has been made in the past on the combining the typical high concentration of fatty acid and mixed cultures. The goal of this study, we try to investigate and stimulate the strategy to enhance the productivity of PHA production. Without depending any co-pure bacterial the system was developed according to competence and dominant bacterial accumulation.
To achieve a high productivity of a desired bioproduct production, fed-batch cultures are usually carried out with the control of the nutrient feeding by monitoring dissolved oxygen (DO), pH or carbon sources (Ryu et al., 1997) as a feedback parameter. In our attention, we tried to evaluate the nitrogen (as a primary nutrient limitation) to determine the productivity of PHB accumulation. In this case, however, high-cell-density fermentation was impossible due to the significant cell lysis caused by the toxicity of NaOH solution, excessively added to control pH. Furthermore, more unsaturated fatty acid has been used and the bacterial cell unable to degrade (or even store) them easily. Until now, the potentially of PHB production using mixed cultures (oily material as substrate) currently was not quite beneficial but interesting to investigate more.
MATERIAL AND METHODS
Experiment operation
The experiments were performed in a double-jacketed laboratory fermentor with working volume of 2 l. The fermentor was equipped with pH and O2 electrode. The
heterotrophic organisms from sewage wastewater was used as inoculums. For determination of the steady-state system, some parameters were used; as representative to the confirmation conditions likes TOC, cell dried weight (CDW) and O2 profiles. Those
observations should be in constant value and indicated that the organisms are capable (metabolism activity) in the dynamic system. During steady state, in one cycle a much higher NH4+ (non-limiting nutrients) were added than
during normal operation. The cycle was modified to ensure the production of growth till up to 48 hour per cycle. The pH was maintained
at 7.00
±
0.1 using 2N HCl or 2N NaOH. The temperature was controlled almost at 30oC by using a water-jacketed and a thermostat bath. In steady state the process was extensively monitored (pH, DO) and sampel (COD, TOC, NH4+, PO42-, PHB/HV, CDW and ash content).The well-aerated reactors were operated with airflow of 2.39 l/min controlled by a mass-flow controller and stirred with two standard geometry six-blade turbines. Almost of the process are conducted in turbulence regime to ensure the well mass transfer and mixtures of oil content using 1000 rpm approximately.
Table 1: Fatty acid composition (wt%) of sunflower oil (SO)
Systematic name Trivial name Omega name Sunflower oil
Hexadecanoic acid Palmitic acid 16:0 5.7
Octadecanoic acid Stearic acid 18:0 4.1
cis-9-Octadecenoic acid Oleic acid 18:1ω9 23.5
cis-9,
cis-12-Octadecadienoic acid Linoleic acid 18:2ω6 64.9
Cis-9, cis-12,
cis-15-Octadecatrienoienoic acid Linolenic acid 18:3ω3 0.2
Others (14:0, 20:0, 20:1ω5,
22:0, 24:0 1.5
Inoculation, medium and fed-batch technique The working volume for this cultivation is 2 l and the same batch reactor also been used for others experimental work. The proportion ratio for inoculums is 1:9 for POME and distilled water respectively. The mixed cultures were first grown in the same batch of SBR that mentioned above and cultivated it for approximately 36 hr at 30oC. A portion of the preculture medium was transferred to the bioreactor at 10% of the working volume after the cells had reached the late exponential stage. The seed of cultures for fermentation were prepared for at least 24 hours in 2 l flasks each containing 1.1 l seed of inoculums, 0.8 l (solution A) of mineral medium and 0.1 l sunflower oil as carbon sources.
Regarding the PHA harvesting and the economics of PHA production, bacterial biomass should be maintained at a high concentration during the peak production of
PHA (during growth phase) in order to maximize its productivity. However, there were large decreases in the total biomass during the nutrient limiting conditions especially when the system is applied to SO cultivation. It is not surprising to observe the rate of decline of the biomass during the nutrient limitation phase because N & P (nitrogen and phosphorus) are essential nutrients required for cellular growth by all living organisms.
In order to measure the SO concentration in culture broth, 2 ml of the culture broth was adopted to a screw tube and then mixed with 5 ml hexane. After vigorous shaken for 1 min, 1 ml of hexane layer was transferred to pre-weighted tube, and then dried at 37oC until the hexane phase evaporated. The SO concentration was estimated as the amount of extract by hexane according to the predetermined calibration curve
Table 2: Nutrient adaptation for fed-batch control system during growth condition Micronutrient* Concentration
(g/l)**
Macronutrient* Concentration (g/l)
FeCl3.6H2O 1.50 NH4Cl 15.5
H3BO3 0.15 KH2PO4 7.59
CuSO4.5H2O 0.03 MgSO4.7H2O 0.2
Micronutrient* Concentration (g/l)**
Macronutrient* Concentration (g/l)
Na2MoO4.2H2O 0.06
ZnSO4.7H2O 0.12
CoCl2.6H2O 0.15
EDTA 10.0
KI 0.18
* Applied during growth condition ** Based on Visniac and Santer solution
Figure 1: Diagram for SBR system (with feast and famine regime)
Analytical Procedures
As the sole carbon source, the fatty acids of SO contained various LCFAs. About 0.15 g cells (in dry weight) were re-suspended in 100 ml mineral solution and cultivated in a rotary shaker at 200 rpm and 30oC for almost 24 hours. SO was added at the pre-determined concentrations to examine their toxic effect on cell growth and PHA synthesis. Batch fermentation was further carried out in a 2 l bioreactor for the time courses. About 3.4 g cell mass harvested from the nutrient-rich culture was re-suspended in 1 liter mineral solutions that contained LCFA. In that case, the initial concentrations of LCFA were controlled at 1.5 – 2.0 g/l. DO concentration was maintained at higher than 20% of air saturation by aeration and agitation.
The dissolved oxygen (DO) concentration in the reactor was measured online with a DO- electrode as percentage of air saturation. Sampels taken from the reactor for analysis of acetate, NH4-N, PO4-P, TOC and
COD and volatile fatty acids (VFAs) were immediately centrifuged and filtered using 0.45
μm filters to separate the bacterial cells from the liquid. The centrifugation was performed using Sorval RC-5B for 15 minute at 9000 rpm at 4oC. The carbon concentration in the supernatant was measured by gas chromatography, while NH4-N
and PO4-P concentration in the supernatant were
measured at 630 nm and 520 nm respectively with auto analyzers (Skalar 5010). The supernatant of VFAs were measured with GC according to the type of carbon chain. The quantification of CDW was performed using the volatile suspended solids (VSS) technique according to the Dutch Standard. Culture (10mL) was centrifuge under high rotation (rpm) to ensure the separation between pellet and supernatant. The ash content of the biomass was determined according to Dutch Standard Method (NEN6621) (NNI. NEN) oxygen content of the biomass was obtained by subtracting the percentages of C, H, N, S and ash from 100%. Sampel taken from the reactor for the PHB determination were added to 10 ml pH sensor
Mineral Discharge
Air DO sensor
1N, HCl 1N, NaOH
Working Volume, 2 l
Discharge Level, 1 1 l l Stirrer
Blade turbines
From waterbath
Carbon feed
tubes containing 2 drops of formaldehyde in order to stop all biological activity immediately. The PHB content of the washed and dried biomass was determined by extraction, hydrolyzation, and esterification in a mixture of hydrochloric acid, 1-propanol, and dichloroethane at 100oC. The resulting organic phase was extracted with water to remove any free acids. The proplyesters were analyzed by GC. Benzoic acid was used as an internal standard throughout the procedure. The PHA content (or PHB content) was given as a percentage of the total biomass dry weight (% PHA or%PHB).
Results and Discussion
In our approach, the medium of SO was re-concentrated based on the solubility data from mixtures of Triton-X 100 solution. Then, we calibrated the reasonable concentration using different dilution factors. It was found that SO solution could only be concentrated up to
120 ml/l by pre-treatment of Triton-X 100. The feeding solution containing 120 ml/l was sufficient to allow high density of cells and relatively high concentration of PHB. Regarding this, we preferred a good enrichment of cultures when the SO solution was acclimatized for a long period.
The initial DO was maintained up to 30% of air saturation. When the CDW reached 20 g/l, the DO was lowered to only 4%. Even though a higher cell concentration of 25 g/l was achieved without removing culture broth by employing favourable SO concentration, the final PHA content was still lower than 40%. The PHB content is a very important factor, which contributes significantly to the cost of production of PHA in large scale cultivation. Therefore, we examined the effect of the DO during the fed-batch culture to achieve high PHA content and cell concentration at the same time. It was considered more significant than cycle length study.
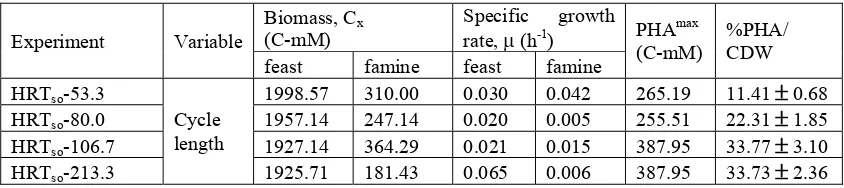
Table 3: Experiments the selected cultivation of fed-batch system under different cycle lengths HRT/SRT
Table 4 shows the suggested strategy on PHA production. Yet, a great accumulation of PHB appeared which reincreased for at least more than 24 hours. It showed that a sufficient time during the active growth phase was important to achieve high final cell and PHB concentrations. The behaviour of the microorganism in the cycles with high SO dosage was comparable to the behaviour during the normal steady state cycles (data not shown). The specific production rates during feast and famine conditions (qpfeast/-qpfamine) were
somewhat higher by increasing the cultivation periods. However, the degradation rates (-kfPHAfamine) were almost the same in a range of
0.2 – 0.6 x 10-3 h-1. The overall observed biomass yield was slightly higher in the normal cycles (i.e. 24 hours). The biomass growth rate in the feast period relative to the overall biomass growth rate was lower in the normal cycles compared to the prolonged cycles with long adaptation periods. It must be noticed that error in the determination of biomass growth in the feast period was relatively large.
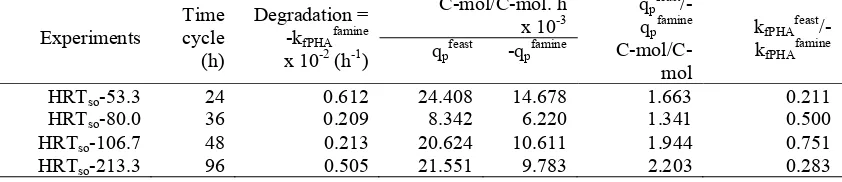
Table 4: Specific observations based on kinetic rates under feast and famine condition Experiments
Data from a single aerobic cycle in the aerobic SBR (the cycle length experiment) was performed three times (Table 5). The specific
specific growth rate, which is in fact the reciprocal of the cycle length. Concerning these data, it is clear that with the exception of the SRT, it is easy to make difference between a feast and famine condition. In comparing the shorter and longer cultivation periods, it appears that the specific growth rate in HRTso-213.3 can
be 10 times higher than in HRTso-53.3.
Obviously, the biomass takes an advantage (for PHA storage) during feast period compared
with famine period because the ratio of specific growth rates (μ/μoverall) seems high during this period. In general, the ratio of biomass accumulation depends on the prolonged cycle of cultivation and adjustment of growth rate. Obviously, when the cultivation is fixed to warm condition, it may increase suddenly because of the excited external energy for biomass.
Table 5: Specific growth rates for aerobic SBR condition at different length of cultivation periods Experiments μfeast
(h-1) μfeast/μfamine μfeast/μoverall μfamine/μoverall HRTso-53.3 0.030
±
0.36 0.729±
2.26 0.843±
0.226 1.157±
1.44HRTso-80.0 0.020
±
1.12 4.038±
1.26 1.603±
1.19 0.397±
2.28HRTso-106.7 0.021
±
1.03 1.368±
2.03 1.155±
2.55 0.845±
1.34HRTso-213.3 0.065
±
0.33 10.758±
2.14 1.830±
1.45 0.170±
2.31(Note: standard deviations are follows after plus/minus sign)
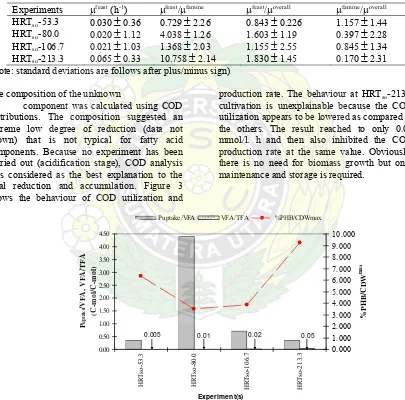
The composition of the unknown
component was calculated using COD distributions. The composition suggested an extreme low degree of reduction (data not shown) that is not typical for fatty acid components. Because no experiment has been carried out (acidification stage), COD analysis was considered as the best explanation to the total reduction and accumulation. Figure 3 shows the behaviour of COD utilization and
production rate. The behaviour at HRTso-213.3
cultivation is unexplainable because the COD utilization appears to be lowered as compared to the others. The result reached to only 0.02 mmol/l. h and then also inhibited the COD production rate at the same value. Obviously, there is no need for biomass growth but only maintenance and storage is required.
0.005 0.01 0.02 0.05
0.00
0.00
HRTso-53.3 HRTso-80.0 HRTso-106.7 HRTso-213.3
Experiment(s)
COD utilization rate COD production rate
Figure 3: COD utilization and production rate based on biomass accumulation and cultivation at different cycle length cultivation
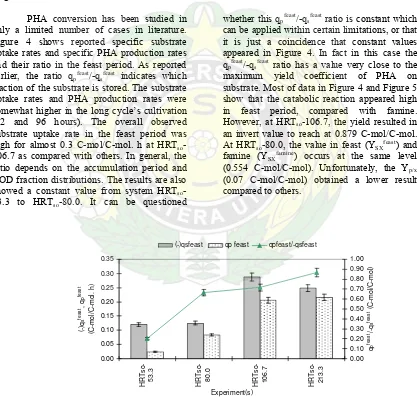
PHA conversion has been studied in only a limited number of cases in literature. Figure 4 shows reported specific substrate uptake rates and specific PHA production rates and their ratio in the feast period. As reported earlier, the ratio qpfeast/-qsfeast indicates which
fraction of the substrate is stored. The substrate uptake rates and PHA production rates were somewhat higher in the long cycle’s cultivation (72 and 96 hours). The overall observed substrate uptake rate in the feast period was high for almost 0.3 C-mol/C-mol. h at HRTso
-106.7 as compared with others. In general, the ratio depends on the accumulation period and COD fraction distributions. The results are also showed a constant value from system HRTso
-53.3 to HRTso-80.0. It can be questioned
whether this qpfeast/-qsfeast ratio is constant which
can be applied within certain limitations, or that it is just a coincidence that constant values appeared in Figure 4. In fact in this case the qpfeast/-qsfeast ratio has a value very close to the
maximum yield coefficient of PHA on substrate. Most of data in Figure 4 and Figure 5 show that the catabolic reaction appeared high in feast period, compared with famine. However, at HRTso-106.7, the yield resulted in
an invert value to reach at 0.879 C-mol/C-mol. At HRTso-80.0, the value in feast (YSXfeast) and
famine (YSXfamine) occurs at the same level
(0.554 C-mol/C-mol). Unfortunately, the Yp/x
(0.07 C-mol/C-mol) obtained a lower result compared to others.
0.00
(-)qsfeast qp feast qpfeast/-qsfeast
0.00
YSXfeast YSX famine YP/X feast
Figure 5: Catabolic and anabolic yield coefficient during feast and famine condition at different length of cultivation.
The results of experiment HRTso-53.3
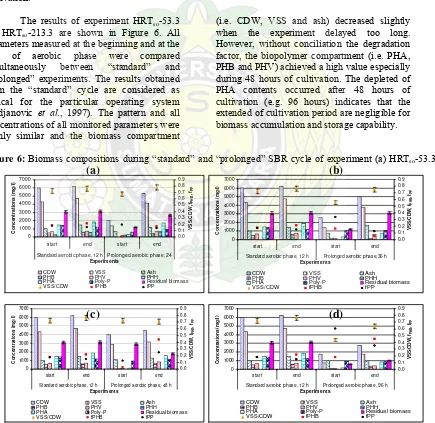
till HRTso-213.3 are shown in Figure 6. All
parameters measured at the beginning and at the end of aerobic phase were compared simultaneously between “standard” and “prolonged” experiments. The results obtained from the “standard” cycle are considered as typical for the particular operating system (Brdjanovic et al., 1997). The pattern and all concentrations of all monitored parameters were highly similar and the biomass compartment
(i.e. CDW, VSS and ash) decreased slightly when the experiment delayed too long. However, without conciliation the degradation factor, the biopolymer compartment (i.e. PHA, PHB and PHV) achieved a high value especially during 48 hours of cultivation. The depleted of PHA contents occurred after 48 hours of cultivation (e.g. 96 hours) indicates that the extended of cultivation period are negligible for biomass accumulation and storage capability.
Figure 6: Biomass compositions during “standard” and “prolonged” SBR cycle of experiment (a) HRTso-53.3,
(b) HRTso-80.0, (c) HRTso-106.7 and (d) HRTso-213.3. Note: Poly-P(mgP/l)=(CDW-VSS)-VSS*5/95,
PHA(mgPHA/l)=PHB+PHV, Residual biomass (mg biomass/l)=CDW-PHB-Poly-P, fPHB (g PHB/ g active
biomass)=PHB/active biomass, fPP (g P/g active biomass)=Poly-P*0.35/active biomass 0
start end start end
Standard aerobic phase, 12 h Prolonged aerobic phase, 24 Experiments
PHA Poly-P Residual biomass
VSS/CDW fPHB fPP
start end start end Standard aerobic phase, 12 h Prolonged aerobic phase,36 h
Experim ents
PHA Poly-P Residual biomass
VSS/CDW fPHB fPP
start end start end Standard aerobic phase, 12 h Prolonged aerobic phase, 48 h
Experim ents
PHA Poly-P Residual biomass
VSS/CDW fPHB fPP
start end start end Standard aerobic phase, 12 h Prolonged aerobic phase, 96 h
Experim ents
PHA Poly-P Residual biomass
VSS/CDW fPHB fPP
(a) (b)
0
Figure 7: Dependence of the amount of PHA produced on HRT/SRT conditions. (♦) experiments used for the fitting the points, (—) model equation developed from fittings.
The experiments of HRT/SRT have been conducted individually to stimulate the “standard” productivity of PHA (∆fPHA) using
SO as substrates. The predictive model (Figure 7) has been used to generate the model equation in a single fed-batch culture. Since there are significant interactions, the model found from those experiments could be used for further studies such as formulation, optimization, factor analysis, or simulation without any bias. Further verification experiment (with this formulation) was not performed because this formulation was for the robust process under the assumed optimized condition. Moreover, no higher results were expected from this experiment. The final optimal formula obtained from different experiment conditions, showed higher PHA productivity at more than 90 hours of HRT/SRT. However, the stationary behaviours have been observed indicating the regular fed-batch cultivation is not much affected the PHA production rate.
The results (Table 6) indicated that peak PHA content (as well as PHB content) can typically be obtained faster during the second limitation of P concentration (famine period), but the maximum PHA content obtained will
probably be less than during the first accumulation phase (feast period). It was noted that the nutrient limitation experiments of this study (especially P) required significantly longer time to obtain maximum PHA content than was required during nitrogen limitation experiments (Chinwetkitvanich et al., 2004). Based on the result obtained by Md Din et al. (2004b), it is probable that, even though the influent contained no phosphorus, the biomass still had phosphorus stored within it that had to be depleted before PHA accumulation would begin. The maximum polymer content in LCFA was maintained in the longer period, therefore possibly to break-down, since the degradation of unsaturated fatty acids taken into account. However, the possibility of the enzymatic polymerization under LCFA has been established using P. oleovorans (pure culture) which clearly demonstrates that P. oleovorans is able to produce variety of PHA, with unit ranging from 6 to 11 carbon atoms, depending on the substrate used for growth (Brandl et al., 1988). No further experiment has been made to confirm these mechanisms but it still significant for overall results based on value-obtained (ratio of VFAs conversion over fatty acids added)
Table 6: Accumulation of PHA content in HRT/SRT conditions under acclimatization of biomass concentration and specific growth rate.
Experiment Variable
feast famine feast famine
HRTso-53.3
Since oil fed mostly contains a high concentration of fatty acid (LCFA), the
formation under steady-state conditions, which reveals the clear relationship between cells and the environment. Since a high final PHB content in cells is desired and can only be achieved in the not-actively-growing cells, an optimal process should have a variation in the cell growth rate by controlling the feeding of growth nutrients with time.
REFERENCES
Beccari, M., Majone, M., Massanisso, P. and Ramadori, R.A. (1998). A bulking sludge with high storage response selected under intermittent feeding. Wat. Res. 32(11). 3403-3413.
Brdjanovic, D., Van Loosdrecht, M.C.M., Hooijmans, C.M., Alaerts, G.J. and Heijnen, J.J. (1997). Temperature effects on physiology of biological phosphorus removal. J. Env. Eng. 123(2). 144-153. Brandl., H., Bachofen, R., Mayer, J. and
Wintermantel, E. (1995). Can. J.
Microbiol. 41(Supll. 1). 143-153.
Byrom, D. (1987). Polymer synthesis by microorganisms: technology and economics. Trends Biotechnol. 5. 246-250.
Chiesa, S.C., Irvine, R.L., Manning, J.F. (1985). Feast/famine growth environments and activated sludge population selection.
Biotechnol. Bioeng. 27. 562-569.
Chinwetkitvanich, S., Randall, C.W. and Panswad, T. (2004). Effects of phosphorus limitation and temperature on PHA production in activated sludge. Wat.
Sci. Tech. 50(8). 135 – 143.
Choi, J. and Lee, S.Y. (1999). Efficient and economical recovery of poly(3-hydroxybutyrate) production by fermentation. Bioprecess. Eng. 17. 335-342.
Doi, Y. (1990). Microbial Polyester. VCH, New York, N.Y.
Holmes, P.A. (1985). Applications of PHB – a microbially produced biodegradable thermoplastic. Phys. Technol. 16. 32-36. Kohno, T., Yoshina, K., Satoh, S. (1991). The
roles of intracellular organic storage materials in the selection of microorganisms in activated sludge. Wat.
Sci. Technol. 23. 889-898.
Krishna, C., Van Loosdrecht, M.C.M. (1999). Effect of temperature on storage polymers and settleability of activated sludge. Wat. Res. 33(10). 2374-2382.
Lee, S.Y. (1996). Bacterial polyhydroxyalkanoates. Biotechnol.
Bioeng. 49. 1-14.
Lee, S.Y. (1996b). Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends
Biotechnol. 14. 431-438.
Lemoigne, M. (1926). Products of dehydration and polymerization of hydroxybutyric acid. Bull Soc Chem Biol. 8. 770-782. Md Din, M. F., Ujang, Z. and Van Loosdrecht,
M.C.M. (2004a). Polyhydroxybutyrate (PHB) production from mixed cultures of sewage sludge and Palm Oil Mill Effluent (POME): The influence of C/N ratio and slow accumulation factor. Wat.
Env. Manag. Series. ISBN: 1 84339 503
7. 115-112.
Md Din, M.F., Ujang, Z. and Loosdrecht, M.C.M. (2004b). Polyhydroxybutyrate (PHB) production from mixed cultures and Palm Oil Mill Effluent (POME) using oxygen modifications. Proceeding of Asiawater. March 30 – 31. Kuala Lumpur, Malaysia: WEMS, 57-65. NNI. NEN 6621 (1982). Bepaling van de asrest.
Nederlands Normalisatie Instituut, Delft Pagni, M., Beffa, T., Isch, C. and Aragno, M.
(1992). Linear growth and poly (β−hydroxybutyrate) synthesis in response to pulse-wise addition of the growth-limiting substrate to steady state heterotrophic continous cultures of
Aquasprillum autotrophicum. J. Gen.
Microbiol. 138. 429-436.
Ryu, H.W., Hahn, S.K., Chang, Y.K. and Chang, H.N. (1997). Production of Poly (β-hydroxybutyric acid) by High Cell Density Fed-Batch Culture of Alcaligenes eutrophus with Phosphate Limitation. Biotechnol. Bioeng. 55(1). 28-32
Smolders, G.J.F., Van Loosdrecht, M.C.M and Heijnen, J.J. (1995). A metabolic model for the biological phosphorus removal process. Wat. Sci. Tech. 31. 79-93. Steibüchel, A., and Füchtenbusch, B. (1998).
Bacterial and other biological systems for polyester production. Trends Biotechnol. 16. 419-427.
Van den Eynde, E., Vriens, I., Wynats, M. and Verachtert, H. (1984). Transient behaviour and time aspects of intermittently and continously fed bacterial cultures with regards to filamentous bulking of activated sludge.
Eur. J. Appl. Microbiol. Biotechnol. 19.
Van Loosdrecht, M.C.M., Pot, M.A., Heijnen, J.J. (1997). Importance of bacterial storage polymers in bioprocess. Wat Sci Tech. 35. 41-47