www.elsevier.com/locate/ibmb
Lipophorin as a yolk protein precursor in the mosquito, Aedes
aegypti
Jianxin Sun, Tsuyoshi Hiraoka
1, Neal T. Dittmer, Kook-Ho Cho, Alexander S.
Raikhel
*Department of Entomology, Michigan State University, East Lansing, MI 48824-1115, USA
Received 1 February 2000; received in revised form 25 April 2000; accepted 5 May 2000
Abstract
We examined expression of the lipophorin (Lp) gene, lipophorin (Lp) synthesis and secretion in the mosquito fat body, as well as dynamic changes in levels of this lipoprotein in the hemolymph and ovaries, during the first vitellogenic cycle of females of the yellow fever mosquito, Aedes aegypti. Lipophorin was purified by potassium bromide (KBr) density gradient ultracentrifugation and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Polyclonal antibodies were produced against individ-ual Lp apoproteins, apolipoprotein-I (apoLp-I) and apolipoprotein-II (apoLp-II), with molecular weights of 240 and 75 kDa, respect-ively. We report here that in the mosquito A. aegypti, Lp was synthesized by the fat body, with a low level of the Lp gene expression and protein synthesis being maintained in pre- and postvitellogenic females. Following a blood meal, the Lp gene expression and protein synthesis were significantly upregulated. Our findings showed that the fat body levels of Lp mRNA and the rate of Lp secretion by this tissue reached their maximum at 18 h post-blood meal (PMB). 20-Hydroxyecdysone was responsible for an increase in the Lp gene expression and Lp protein synthesis in the mosquito fat body. Finally, the immunocytochemical localization of Lp showed that in vitellogenic female mosquitoes, this protein was accumulated by developing oocytes where it was deposited in yolk granules. 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Lipophorin; Mosquito; Vitellogenesis; Fat body; Ovary; Gene expression; 20-Hydroxyecdysone; Yolk protein
1. Introduction
In insects, lipophorin (Lp) is the major hemolymph lipoprotein, which is composed of two apolipoproteins, 230–250-kDa apoLp-I and 70–85-kDa apoLp-II. A third small apoprotein (apoLp-III) of 18–20-kDa is associated with Lp in a reversible manner. In a high-density lipo-protein (HDLp), one apoLp-I and one apoLp-II are asso-ciated with two apoLp-III molecules (Kawooya et al. 1984, 1986; Ryan, 1990; Van der Horst, 1990; Surholt et al., 1992; Blacklock and Ryan, 1994; Soulages and Wells, 1994).
The main function of Lp is to transport lipids
through-* Corresponding author. Tel.: 7144; fax: 1-517-353-3396.
E-mail address: [email protected] (A.S. Raikhel).
1 Present address: Division of Applied Biological Science, Faculty
of Agriculture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-Cho, Fuchu, Tokyo 13-5-83, Japan.
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 9 3 - X
out the insect body, loading dietary lipids in the midgut and delivering them to the sites of their metabolism and storage. Lp also transports lipids from storage sites to utilization sites such as muscles (Kanost et al., 1990). Insect Lp carries mainly diacylglycerol and phospholipid (Van der Horst, 1990; Soulages and Wells, 1994).
donor or recipient cells. Thus, this mechanism allows an insect to meet an increased requirement for lipid trans-port without additional change in the Lp hemolymph concentration (Wells et al., 1987; Shapiro et al., 1988; Gondim et al., 1992; Ryan, 1996).
Oocyte development in insects involves the accumu-lation of large amounts of lipid, most of which is extra-ovarian in origin and is delivered by Lp; vitellogenin (Vg) contributes only about 5% of the oocyte lipid. A dual mechanism accounts for the Lp-mediated lipid delivery into the developing oocytes. The major vehicle for lipid delivery is the LDLp particle; however, some lipid is delivered by HDLp, which is internalized by developing oocytes via receptor-mediated endocytosis. Internalized HDLp, stripped of most of its lipid and apoLp-III molecules, is converted to a very high-density lipophorin (VHDLp), which is deposited in developing oocytes (Kawooya and Law, 1988; Kawooya et al., 1988; Liu and Ryan, 1991).
In anautogenous mosquitoes, a regulatory cascade linked to a blood meal controls egg maturation. Only after a mosquito female ingests blood are major events of egg maturation activated, including the accumulation of yolk proteins and lipids by developing oocytes. As a consequence of blood feeding, mosquitoes are vectors of numerous devastating diseases of humans and domestic animals (Collins and Pasketwitz, 1995; Bruno et al., 1997; Butler, 1997; Beier, 1998). It is this link to blood feeding and pathogen transmission that explains our keen interest in the molecular mechanisms underlying egg maturation in anautogenous mosquitoes (Raikhel, 1992).
Lipophorin of the mosquito, Aedes aegypti, has been reported to be an HDLp, with a density of 1.112–1.114 g/ml. Its apoprotein composition is similar to that of HDLp from other insect species: it contains two apoprot-eins, apoLp-I and apoLp-II with molecular weights of 240 and 70 kDa, respectively (Capurro et al., 1994; Ford and Van Heusden, 1994; Van Heusden et al., 1998). However, the lipid composition of mosquito Lp differs from that of other insect Lps studied so far; in addition to 32% phospholipid, it contains different neutral lipids of which triacylglycerol is the most abundant with 41%. This is in contrast with Lps from other insect species, in which diacylglycerol is usually the major neutral lipid.
A. aegypti Lp contains only 7% diacylglycerol (Ford and
Van Heusden, 1994; Pennington et al., 1996; Van Heusden et al., 1997).
A. aegypti Lp concentrations increase upon ingestion
of a blood meal, when the mosquito needs an increased rate of lipid transport to the developing ovaries. It has been reported by two laboratories that lipophorin reaches its maximal levels by 40–48 h post-blood meal (PBM) when major events of egg yolk and lipid deposition have been completed (Capurro et al., 1994; Van Heusden et al., 1997). In both studies, however, determinations of
Lp levels have been performed in whole bodies of mos-quitoes. Therefore, in order to understand the role of lipophorin in development of mosquito oocyte, further studies should take into account the complexity of the physiological state of vitellogenic female mosquitoes.
In the present study, we analyzed in detail the expression of the Lp gene, Lp synthesis and secretion in the mosquito fat body, as well as dynamic changes in levels of this protein in the hemolymph and ovaries dur-ing the first vitellogenic cycle of A. aegypti females. We also investigated the regulation of the Lp gene by 20-hydroxyecdysone (20E) in the fat body. Finally we localized the Lp synthesis and accumulation by immuno-cytochemistry. Our findings showed that the fat body levels of Lp mRNA and the rate of Lp secretion by this tissue reached their maximum at 18 h PMB and the ovar-ies accumulated Lp where it was deposited in yolk gran-ules of developing oocytes.
2. Materials and methods
2.1. Insects
Mosquitoes, A. aegypti, were reared as described by Hays and Raikhel (1990). Three to five days after eclo-sion, adult females were allowed to feed on white rats to initiate vitellogenesis. All dissections were performed in Aedes physiological saline (APS) (Hagedorn et al., 1977) at room temperature.
2.2. Materials
All analytic grade chemicals and protease inhibitors were purchased from Sigma and Calbiochem, respect-ively, unless stated otherwise.32P-dATP (3000 Ci/mmol) for labeling nucleotide probes, 35S-sulphur reagent for protein labeling in vitro and 35S-methionine (1120 Ci/mmol) for fat body culture in vitro were from NEN Life Science Products Inc., Amersham Life Science Pro-ducts and ICN Radiochemicals, respectively. DEAE-sepharose CL-6B was from Pharmacia. Protein Assay reagent, Protein A-sepharose for immunoprecipitation and molecular weight standards for sodium dodecyl sulf-ate–polyacrylamide gel electrophoresis (SDS–PAGE) were purchased from Bio-Rad. Safety Solve II scintil-lation cocktail was supplied by Research Products Inter-national. RNA ladder (0.24–9.5 kb) was purchased from Life Technologies, Inc.
2.3. Lipophorin purification and antiserum preparation
µM pepstatin (Roche Molecular Biochemicals), 4µg/ml each leupeptin, chymostatin and antipain, 10µg/ml apro-tinin, 5 mM e-amino-n-caproic acid (ACA), 1 mM
benzamidine, 12.5 mM EDTA and 1.0 mM phenylme-thylsulfonylfluoride (PMSF) (Roche Molecular Biochemicals). The homogenate was centrifuged briefly and the clear supernatant was subjected to a potassium bromide (KBr) density gradient ultracentrifugation as described previously (Pennington et al., 1996). Partially purified Lp fractions were further separated with 8% SDS–PAGE, and then specific Lp bands (apoLp-I and apoLp-II) were excised from the gel. The two different apoproteins were separately injected into rabbits with complete adjuvant. The specificity of the sera was tested by immunoblotting, using peroxidase-labeled goat anti-rabbit IgG (Cappel Organon Teknika Corp.) as second-ary antibody, SuperSignal Substrate (Western Blotting Kit, Pierce) and film fluorography.
2.4. Fat body incubation in vitro and immunoprecipitation
Abdominal walls with adhering fat body from blood-fed or 3–5-day-old previtellogenic females were isolated and placed in a tissue culture system as described pre-viously (Raikhel et al., 1997). To investigate the changes of Lp during vitellogenesis, synthesized and secreted Lp from fat body was radiolabeled with 35S-methionine according to the method described previously in a pulse-chase manner, 1.0 h and 2.0 h respectively, for radioim-munoassay and Western blot analysis (Hays and Raikhel, 1990). To examine the effects of ecdysone on lipophorin synthesis in the fat body, 20E (Sigma) and the protein translation inhibitor, cycloheximide (Chx, Calbiochem) were added into the culture medium as described pre-viously (Deitsch et al., 1995).
Immunoprecipitation was performed according to the method described by Hays and Raikhel (1990). To pre-vent a non-specific cross-reaction of vitellogenin with Lp, Vg was removed with DEAE-sepharose CL-6B before analyzing the samples. After removal of Vg, the medium was incubated with the above polyclonal anti-body (apoLp-I antianti-body) and the antianti-body-bound com-plexes were precipitated with Protein A-sepharose. The precipitates were applied to radioimmunoassays or fluo-rography as described previously (Hays and Raikhel, 1990).
2.5. Protein preparation and electrophoresis
Fat body, hemolymph and ovary proteins were pre-pared as described previously (Hays and Raikhel, 1990). Unless otherwise noted, all solutions contained the fol-lowing protease inhibitors: 1 mM 4-(2-aminoethyl)-ben-zenesulfonylfluoride, HCl (AEBSF), 1 mM PMSF, 5 mM ACA, 1 mM benzamidine, 10 mM EDTA, 10µg/ml
aprotinin, and 2µg/ml each antipain, leupeptin, pepstatin and chymostatin.
Hemolymph at different time points was collected from five mosquitoes. The mosquitoes were carefully dissected in 25 µl APS containing protease inhibitors. The ovaries and midguts were removed gently, and the remaining liquid was reserved as the hemolymph sam-ple. Proteins in the ovary and the fat body at each time point were extracted from 10 mosquitoes. Dissected ovaries and fat bodies were homogenized in 150 µl of the above APS solution and then centrifuged at 14,000 rpm for 15 min at 4°C, the supernatant was saved as the ovary and fat body extract respectively.
For quantification of Lp accumulated in the ovary, ovaries at 48 h PBM were homogenized as described above. Tenµl purified35S-methionine metabolically lab-eled Lp (specific activity, 38003.3 cpm/µg) was added to the homogenate to serve as a reference for Lp purifi-cation. Lipophorin was then purified with density gradi-ent ultracgradi-entrifugation and the total Lp in the ovary extract was estimated by adjusting the amount of Lp measured to the percent of labeled Lp recovered.
SDS–PAGE was performed using either 8% or 6–15% gradient gels by the method of Laemmli (1970). Proteins were visualized by staining with either Coomassie Brilli-ant Blue R-250 or were processed for fluorography. Pro-tein concentration was measured by Bio-Rad proPro-tein assay regent according to the instruction using BSA as a protein standard.
2.6. In vitro protein labeling
Lipophorin synthesized by fat body cultures was lab-eled with 35S-methionine as described above (Section 2.4), Lp purified from pupae was labeled with 35 S-sul-phur labeling regent SJ440 as suggested by the manufac-turer. Briefly, 50 µl (50 µCi) of SJ440 were aliquoted into an Eppendorf tube and a steady stream of nitrogen gas was blown across the top of the tube to evaporate the benzene in the solution. Next, 250µg of protein dis-solved in 100 mM sodium borate buffer (pH 8.6) were added and incubated on ice for 30 min. The reaction was stopped by the addition of 100 µl 200 mM glycine in the above borate buffer. The reaction mixture was separ-ated by chromatography through a PD-10 column equi-librated in borate buffer.
2.7. RNA isolation and Northern blot analysis
LS Reagent (Molecular Research Center, Inc.) according to the instructions from the manufacturer. Twenty-five to 35 fat body equivalents of mRNA or 20 µg of total RNA were fractionated by electrophoresis in 1% agarose/formaldehyde gel and transferred to a nitrocellu-lose membrane (Hybond) by conventional capillary blot-ting. A 1.0-kb fragment of the lipophorin gene was amplified from the genomic DNA by the polymerase chain reaction (PCR) described previously (Bej et al., 1991) and used as a probe. The primers for PCR were designed based on the partial nucleotide sequence of A.
aegypti lipophorin cDNA (Van Heusden et al., 1998).
The primer sequences were: upstream primer, 59
-CTTTGACTGCCGGTGCTCCACGATC-39; downstream
primer, 59
-GAAGTCGAAGGGAAATTGGTTGGTGG-39. PCR was conducted for 30 cycles of 94°C for 1 min,
68°C for 1 min, and 72°C for 2 min. The amplified frag-ment showed an expected size and nucleotide sequence. Random-primed probes were prepared with a DNA labe-ling kit (Boehringer Mannheim). The membranes were prehybridized in 50% formamide, 5×SSC (saline-sodium citrate), 50 mM sodium phosphate (pH 6.7), 100 µg/ml salmon sperm DNA and 5×Denhardt’s solution at 42°C for 4 h and hybridized with32
P-dATP labeled lipophorin DNA probe. The hybridized membranes were then extensively washed three times with 2×SSC, 0.1% SDS for 10 min at 42°C, and three times with 0.1×SSC, 0.1% SDS for 20 min at 65°C. Finally, the membranes were exposed to Kodak films at280°C. The same membranes
were striped with two washes in 0.1×SSC, 0.05% SDS for 2 min at 95°C and rehybridized with mosquito actin (Deitsch et al., 1995) and/or vitellogenic carboxypeptid-ase (VCP) (Cho et al., 1991) DNA probes.
2.8. Immunocytochemical localization of lipophorin
Immunocytochemical observations using A. aegypti apoLp-I polyclonal antibody and a mixture of mono-clonal antibodies specific against small Vg subunits were conducted to localize Lp, Vg and Vitellin in the fat bod-ies and ovarbod-ies. Cryosections of 6–8 µm were applied to Poly-prep coated slides (Sigma), and processed by the method of Raikhel and Lea (1983). The results were visualized by fluorescent photomicroscopy using a Zeiss Axiophot microscope equipped with phase contrast and epifluorescence, and Zeiss 210 laser scanning micro-scope, and recorded on Kodak film.
3. Results
3.1. Purification and characterization of mosquito lipophorin
A. aegypti Lp was purified from secretions of the
vitel-logenic fat body cultured in vitro and from pupae by the
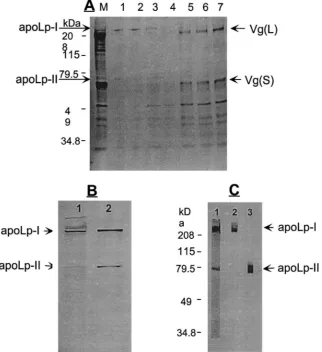
KBr density gradient ultracentrifugation (Fig. 1A). The KBr density gradient ultracentrifugation showed that Lp from these two sources possessed the same density (data not shown). The SDS–PAGE analysis confirmed that Lp secreted from the vitellogenic fat body cultured in vitro had a similar size to the lipophorin purified from mos-quito pupae. In both cases, the mosmos-quito Lp was com-posed of two apoproteins: the 240-kDa apoLp-I and the 75-kDa apoLp-II (Fig. 1B). We prepared polyclonal anti-bodies against each apoprotein, apoLp-I and apoLp-II. Immunoblotting analysis showed that these antibodies were specific to their respective Lp apoproteins (Fig. 1C). In all subsequent assays, we utilized the anti-apoLp-I antibody for detecting mosquito Lp.
3.2. Kinetics of lipophorin secretion by the mosquito fat body during the first vitellogenic cycle
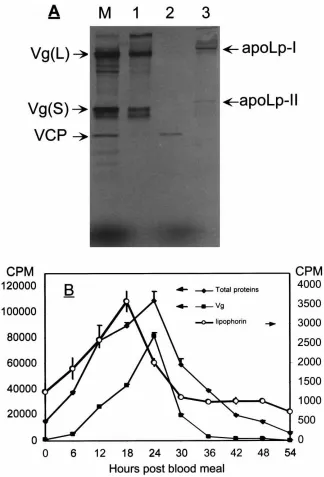
To investigate changes in the rate of Lp secretion in the mosquito fat body during vitellogenesis, the fat bod-ies were cultured in vitro in a pulse-chase manner in a culture medium supplemented with either 35S-labeled or cold methionine, respectively. Fat bodies from pre- and postvitellogenic females collected at different times PBM with 6-h intervals, and were incubated as described in Section 2.4. The radiolabeled secreted proteins were collected from the chase media (Fig. 2A, lane M). Vg was separated by DEAE-Sepharose (Fig. 2A, lane 1). Lipophorin was immunoprecipitated by anti-apoLp-I antibodies (Fig. 2A, lane 3), and its levels were meas-ured by radioimmunoassay (Hays and Raikhel, 1990). Vg was immunoprecipitated using a mixture of mono-clonal antibodies against the Vg small subunit, and its levels were measured by the same method as Lp. The levels of total protein secreted by these fat bodies were also measured. In contrast to Vg, synthesis and secretion of which were initiated by a blood meal, the previtellog-enic fat bodies produced Lp at a relatively high level (Fig. 2B). However, the rate of Lp secretion significantly increased after a blood meal, reaching a peak at 18 h PBM, and decreased thereafter to the previtellogenic lev-els by 30 h PBM (Fig. 2B). The rate of Vg synthesis and secretion exhibited much more dramatic PBM activation than that of Lp, reaching its peak at 24 h PBM but then dropping to background levels by 36 h PBM (Fig. 2B).
3.3. Changes in lipophorin levels in the fat body, hemolymph and ovary during the first vitellogenic cycle
Fig. 1. Characterization of the mosquito lipophorin (Lp). (A)35S-Methionine-labeled Lp from the fat body culture medium, subjected to the KBr
density gradient ultracentrifugation. Gradient centrifugation fractions were analyzed by 8% SDS–PAGE and fluorography. M, fat body culture medium; 1–7, each gradient fraction from the top to bottom. (B) Comparison of Lp secreted by the fat body of vitellogenic female mosquito (1) with Lp isolated from pupae (2). Lipophorin from the female fat body was metabolically labeled with35S-methionine, while Lp from pupae was
labeled in vitro with35S-sulphur labeling reagent. (C) Western blot analysis using polyclonal antibodies specific against either I or
apoLp-II. 1, Purified Lp separated by 8% SDS–PAGE and stained with Coomassie Blue R250; 2, immunoblot probed with anti-apoLp-I antibody; 3, immunoblot probed with anti-apoLp-II antibody. (A and C) Protein molecular weight standards (in kilodaltons) are noted on the left.
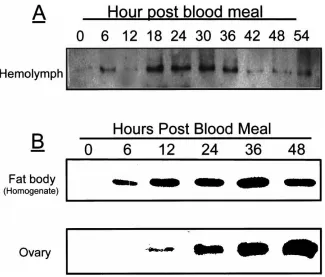
gradient SDS–PAGE and transferred onto a nitrocellu-lose membrane, and then detected with the apoLp-I spe-cific antibody. Fig. 3A showed that Lp levels in the hem-olymph gradually increased after the blood meal, reaching a peak at 18 h PBM, and then decreased to the previtellogenic level by 42 h PBM. Interestingly, the concentration of Lp in the fat body continued to increase, reaching its maximum at 36 h PBM, suggesting the possibility of reabsorption of Lp by the fat body from the hemolymph during postvitellogenesis (Fig. 3B). The previtellogenic and early vitellogenic ovaries had no detectable Lp; it was first detected at 6–12 h PBM and then increased gradually until 48 h PBM. These results suggested that following a blood meal, the Lp secreted into the hemolymph was probably sequestered by developing oocytes (Fig. 3B).
To further demonstrate the identity of ovarian Lp, we purified Lp from ovaries at 48 h PBM by density
gradi-ent ultracgradi-entrifugation, analyzed it using SDS–PAGE and Western blotting with apoLp-I and apoLp-II poly-clonal antibodies. These analyses showed that ovarian Lp was similar to that from the fat body culture medium and consisted of apoLp-I and apoLp-II (Fig. 4). Prelimi-nary quantitative analysis showed that at the time of ter-mination of protein uptake (48 h PBM), Lp was 3% of the total ovarian protein (data not shown).
3.4. Expression of the mosquito Lp gene during the first vitellogenic cycle
Fig. 2. Changes of Lp synthesis rate in the female fat body during vitellogenesis. (A) Removal of vitellogenin (Vg) from the fat body culture medium using DEAE-Sepharose: M, the proteins secreted by the fat body; 1, Vg-bound fraction; 2, unbound fraction; 3, lipophorin, immunoprecipit-ated by the protein A-agarose– anti-apoLp-I antibody complexes from the unbound fraction. 35S-Methionione labeling and fluorography. (B)
Radioimmunoassay determination of Lp, Vg and total protein secreted by the fat body at different times after a blood meal. The values are means±SE (n=3). Arrows point to the scales used for the proteins indicated.
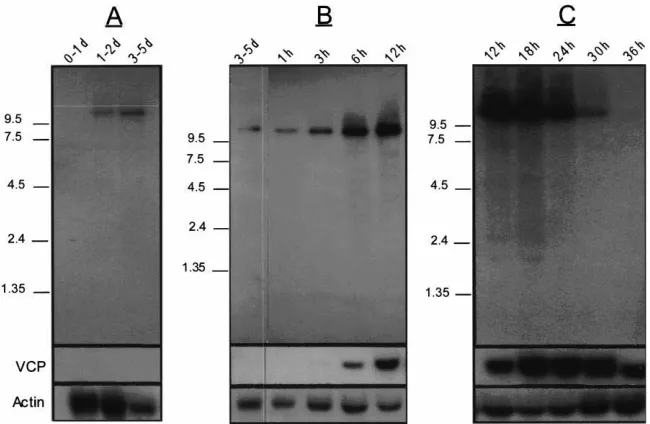
to increase between 3 and 6 h PBM and reached peak levels between 12 and 18 h PBM, before dropping to a barely detectable level by 36 h PBM (Fig. 5B,C). VCP cDNA, reverse transcripted from the gene encoding a second yolk protein precursor in the mosquito, was used as a vitellogenic control in hybridization to the prep-arations of the same tissues (Fig. 5). The VCP gene was not expressed in the previtellogenic period, its transcript became detectable at 6 h PBM, reached its peak expression at 24 h PBM and then declined to
undetect-able levels after 36 h PBM (Fig. 5). Neither the VCP nor the Lp gene was expressed in the ovary (not shown).
3.5. 20-Hydroxyecdysone increases the Lp gene expression and protein synthesis in cultured previtellogenic fat bodies
Previtellogenic fat bodies from 3–5-day-old females were incubated for 6 h at 27°C in the culture medium in the presence or absence of 1026
Fig. 3. Western blot analysis of Lp levels in the fat body, hemolymph and ovary of the mosquito female at different times after a blood meal. (A) Lipophorin content in the hemolymph. (B) Lipophorin extracted from the fat bodies (upper panel) and the ovaries (lower panel). Proteins were resolved by 6–15% SDS–PAGE, transferred onto the nitrocellulose membrane and probed with anti-apoLp-I antibodies.
Fig. 4. Characterization of the ovarian Lp. (A) Lipophorin isolated from the ovaries at 48 h PBM using the KBr density gradient ultracentrifugation. Each fraction from the centrifugation was resolved by 6% SDS–PAGE and stained with Coomassie Blue R250. Lanes 1–9 were fractions from high to low density; M, SDS–PAGE standard protein molecular weight markers. (B) Partially purified Lp (fraction 1) from the ovary was analyzed by Western blotting using A. aegypti apoLp-I and apoLp-II polyclonal antibodies (lane 1) and compared with Lp density gradient-purified from the fat body culture media (lane 2).
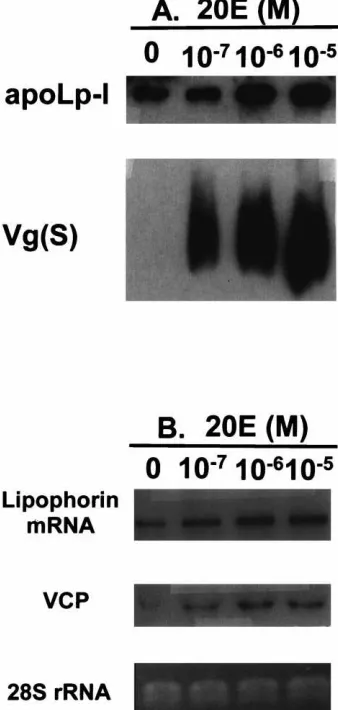
experiments, we also monitored the effect of 20E on pro-duction of Vg as a positive control. Culture medium from each sample was analyzed by Western blotting, uti-lizing polyclonal antibodies against apoLp-I and a mix-ture of monoclonal antibodies against the Vg small sub-unit as primary antibodies. The previtellogenic fat bodies secreted a considerable amount of Lp, but not Vg. 20-Hydroxyecdysone increased the Lp production in a dose-dependent manner similar to the response of the Vg gene to 20E; however, this enhancement of Lp production was not as dramatic as that of Vg (Fig. 6A).
The effect of 20E on the Lp gene was also examined at the mRNA level. The VCP gene was used as a positive control for induction by 20E. In contrast to VCP, Lp mRNA was present in the previtellogenic fat bodies. The levels of both VCP and Lp transcripts increased in response to 20E in a dose-dependent manner (Fig. 6B). However, the induction of the VCP gene and an increase in Lp mRNA levels by 20E in cultured fat bodies was blocked by addition of cycloheximide (Chx), a protein translational inhibitor, to the medium. The Chx inhi-bition was dose-dependent with 1025
Fig. 5. Lipophorin mRNA expression in the fat body of the female mosquito during the first vitellogenic cycle. Northern blot analysis of mRNA prepared from dissected fat bodies at the indicated times. (A and C) 35 mosquito-equivalent loaded per lane. (B) 25 mosquito-equivalent loaded per lane. After hybridization with Lp cDNA, the membranes were striped and successively probed with actin cDNA (Deitsch et al., 1995) and the yolk protein precursor vitellogenic carboxypeptidase (VCP) cDNA (Cho et al., 1991). An RNA ladder (in kilobases) is noted on the left; d, days post-eclosion; h, hours post-blood meal.
abolishing the effect of 1026
M 20E, which otherwise maximally increases Lp gene expression (data not shown).
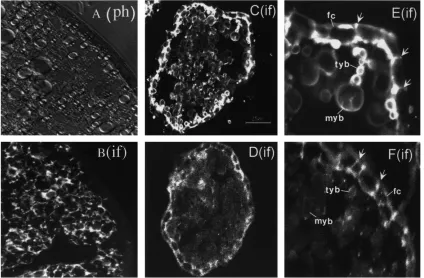
3.6. Localization of the synthesis and accumulation of Lp
Immunocytochemistry using the apoLp-I anti-body and rhodamine-conjugated secondary antibodies revealed the distribution of Lp in tissue sections (Fig. 7). In the fat bodies of vitellogenic females, this protein exhibited localization similar to that of Vg and VCP (Fig. 7B). However, in contrast to these yolk protein pre-cursors, Lp was also present in the previtellogenic and late-vitellogenic fat bodies (not shown).
In contrast to the fat body, no Lp was found in the previtellogenic ovaries (not shown). In follicles of vitel-logenic ovaries, Lp was found in inter-follicular and the peri-oocytic spaces as well as in yolk bodies of oocytes (Fig. 7D,F). Vitellogenin, used as a positive control, appeared in similar compartment of the same follicles, but with much higher intensity of labeling (Fig. 7C,E).
4. Discussion
Our findings are in agreement with previous reports showing that in insects, including mosquitoes, the fat body is the site of Lp synthesis and secretion. Likewise, we show that the mosquito apoLp-I and apoLp-II orig-inate from the same Lp precursor gene, expression of which leads to the Lp transcript of 10 kb in size (Weers
et al., 1992a,b; Sundermeyer et al., 1996; Van Heusden et al., 1998). However, we demonstrate here that in the mosquito A. aegypti, the fat body levels of Lp mRNA and the rate of Lp secretion by this tissue reached their maximum at 18 h PMB. Our conclusion rests on several lines of evidence which are in very good correlation with each other: the rate of secretion of the newly synthesized fat body Lp detected by the radioimmunoassay, the amounts of Lp secreted by the fat bodies, the Lp levels in collected samples of hemolymph detected by immu-noblots, and finally, the Lp transcript levels in the fat body, demonstrated by the Northern blot analysis. Our results are in agreement with those from previous studies in which Lp was reported to reach its highest level at 40–48 h PBM (Capurro et al., 1994; Van Heusden et al., 1997). However, our study clearly showed the underly-ing complexity of the Lp metabolism in the vitellogenic female mosquito, with Lp being synthesized by the fat body and accumulated by the ovaries. Furthermore, the increased level of Lp in the fat body at the postvitellog-enic period suggests that this tissue internalized Lp from the hemolymph after the completion of the yolk accumu-lation cycle by developing oocytes.
Fig. 6. Effect of 20-hydroxyecdysone (20E) on the expression of the mosquito Lp gene and synthesis of Lp in the fat body culture. (A) Western blot analysis of the effect of different concentrations of 20E on the Lp synthesis. Induction of the Vg synthesis by 20E was meas-ured as positive control. (B) Northern blot analysis of the 20E effect on the Lp gene expression in the mosquito fat body in vitro. Induction of the VCP gene by 20E was measured as a positive control. 28S rRNA was used as a marker for RNA loading.
in vitro fat body culture experiments demonstrated that 20E is involved in elevating the Lp gene expression in a dose-dependent manner similar to that of Vg and VCP genes (Deitsch et al., 1995), with 1026 M 20E to be required for maximal activation of all these genes. Fur-thermore, tests with the protein synthesis inhibitor, cycloheximide, show that similar to the Vg and VCP genes, the 20E activation of the Lp gene was completely inhibited by this reagent, indicating the requirement of protein synthesis for mediating the 20E regulation of these genes (Deitsch et al., 1995). Further analysis of regulatory regions of these genes should be conducted in order to elucidate the settled differences in regulatory circuitry of these genes determining the precise timing of their expression.
A blood meal taken by an anautogenous mosquito triggers a cascade of physiological events, which lead to
rapid and synchronous development of oocytes into mature eggs ready for oviposition by 72 h PBM. The dramatic enlargement of oocytes in such a short period of time is a consequence of rapid accumulation of large quantities of extraovarian yolk protein precursors as well as lipid. In A. aegypti, three yolk protein precursors, Vg, VCP and VCB, are synthesized by the fat body and specifically accumulated by developing oocytes (Dhadialla and Raikhel, 1990; Hays and Raikhel, 1990; Cho et al. 1991, 1999). The data we report here, showing accumulation of Lp in developing oocytes, suggest that Lp also serves as a yolk protein precursor in this mos-quito. Lipophorin has been implicated as a yolk protein precursor in eggs of lepidopteran insects, where it is deposited as VHDLp (Chino et al., 1977; Kawooya and Law, 1988; Kawooya et al., 1988; Telfer and Pan, 1988; Kulakosky and Telfer, 1990; Telfer et al., 1991).
In insect oocytes, accumulation of Vg is mediated by the specific Vg receptor (Sappington and Raikhel, 1998). The mosquito Vg receptor has been cloned and shown to represent a unique class of LDLp receptor family (Sappington et al. 1995, 1996; Sappington and Raikhel, 1998). Specific Lp receptors have been identified in the fat body of several insects (Tsuchida and Wells, 1990; Dantuma et al. 1996, 1997). More recently, cloning of the Locusta Lp receptor, homologous to the vertebrate VLDLp receptor, has been reported. The Locusta Lp receptor mediates Lp endocytosis by the fat body (Dantuma et al., 1999). The information concerning the Lp receptor in insect oocytes is limited. Osir and Law (1986) have reported that Manduca Lp does not compete with Vg for binding to oocyte membranes indicating that the internalization of these lipoproteins occurs via separ-ate receptors. However, Kulakosky and Telfer (1990) have reported the competition between Vg and Lp during their internalization by Hyalophora vitellogenic follicle, suggesting the presence of a common receptor for both lipoproteins. Our preliminary data indicate that a specific Lp receptor exists in the mosquito oocytes, which is dis-tinct from the Vg receptor and is homologous to the
Locusta fat body Lp receptor and to the vertebrate
VLDLp receptor (Sook-Jae Seo, Hyang-Mi Jun and Alexander S. Raikhel, unpublished observation).
In conclusion, we found that in the mosquito A.
aegypti, Lp is synthesized by the fat body, with a low
Fig. 7. Immunocytochemical localization of Lp in frozen sections of tissues, using either anti-apoLp-I polyclonal antibodies or a mixture of monoclonal antibodies against the Vg small subunit, followed by rhodamine-conjugated rabbit IgG or fluorescein-conjugated goat-anti-mouse IgG secondary antibodies. (A and B) The fat body at 12 h PBM phase contrast image (ph) and immunostained image (if) with apoLp-I antibodies, respectively. (C–F) Confocal images of ovarian follicles at 12 h PBM immunostained with Vg antibodies (C and E) or anti-apoLp-I (D and F), respectively. FC, follicular cells; MYB, mature yolk body; TYB, transitional yolk body. Arrows point to positive immunostaining between follicular cells.
constitutes only 3% of total ovarian proteins after the completion of protein accumulation. Considering that lipids make up approximately 35–40% of the insect egg dry weight (Kawooya and Law, 1988; Briegel, 1990), internalization of Lp is unlikely the major route of lipid delivery to the developing oocyte. Thus, elucidation of the precise role of Lp as a yolk protein precursor in the mosquito oocyte requires further studies.
Acknowledgements
The authors thank A.R. Hays for his excellent techni-cal assistance, Dr J.S. Zhu for crititechni-cal reading, and Michel Trail for editing the manuscript. This work was supported by the NIH grant AI24716 to ASR.
References
Beier, J.C., 1998. Malaria parasite development in mosquitoes. Annu. Rev. Entomol. 43, 519–543.
Bej, A.K., Mahbubani, M.H., Atlas, R.M., 1991. Amplification of nucleic acids by polymerase chain reaction (PCR) and other methods and their applications. Crit. Rev. Biochem. Mol. Biol. 26, 301–334.
Blacklock, B.J., Ryan, R.O., 1994. Hemolymph lipid transport. Insect Biochem. Mol. Biol. 24, 855–873.
Bruno, J.M., Feachem, R., Godal, T., Nchinda, T., Ogilvie, B., Mshana, R. et al., 1997. The spirit of Dakar: a call for action on malaria. Nature 386, 541.
Butler, D., 1997. Time to put malaria control on the global agenda. Nature 386, 535–536.
Capurro, M.de L., de Bianchi, A.G., Marinotti, O., 1994. Aedes aegypti lipophorin. Comp. Biochem. Physiol. 108, 35–39.
Chino, H., Downer, R.G., Takahashi, K., 1977. The role of diacylgly-cerol-carrying lipoprotein I in lipid transport during insect vitellog-enesis. Biochim. Biophys. Acta 22, 508–516.
Cho, W.L., Deitsch, K.W., Raikhel, A.S., 1991. An extraovarian pro-tein accumulated in mosquito oocytes is a carboxypeptidase acti-vated in embryos. Proc. Natl. Acad. Sci. U. S. A. 88, 10821. Cho, W.L., Tsao, S.M., Hays, A.R., Walter, R., Chen, J.S.,
Snigirev-skaya, E.S. et al., 1999. Mosquito cathepsin B-like protease involved in embryonic degradation of vitellin is produced as a lat-ent extraovarian precursor. J. Biol. Chem. 274, 13311–13321. Collins, F.H., Pasketwitz, S.M., 1995. Malaria: current and future
pros-pects for control. Annu. Rev. Entomol. 40, 195–219.
Dantuma, N.P., Van Marrewijk, W.J., Wynne, H.J., Van der Horst, D.J., 1996. Interaction of an insect lipoprotein with its binding site at the fat body. J. Lipid Res. 37, 1345–1355.
Dantuma, N.P., Pijnenburg, M.A., Diederen, J.H., Van der Horst, D.J., 1997. Developmental down-regulation of receptor-mediated endo-cytosis of an insect lipoprotein. J. Lipid Res. 38, 254–265. Dantuma, N.P., Pijnenburg, M.A., Diederen, J.H., Van der Horst, D.J.,
Dantuma, N.P., Potters, M., De Winther, M.P., Tensen, C.P., Kooiman, F.P., Bogerd, J. et al., 1999. An insect homolog of the vertebrate very low density lipoprotein receptor mediates endocytosis of lipo-phorins. J. Lipid Res. 40, 973–978.
Deitsch, K.W., Chen, J.S., Raikhel, A.S., 1995. Indirect control of yolk protein genes by 20-hydroxyecdysone in the fat body of the mos-quito, Aedes aegypti. Insect Biochem. Mol. Biol. 25, 449–454. Dhadialla, T.S., Raikhel, A.S., 1990. Biosynthesis of mosquito
vitello-genin. J. Biol. Chem. 265, 9924–9933.
Ford, P.S., Van Heusden, M.C., 1994. Triglyceride-rich lipophorin in
Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 31, 435–441.
Gondim, K.C., Atella, G.C., Kawooya, J.K., Masuda, H., 1992. Role of phospholipids in the lipophorin particles of Rhodnius prolixus. Arch. Insect Biochem. Physiol. 20, 303–314.
Hagedorn, H.H., Turner, S., Hagedorn, E.A., Pontecorvo, D., Green-baum, P., Pfeiffer, D. et al., 1977. Postemergence growth of the ovarian follicles of Aedes aegypti. J. Insect Physiol. 23, 203–206. Hays, A.R., Raikhel, A.S., 1990. A novel protein produced by the vitellogenic fat body and accumulated in mosquito oocytes. Roux’s Arch. Dev. Biol. 119, 114–121.
Kanost, M.R., Kawooya, J.K., Law, J.H., Ryan, R.O., Van Heusden, M.C., Ziegler, R., 1990. Insect hemolymph proteins. Adv. Insect Physiol. 22, 229–396.
Kawooya, J.K., Law, J.H., 1988. Role of lipophorin in lipid transport to the insect egg. J. Biol. Chem. 263, 8748–8753.
Kawooya, J.K., Keim, P.S., Ryan, R.O., Shapiro, J.P., Samaraweera, P., Law, J.H., 1984. Insect apolipophorin III. Purification and properties. J. Biol. Chem. 259, 10733–10737.
Kawooya, J.K., Meredith, S.C., Wells, M.A., Kezdy, F.J., Law, J.H., 1986. Physical and surface properties of insect apolipophorin III. J. Biol. Chem. 261, 13588–13591.
Kawooya, J.K., Osir, E.O., Law, J.H., 1988. Uptake of the major hem-olymph lipoprotein and its transformation in the insect egg. J. Biol. Chem. 263, 8740–8747.
Kulakosky, P.C., Telfer, W.H., 1990. Lipophorin as a yolk precursor in Hyalophora cecropia: uptake kinetics and competition with vit-ellogenin. Arch. Insect Biochem. Physiol. 14, 269–285.
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685.
Liu, H., Ryan, R.O., 1991. Role of lipid transfer particle in transform-ation of lipophorin insect oocytes. Biochem. Biophys. Acta 1085, 112–118.
Osir, E.O., Law, J.H., 1986. Studies on binding and uptake of vitellog-enin by follicles of the tobacco hornworm, Manduca sexta. Arch. Insect Biochem. Physiol. 3, 513–528.
Pennington, J.E., Nussenzveig, R.H., Van Heusden, M.C., 1996. Lipid transfer from insect fat body to lipophorin: comparison between a mosquito triacylglycerol-rich lipophorin and a sphinx moth diacyl-glycerol-rich lipophorin. J. Lipid Res. 37, 1144–1152.
Raikhel, A.S., Lea, A.O., 1983. Previtellogenic development and vitel-logenin synthesis in the fat body of a mosquito: an ultrastructural and immunocytochemical study. Tissue Cell 15, 281–300. Raikhel, A.S., 1992. Vitellogenesis in mosquitoes. Adv. Dis. Vector
Res. 9, 1–39.
Raikhel, A.S., Deitsch, K.W., Sappington, T.W., 1997. Culture and analysis of insect fat body. In: Crampton, J.M., Beard, C.B., Louis,
C. (Eds.), The Molecular Biology of Insect Disease Vectors: A Method Manual. Chapman & Hall, London, pp. 507–522. Ryan, R.O., 1990. Dynamics of insect lipophorin metabolism. J. Lipid
Res. 31, 1725–1739.
Ryan, R.O., 1996. Merck Frosst award lecture 1995. La conference Merck Frosst 1995. Structural studies of lipoproteins and their apol-ipoprotein components. Biochem. Cell Biol. 74, 155–164. Sappington, T.W., Raikhel, A.S., 1998. Molecular characteristics of
insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 28, 277–300.
Sappington, T.W., Hays, A.R., Raikhel, A.S., 1995. Mosquito vitellog-enin receptor: purification, developmental and biochemical charac-terization. Insect Biochem. Mol. Biol. 25, 807–817.
Sappington, T.W., Kokoza, V.A., Cho, W.L., Raikhel, A.S., 1996. Molecular characterization of the mosquito vitellogenin receptor reveals unexpected high homology to the Drosophila yolk protein receptor. Proc. Natl. Acad. Sci. U. S. A. 93, 8934–8939. Shapiro, J.P., Law, J.H., Wells, M.A., 1988. Lipid transport in insects.
Annu. Rev. Entomol. 33, 297–318.
Soulages, J.L., Wells, M.A., 1994. Lipophorin: the structure of an insect lipoprotein and its role in lipid transport in insects. Adv. Protein Chem. 45, 371–415.
Sundermeyer, K., Hendricks, J.K., Prasad, S.V., Wells, M.A., 1996. The precursor protein of the structural apoproteins of lipophorin: cDNA and deduced amino acid sequence. Insect Biochem. Mol. Biol. 26, 735–738.
Surholt, B., Van Doorn, J.M., Goldberg, J., Van der Horst, D.J., 1992. Insect lipophorin conversions. Compositional analysis of high- and low-density lipophorin of Acherontia atropos and Locusta
migratoria. Biol. Chem. Hoppe–Seyler 373, 13–20.
Telfer, W.H., Pan, M.L., 1988. Adsorptive endocytosis of vitellogenin, lipophorin, and microvitellogenin during yolk formation in
Hyalo-phora. Arch. Insect Biochem. Physiol. 9, 339–355.
Telfer, W.H., Pan, M.L., Law, J.H., 1991. Lipophorin in developing adults of Hyalophora cecropia: support of yolk formation and prep-aration for flight. Insect Biochem. 21, 653–663.
Tsuchida, K., Wells, M.A., 1990. Isolation and characterization of a lipoprotein receptor from the fat body of an insect, Manduca sexta. J. Biol. Chem. 265, 5761–5767.
Van der Horst, D.J., 1990. Lipid transport function of lipoproteins in flying insects. Biochim. Biophys. Acta 1047, 195–211.
Van Heusden, M.C., Erickson, B.A., Pennington, J.E., 1997. Lipopho-rin levels in the yellow fever mosquito, Aedes aegypti, and the effect of feeding. Arch. Insect Biochem. Physiol. 34, 301–312. Van Heusden, M.C., Thompson, F., Dennis, J., 1998. Biosynthesis of
Aedes aegypti lipophorin and gene expression of its
apolipoprote-ins. Insect Biochem. Mol. Biol. 28, 733–738.
Weers, P.M.M., Van Marrewijk, W.J.A., Beenakkers, A.M.TH., Van der Horst, D.J., 1992a. Biosynthesis of locust lipophorin. J. Biol. Chem. 268, 4300–4303.
Weers, P.M.M., Van der Horst, D.J., Van Marrewijk, W.J.A., Van der Eijinder, M., Van Doom, J.M., Beenakkers, A.M.TH., 1992b. Biosynthesis and secretion of insect lipophorin. J. Lipid Res. 33, 485–491.
Wells, M.A., Ryan, R.O., Kawooya, J.K., Law, J.H., 1987. The role of apolipophorin III in vivo lipoprotein interconversions in adult