Summary We studied the responses to light and nutrient availability of the two Afromontane conifers Juniperus procera Hochst. ex Endl., reputedly a shade-intolerant species, and Afrocarpus gracilior Polger C.N. Page, a shade-tolerant spe-cies. The species behaved similarly in response to light and nutrient availability. Both species showed positive growth rates at photon fluxes of 0.6--0.8 mol m−2 day−1. Maximum relative growth rates of 0.15 week−1 were attained at an irradiance of 8 mol m−2 day−1 in the high-nutrient supply treatment. The growth response to high-nutrient supply was achieved by an increase in leaf area ratio rather than by an increase in net assimilation rate.
In a 10-week growth experiment, both species displayed an increase in stem extension rate in response to a low red/far-red ratio (0.09) at low irradiance, and A. gracilior also responded by increasing its specific leaf area. Similiar findings were observed in a short-term experiment in which internodes were laterally irradiated.
Keywords: Afrocarpus gracilior, Afromontane coniferous for-est, climax species, Ethiopia, forest understory, Juniperus pro-cera, pioneer species, photosynthetic photon flux, red/far-red ratio, seedling establishment.
Introduction
Plants in the forest understory exhibit a variety of photosyn-thetic characteristics that enable them to maintain a positive carbon balance at low irradiances (Boardman 1977, Björkman 1981). Although many studies on the physiological and eco-logical significance of shade have shown that the capacity of understory plants to change their pattern of morphological development in response to dense vegetation is largely trig-gered by a change in the red to far-red ratio (R/Fr) (Smith 1986, Lee 1988, Ballaré et al. 1990, Turnbull 1991, Schmitt and Wulff 1993, Aphalo and Ballaré 1995, Tinoco-Ojanguren and Pearcy 1995), few studies have focused on the morphogenetic responses of tree seedlings to a low R/Fr ratio (Morgan et al. 1983, Kwesiga and Grace 1986, Warrington et al. 1989, Kamaluddin 1991, Kamaluddin and Grace 1993).
Many studies have focused on the effects of irradiance on seedling growth in forest clearings; however, the effects of nutrient supply may be equally important. There is evidence that leaf morphology and physiology and hence leaf photosyn-thetic capacity change with increasing nutrient supply, particu-larly at high irradiance (Thompson et al. 1988, Riddoch et al. 1991). Although the supply of all nutrients is important (Grubb 1977), the supply of N is particularly important because leaf photosynthesis is highly and positively correlated with N sup-ply (Field and Mooney 1986, Thompson et al. 1988, Evans 1989, Riddoch et al. 1991). Thus, plants acclimate to a given irradiance and nutrient availability by physiological adjust-ments that increase carbon gain.
Previous studies suggest that gap species and climax species differ in their growth responses to light (Fetcher et al. 1983, Popma and Bongers 1988). Because differential responses of species to irradiance and nutrient availability may influence colonization patterns in forests, we have investigated possible interactions between light availability and nutrient supply on the growth of two Afromontane coniferous tree species. Spe-cifically, we tested the hypothesis that a fast-growing gap species needs an enhanced supply of nutrients to exploit full light. We grew seedlings of Juniperus procera Hochst. ex Endl. (formerly Juniperus excelsa M. Bieb.) and Afrocarpus gracilior (Pilger) C.N. Page (formerly Podocarpus gracilior Pilger and/or Decussocarpus gracilior (Pilger) de Laub.) un-der light conditions simulating an unun-derstory habitat, a forest clearing and disturbance gaps at two rates of nutrient supply. Because it is unclear whether the response of tree seedlings to shade is dependent on a low R/Fr ratio or a low photon flux density, we investigated the influence of R/Fr ratio on stem extension at low irradiances.
Both Juniperus procera and Afrocarpus gracilior are wide-spread constituents of the Afromontane forests. Juniperus pro-cera is naturally distributed in the mountainous regions of East Africa and on the mountains alongside the shore of the Red Sea (in Saudi Arabia and Yemen) (Kerfoot 1966, Farjon 1992). It has been classified as shade intolerant, because it does not regenerate under shade, and its seedlings are unable to pene-trate a deep layer of humus on the surface of the soil (Gardner 1926). The natural distribution of A. gracilior extends from
Response of two Afromontane coniferous tree species to light and
nutrient supply
HAILU SHAREW, JOHN GRACE and COLIN J. LEGG
Institute of Ecology and Resource Management, Division of Biological Sciences, The University of Edinburgh, Darwin Building, Mayfield Road, Edinburgh, EH9 3JU, U.K.
Received June 28, 1995
Ethiopia to Zaïre (Melville 1954, Gaussen 1974). The species is considered shade tolerant because it regenerates naturally in the undisturbed part of the forest (Chaffey 1979) and under pure J. procera stands (Sharew 1994). Both are economically important timber species (Dale and Greenway 1961, Chaffey 1979, Hall 1984, Kigomo 1985).
Material and methods
Plant materials
Seeds of J. procera and A. gracilior collected from Ethiopia were sown in germination trays containing equal parts by volume of vermiculite and perlite. When the seedlings were 2--3 weeks old and ranged in height from 24 to 45 mm for J. procera and from 38 to 70 mm for A. gracilior, they were transplanted to plastic tubes (210-mm tall and 65-mm in di-ameter) filled with equal volumes of vermiculite and perlite. The tubes were embedded in pots of perlite to permit drainage.
Shade and nutrient supply experiment
Growth conditions and treatments Four light regimes, (i) deep shade, (ii) low, (iii) intermediate and (iv) high photon irradiances, and two nutrient regimes, (i) low and (ii) high, were each applied to one block of 10 seedlings in a randomized factorial design in a greenhouse. The seedlings were grouped according to initial size to minimize the within-block variation. Each plant was provided with an individual shade-cover consisting of a plastic tube (240-mm long and 75-mm in diameter), covered at one end with celluloid filters (Strand, Northern Light, Edinburgh) and fixed at the distal end with masking tape. The outer part of the tube was covered with aluminum foil to reflect transmitted light. The advantage of individual shade covers is that each plant is independent of all the others, thus avoiding the problem of pseudoreplication that arises when large enclosures are utilized. The celluloid filters simulated forest shade by simultaneously reducing photosyn-thetic photon flux density (PPFD) and R/Fr ratios (Kamalud-din 1991). Spectral transmission of the filters was scanned with a spectroradiometer (Model 6000, Monolite Instruments Ltd., Surrey, UK) comprising a system controller, scanning mono-chrometer Model 6162, tungsten halogen light source with IR filter 6130, integrating sphere Model 6118 and PMT unit Model 6771+6173A.
For the shade treatments, two layers of Chromoid 211 (Chromoid, Strand Lighting, Middlesex, UK) plus one layer of Chromoid 209 were used for the deep-shade treatment; one layer of Chromoid 211 0.9 ND was used for the low-light treatment, and one layer of Chromoid 209 plus one layer of plastic netting were used for the intermediate-light treatment. To maintain a similar microclimate inside the tubes in all treatments, the tubes in the high-light treatment were covered with one layer of clear filter No. 430. The PPFD under each treatment was measured with a quantum sensor (LI-190 SB, Li-Cor Inc., Lincoln, NE) and the R/Fr ratio was measured hourly with a red/far-red sensor (SKR 110, Skye Instruments, Ltd., Powys, Wales). Data were stored on a micrologger
(Delta-T Devices, Cambridge, U.K.), and the mean ratio for each treatment was calculated from 12 readings.
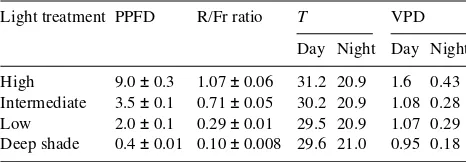
The mean daily total PPFD ranged from 0.4 ± 0.01 in the deep-shade treatment to 9.0 ± 0.3 mol m−2 day−1 in the high-light treatment (Table 1). The mean daily day/night air temperature was 28.8 ± 0.3/21.0 ± 0.1 °C. There was no statistically significant difference in air temperature between treatments, although it was slightly higher in the high-light treatment during the day (Table 1).
The seedlings were fed with modified Ingestad’s solution (Ingestad 1979). Solution B was modified to exclude nitrogen, potassium and phosphorus salts to allow the application of the same amounts of minor and trace elements for all treatments. The amounts of the other elements were raised to maintain the same ionic balance as in the original Solution B. Stock Solut-ion A contained (g dm−3), NH
4NO3 140.2, KNO3 37.2, KH2PO4 41.3, K2SO4 14.0; and Stock Solution B contained: H2SO4 1.47, H3BO3 0.57, FeC6H5O7.5H2O 4.19, (CH3COO)2 -Mg.4H2O 37.53, MnSO4 0.55, CuCl2 0.032, ZnSO4 0.036, NaMoO4 0.007. Solutions A and B were diluted with deionized water in the ratios of 0.9/1 and 0.03/1 to provide 30 and 1 mg dm−3 of N, respectively, for the high- and low-nutrient regimes. The diluted stock solutions were mixed in equal proportions and applied daily to each planting tube until the solution began to flow from the drainage hole.
At the start of the experiment (t1), 10 seedlings of each species were harvested and their leaf area (A) measured. The seedlings were then dried at 100 °C for 24 h and leaves, stem and roots weighed.
Leaf anatomy After 20 weeks of growth, stomatal density, and thickness of leaf palisade tissue and spongy mesophyll were measured. To determine stomatal density, a surface rep-lica impression was made on both leaf surfaces with silicone rubber and transparent varnish. Stomatal densities were counted with a calibrated grid in the eyepiece of a light micro-scope covering a microscopic field of 1 mm2 on a sample of five seedlings of each treatment.
Growth analysis The height and number of leaves of the seedlings were recorded weekly. Finally, after 20 weeks of growth (t2), the remaining plants were harvested and height, hypocotyl length, number of leaves and leaflets of the 80 seedlings were recorded. Projected leaf area was measured with a leaf area meter (LI-1300, Li-Cor, Inc.) and dry weight of the leaves determined.
Table 1. Summary of treatment conditions. Photosynthetic photon flux density (PPFD, µmol m−2 day−1), Red/far-red (R/Fr) ratio, air
tem-perature (T, °C) and vapor pressure deficit (VPD, kPa).
Light treatment PPFD R/Fr ratio T VPD
Day Night Day Night
Mean relative growth rates of biomass (RGRb) and stem length (RGRs), and net assimilation rate (NAR), were calcu-lated for the period t1 to t2. Leaf area ratio(LAR), specific leaf area (SLA), leaf weight ratio (LWR), specific stem length (SSL), stem weight ratio (SWR) and root weight ratio (RWR) were determined at final harvest (t2). Plant dry weight and leaf area were highly and positively correlated (J. procera: r = 0.93, n = 60, P < 0.001; A. gracilior: r = 0.82, n = 60, P < 0.001).
The variation in each parameter was determined by analysis of variance (ANOVA). Duncan’s New Multiple Range Test was used to locate significant differences between treatments (Steel and Torrie 1981).
Leaf nitrogen and chlorophyll Leaf nitrogen was determined with a flow injection analysis (5020 spectrophotometer and 5032 analyser, Tecator, Bristol, U.K.) after grinding leaf tissue in a Mixer mill (Glen Creston, Stanmore, U.K.) and digesting with acid.
Leaf chlorophyll was determined in mature leaves of J. pro-cera and A. gracilior. Pairs of leaves were taken from opposite sides of the mid-point of the stem of five seedlings of each treatment. The leaf area of half of each sample was measured and the leaves were weighed after drying at 100 °C for 24 h. Chlorophyll was extracted from the other half of each leaf sample with 3 cm3 of N,N′-dimethylformamide (DMF) for 24 h in the dark as described by Porra et al.(1989). Concentra-tions of chlorophylls a and b were calculated from the absor-bance values at 647 and 664 nm by the equations of Ziegler and Egle (1965) cited by Šesták (1971).
Red/far-red ratio experiments
Stem extension under contrasting R/Fr ratios
Two-week-old, greenhouse-grown potted seedlings were placed in a growth cabinet for 1 week under white light. At the start of the experiment, seedling heights ranged from 28 to 49 mm. The seedlings were grouped in eight blocks of more or less uniform height and each group of eight seedlings was subjected to a high, intermediate or low R/Fr treatment (1.27 ± 0.02, 0.51 ± 0.04 and 0.09 ± 0.01, respectively) in a random-ized block design for 10 weeks in a growth cabinet.
Each seedling was provided an individual shade-cover and subjected to a high, intermediate or low R/Fr ratio obtained by covering the end of the shade-cover tube with Chromoid 209 ND, Chromoid 422 moss green or Chromoid 116 blue green acetate filters, respectively. Silver black scrim (Cinelux 270), neutral muslin and nylon fabrics were used where necessary, to obtain the same PPFD of 39 µmol m−2 s−1 in all of the R/Fr ratio treatments.
Growth room conditions included a 12-h photoperiod and a PPFD on the bench of 470 ± 18.5 µmol m−2 s−1 provided by 400-W metal halide lamps (Kolorarc MBIF/H, Thorn Light-ing, London, UK) supplemented with 60-W incandescent bulbs to enrich the far-red portion of the spectrum. Day/night temperature was 25/20 °C. Relative humidity was about 70%. The seedlings were fed with Ingestad’s solution to provide 30 mg dm−3 of N. About 50 ml of nutrient solution was applied once a day.
Eight seedlings were harvested at the beginning of the ex-periment and leaf area and dry weight measured. Seedling height was recorded weekly throughout the experiment. At the end of the 10-week treatment period, the seedlings were har-vested and stem length, leaf area and the dry weight of the different plant organs of each seedling measured.
Stem extension in response to added far-red One-week-old greenhouse-grown seedlings were transferred to a growth cabi-net and grown in 120 µmol m−2 s−1 of white light (WL) for 2 to 3 weeks before the experiment. The photoperiod was 12 h and day/night temperature was 25/20 °C. Relative humidity was 70%.
Six seedlings were grown in continuous white light (WL) as a control, and 12 seedlings were grown in a sequence of 24 h of white light, 24 h of supplementary far-red and 24 h of white light (WL1-FR-WL2). The supplementary far-red was supplied laterally to the terminal internode of each seedling from a fiber-optic light guide mounted with an interference filter (far-red maximum = 730 nm, 25 mm diameter, Glen Spectra, Middlesex, UK). Far-red radiation at about 35 µmol m−2 s−1 in a background WL of 120 µmol m−2 s−1 resulted in a drop in the R/Fr ratio from 1.75 to 0.12. The light source was a slide projector with a tungsten-halogen bulb, with light focused by a lens onto an interference filter.
Stem extension rates of the terminal internode were moni-tored with a linear voltage displacement transducer (LVDT, type DFG/5, ± 5.0 mm stroke, RS Components, Corby, UK). The LVDT had a sensitivity of 54.34 mV mm−1. The LVDT was mounted in an adjustable supporting stand, with the arma-ture running freely through the barrel. Plants and transducers were coupled by a balance and cotton thread tied around the first internode just below the primary leaf node. The thread was passed through a small pulley wheel. Two LVDTs were used allowing one seedling of each species to be monitored simul-taneously. The outputs from the LVDTs were logged with a Delta-T datalogger. The extension rates were sampled every 5 s and averaged over 5-min intervals. Anti-vibration pads were provided under the supporting stand.
Results
Shade and nutrient supply experiment
positive interaction between light and nutrient supply on RGRb in both species (Table 2).
An increase in irradiance resulted in an increase in NAR but a decrease in leaf area ratio (LAR), particularly in A. gracilior (Figure 1). Nutrient supply had no significant effect on NAR in either species (Table 2).
Differences in LAR were the result of differences in both specific leaf area (SLA) and leaf weight ratio (LWR) (Fig-ure 1). The SLA of J. procera responded to nutrients but not to light, whereas SLA of A. gracilior responded to light but not to nutrients (Table 2). Irradiance had only a slight effect on LWR, whereas nutrient supply significantly increased LWR in both species (Table 2, Figure 1).
The high-nutrient treatment significantly increased height growth in both species (Table 2). Light had no significant effect on height growth of J. procera seedlings, but there was a significant and progressive increase in RGRs of A. gracilior
seedlings with increasing irradiance in the high-nutrient treat-ment (Figure 2).
Both light and nutrient supply affected relative growth rates and allocation patterns of dry matter (Figure 2). Species showed similar patterns of response in specific stem length (SSL) to light availability, but contrasting SSL responses to nutrient supply (Table 2). Juniperus procera had much greater SSL in the low-nutrient and low-light treatments than in the high-light and high-nutrient treatments. In contrast, SSL in A. gracilior showed relatively little response to nutrients, al-though it was highest at low irradiances and high nutrient availability.
Irradiance had opposite effects on stem weight ratio (SWR) and root weight ratio (RWR) in J. procera seedlings, increas-ing light availability significantly decreased SWR and signifi-cantly increased RWR in both nutrient regimes (Table 2 and Figure 2). Only the deep-shade treatment significantly affected SWR in A. gracilior and nutrient supply had no significant effect on SWR. Irradiance had no significant effect on RWR in A. gracilior. In both species, RWR was significantly reduced in the high-nutrient treatment (Figure 2; Table 2).
Figure 1. Effects of PPFD and nutrient supply on relative growth rate (RGRb), net assimilation rate (NAR), leaf area ratio (LAR), specific
leaf area (SLA) and leaf weight ratio (LWR) of J. procera (left panel) and A. gracilior (right panel) seedlings grown in a high-nutrient supply (j) or low-nutrient supply (h) treatment for 20 weeks in a green-house. Means of 10 seedlings, except for the lowest PPFD treatment where mortality was widespread and dead seedlings were not included in the mean, vertical bars indicate the standard error of the mean.
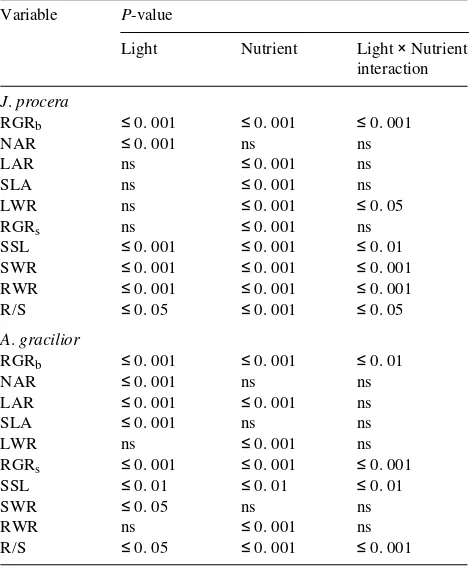
Table 2. Three-way ANOVA (residual degrees of freedom = 45) of the effects of PPFD and nutrient supply on the growth of J. procera and
A. gracilior seedlings. Seedlings in the deep-shade treatment were
excluded from the analysis. Because there was no significant effect of plant position in the greenhouse, the data are not shown, but they were included in the ANOVA. Abbreviations: RGRb = relative growth rate;
NAR = net assimilation rate; LAR = leaf area ratio; SLA = specific leaf area; LWR = leaf weight ratio; RGRs = relative growth rate of
stem; SSL = specific stem length; SWR = stem weight ratio; RWR = root weight ratio; and R/S = root length/stem length ratio.
Variable P-value
Light Nutrient Light × Nutrient interaction
J. procera
RGRb ≤ 0. 001 ≤ 0. 001 ≤ 0. 001
NAR ≤ 0. 001 ns ns
LAR ns ≤ 0. 001 ns
SLA ns ≤ 0. 001 ns
LWR ns ≤ 0. 001 ≤ 0. 05
RGRs ns ≤ 0. 001 ns
SSL ≤ 0. 001 ≤ 0. 001 ≤ 0. 01 SWR ≤ 0. 001 ≤ 0. 001 ≤ 0. 001 RWR ≤ 0. 001 ≤ 0. 001 ≤ 0. 001 R/S ≤ 0. 05 ≤ 0. 001 ≤ 0. 05
A. gracilior
RGRb ≤ 0. 001 ≤ 0. 001 ≤ 0. 01
NAR ≤ 0. 001 ns ns
LAR ≤ 0. 001 ≤ 0. 001 ns
SLA ≤ 0. 001 ns ns
LWR ns ≤ 0. 001 ns
RGRs ≤ 0. 001 ≤ 0. 001 ≤ 0. 001
SSL ≤ 0. 01 ≤ 0. 01 ≤ 0. 01
SWR ≤ 0. 05 ns ns
RWR ns ≤ 0. 001 ns
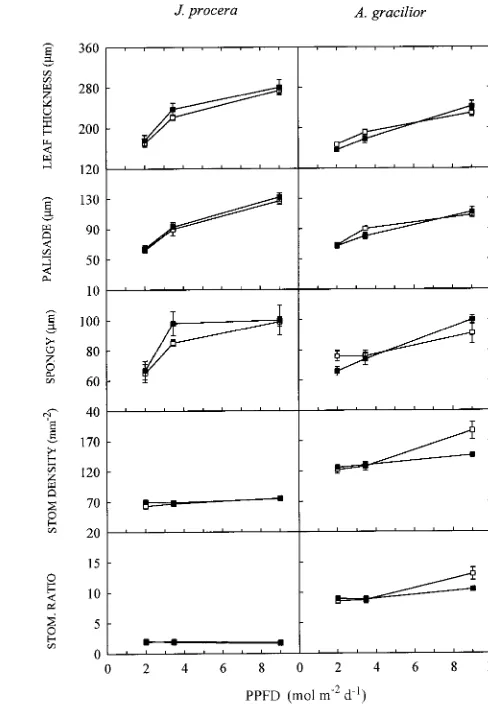
Leaf anatomy In general, there was a significant increase in leaf thickness with increasing irradiance in the study species (Figure 3), both palisade tissue and spongy mesophyll showing significant increases. The relative increases in thickness were proportionately greater in J. procera than in A. gracilior. In general, nutrient supply had no significant influence on leaf thickness.
The leaves of both species are amphistomatous but there were many more stomata on the lower leaf surface than on the upper leaf surface in A. gracilior seedlings, particularly in the low-nutrient plus high-light treatment (Figure 3). Overall, sto-matal density was not affected by nutrient treatments but it increased with increasing irradiance, and the increase was highly significant in A. gracilior and just significant in J. pro-cera (Figure 3).
Leaf chlorophyll content In both species, chlorophyll content on a leaf area basis was strongly and significantly (P ≤ 0.001) influenced by nutrient regime, whereas light availability only weakly (P ≤ 0.05) affected chlorophyll content in A. gracilior and the effect was not significant in J. procera (Figure 4). In both species, chlorophyll concentration increased significantly
(P ≤ 0.05) with increasing light availability and nutrient supply (Figure 4). There were no significant interactions between light availability and nutrient supply on chlorophyll content or con-centration in either species.
Leaf nitrogen content Leaf nitrogen concentration was not significantly affected by light availability in J. procera. In contrast, nitrogen concentration of A. gracilior seedlings was significantly reduced with increasing irradiance (Table 3). In both species, leaf nitrogen concentrations were significantly increased by nutrient supply. The relationship between NAR and nitrogen content per leaf area was weak in both species (Table 4). Moreover, nutrient addition had no influence on nitrogen content on a leaf area basis.
Figure 2. Effects of PPFD and nutrient supply on relative stem exten-sion rate (RGRs), specific stem length (SSL), stem weight ratio (SWR)
and root weight ratio (RWR) of J. procera (left panel) and A. gracilior
(right panel) seedlings grown in a high-nutrient supply (j) or low-nu-trient supply (h) treatment for 20 weeks in a greenhouse. Means of 10
seedlings; vertical bars indicate the standard error of the mean. Figure 3. Effects of PPFD and nutrient supply on leaf thickness, palisade thickness, spongy mesophyll thickness, stomatal density and upper/lower stomatal ratio of J. procera (left panel) and A. gracilior
R/Fr ratio experiments
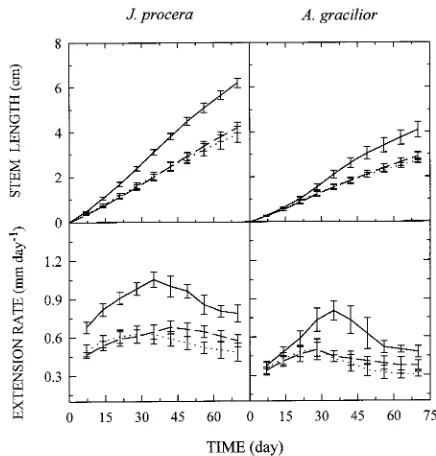
Seedling stem extension and the R/Fr ratio Stem extension growth increased with decreasing R/Fr ratio in both species (Figure 5). In J. procera, a low R/Fr ratio induced a signifi-cantly higher SWR and signifisignifi-cantly lower LWR than a high R/Fr ratio but no other response was detected (Table 5). In A. gracilior, there were additional responses to the low R/Fr ratio treatment including an increase in specific leaf area and a decrease in specific stem length.
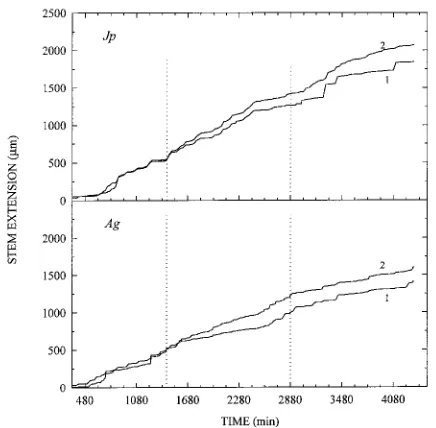
Seedling stem extension rate in response to supplementary far-red light Enriching white light with far-red light caused fluctuations in stem extension rates of both J. procera and A. gracilior seedlings grown in 120 µmol m−2 s−1 of equili-brated white light. On average, the application of FR resulted in a higher stem extension rate compared to the rates during exposure to the periods of white light, WL1 and WL2 (Table 6).
Figure 4. Effects of PPFD and nutrient supply on chlorophyll content and concentration of J. procera (left panel) and A. gracilior (right
panel) seedlings grown in a high-nutrient supply (j) or low-nutrient supply (h) treatment for 20 weeks in a greenhouse. Means of 10 seedlings; vertical bars indicate standard error of mean.
Table 3. Effects of irradiance (PPFD) and nutrient concentration on percent leaf nitrogen of J. procera and A. gracilior seedlings. Residual degrees of freedom = 45.
PPFD J. procera A. gracilior
(mol m−2 day−1) 1 mg l−1 30 mg l−1 1 mg l−1 30 mg l−1
2.0 0.85 b 2.20 a 1.33 b 2.44 a
3.5 1.00 b 2.18 a 0.95 c 2.15 a
9.0 0.83 b 2.18 a 0.68 d 2.06 a
Table 4. Relationship between net assimilation rate (NAR, g m−2 week−1, mean ± SE) and leaf nitrogen content (N, g m−2) of J. procera and
A. gracilior seedlings.
PPFD J. procera A. gracilior
(mol m−2 day−1) 1 mg l−1 30 mg l−1 1 mg l−1 30 mg l−1
N NAR N NAR N NAR N NAR
2.0 0.51 0.71 ± 0.03 0.50 0.61 ± 0.02 0.54 0.90 ± 0.03 0.92 0.81 ± 0.04
3.5 0.70 1.21 ± 0.04 1.1 1.14 ± 0.07 0.41 1.38 ± 0.09 1.1 1.25 ± 0.21
9.0 0.54 1.63 ± 0.12 1.2 1.68 ± 0.12 0.20 2.06 ± 0.13 1.5 2.10 ± 0.21
Figure 5. Effects of R/Fr ratio on stem length (upper panel) and stem extension rate (lower panel) of J. procera (left panel) and A. gracilior
The difference was significant in A. gracilior seedlings but not in J. procera seedlings (Table 6). Overall, stem extension was higher in J. procera than in A. gracilior (Figure 6).
Discussion
Effects of shade and nutrients
The experimental light conditions were designed to reflect those in the Ethiopian forest of Arba-gugu, which is a typical Afromontane coniferous forest. Irradiance of the low-light treatment was about 7% of that measured in a large clearing, and was comparable to the low light conditions of the under-story of Arba-gugu forest and the high-light treatment was equivalent to that observed at the forest margin (Table 1) (Sharew 1994). Irradiance of the deep-shade treatment (1% of the open PPFD treatment, 0.4 mol m−2 day−1) was lower than that measured in the forest understory and none of the seed-lings tolerated the deep-shade treatment. The low R/Fr ratios used here were lower than those in the forest; however, they were comparable to values from other tropical forests (Vázques-Yánes and Smith 1982).
The species behaved similarly in response to irradiance and nutrient supply. Both species showed negative growth rates at the lowest PPFD, and a rapid increase in RGRb over the range 0 to 4 mol m−2 day−1. The enhanced nutrient supply substan-tially increased relative growth rates especially at high irradi-ances, and the increase was brought about by an increase in leaf area ratio rather than by an increase in net assimilation rate. The high-nutrient treatment had no effect on nitrogen content on a leaf area basis and no effect on leaf thickness or stomatal density; however, it did change chlorophyll content Table 5. Morphogenetic adjustments in response to contrasting R/Fr ratios of J. procera and A. gracilior seedlings grown in a high, intermediate
or low (1.27, 0.51 and 0.09, respectively) R/Fr ratio at a PPFD of 39 µmol m−2 s−1 for 10 weeks in a growth cabinet. Mean ± SE of eight seedlings.
The ANOVA was conducted with 2 and 21 degrees of freedom. Duncan’s New Multiple Range Test; means followed by the same letter are not significantly different from each other at P = 0.05.
Variable Red/Far-red ratio ANOVA
High Intermediate Low F P
J. procera
SLA (cm2 mg−1) 0.123 ± 0.014 a 0.127 ± 0.016 a 0.134 ± 0.022 a 0.75 0.485 SSL (cm mg−1) 0.172 ± 0.024 a 0.189 ± 0.031 a 0.166 ± 0.032 a 1.21 0.318
SWR 0.201 ± 0.037 b 0.209 ± 0.020 ab 0.245 ± 0.035 a 3.85 0.038
LWR 0.541 ± 0.049 a 0.526 ± 0.027 ab 0.491 ± 0.017 b 4.06 0.032
A. gracilior
SLA (cm2 mg−1) 0.200 ± 0.005 b 0.205 ± 0.006 b 0.233 ± 0.016 a 4.05 0.033
SSL (cm mg−1) 0.208 ± 0.011 a 0.207 ± 0.004 a 0.178 ± 0.009 b 4.73 0.020
SWR 0.190 ± 0.006 b 0.209 ± 0.011 ab 0.267 ± 0.003 a 27.09 <0.001
LWR 0.594 ± 0.013 a 0.578 ± 0.014 b 0.511 ± 0.004 13.52 <0.001
Table 6. Stem extension rates (µm min−1) of J. procera and
A. gracilior seedings before FR (WL1), during FR and after FR (WL2) treatment. Means ± SE of 12 seedlings. Means followed by the same letter are not significantly different from each other at P = 0.05
(ANOVA with 2 and 23 degrees of freedom, and Duncan’s New Multiple Range Test).
Variable J. procera A. gracilior
Rate during WL1 phase 0.42 ± 0.06 a 0.35 ± 0.04 b Rate during FR phase 0.56 ± 0.04 a 0.49 ± 0.03 a Rate during WL2 phase 0.44 ± 0.05 a 0.26 ± 0.02 c
F statistics (P-value) 2.22 (0.125) 13.69 (<0.001)
Figure 6. Effects of supplementary illumination with far-red light on stem extension of J. procera (Jp) and A. gracilior (Ag) seedlings. Control seedlings (2) were grown in continuous white light (n = 6),
on a leaf area basis. Under high-light conditions, the photosyn-thetic apparatus of plants receiving a low nutrient supply is less efficient than that of plants receiving a high nutrient supply (Robson and Parsons 1978). Nutrient supply often increases leaf N status regardless of irradiance during growth, and pho-tosynthesis responds accordingly (Thompson et al. 1988).
A comparison of the treatment effects on the weight ratios of leaf, stem and root (LWR, SWR and RWR) indicated that carbon allocation was sensitive to both light and nutrient sup-ply. The increase in nutrient supply caused a shift in allocation to the shoot from the root. The stem fraction was only slightly changed by nutrient supply (in J. procera) or not changed at all (in A. gracilior). The form of the plant was notably dependent on light (e.g., the specific stem length of J. procera in the high-nutrient treatment was increased fourfold by high irradi-ance). This tendency to stem etiolation under high irradiance may be a characteristic of pioneer species, whose height in-creases with decreasing R/Fr ratio. The higher SSL in the high-light treatment in the pioneer J. procera compared with A. gracilior may be a result of the effect of light availability on biomass partitioning. In shade-tolerant species, photosynthate is allocated preferentially to leaf growth, and then to roots, storage and stem growth as carbon availability increases. In seedlings of the pioneer J. procera growing in high light, preferential allocation of biomass to stem growth would facili-tate increased height growth and competitive ability, whereas shade-tolerant species growing in shade would not benefit from height growth but may preferentially allocate photosyn-thate to leaves to increase the capture of light energy (Waring and Pitman 1985). We conclude that J. procera is more respon-sive to changes in environment than A. gracilior and channels more of its resources to roots at low irradiances, whereas it preferentially allocates biomass to stem growth at high irradi-ances (Figure 2). This conclusion is supported by other studies showing that changes in environmental conditions are met by allocation of growth resources to the organ that is capable of alleviating the limitation imposed by the new condition (Corré 1983b,1983c, Thompson et al. 1988).
Leaf characteristics and chlorophyll
Leaf thickness was reduced by growth at low PPFD, and the thinner leaves contained only a rudimentary palisade layer and loosely arranged mesophyll tissues. However, chlorophyll content was not influenced by light availability and so the total mesophyll volume contained a greater concentration of chlo-rophyll at low irradiances than at high irradiances, presumably enabling more effective light harvesting. Low maximum pho-tosynthetic rates in thin shade leaves have been observed for non-pioneer and pioneer forest tree seedlings of Flindersia brayleyana F. Muell. (Thompson et al. 1988) and Nauclea diderrichii (De Wild. and Th. Durand) Merr. (Riddoch et al. 1991), respectively.
The total chlorophyll content of the Afromontane coniferous tree species was similar to that reported by others (100--1000 mg m−2) (Šesták 1971, Eliáš and Masarovicová 1980, Bjork-man 1981, Morales et al. 1982, Kwesiga 1984, Kamaluddin 1991). Juniperus procera generally had a higher chlorophyll
content, whereas A. gracilior had a higher chlorophyll concen-tration. Boardman (1977) suggested that chlorophyll content is lower in shade species and shade leaves than in sun species and sun leaves, but this may not be true for coniferous species (Lewandowska and Jarvis 1977).
Response to R/Fr ratios
Because response to R/Fr is a means by which seedlings perceive shade and proximity of neighbors (Aphalo and Bal-laré 1995), we assessed the sensitivity of the species to a reduced R/Fr ratio while holding photon flux constant at a low value representative of forest shade. In general, seedlings of both J. procera and A. gracilior showed an increase in stem extension with decreasing R/Fr ratios (Figure 5). However, the increase was significant only at a R/Fr ratio of 0.09. A similar response was observed in short-term experiments which showed higher stem extension rates over a period of hours when the terminal internode was irradiated with added far-red (Figure 6 and Table 6).
The reduction in stem extension rates in both species after 5 weeks of growth at a low R/Fr ratio (Figure 5) may indicate a restriction brought about by lack of assimilates (cf. Child and Smith 1987). Sun-adapted plants respond to shade by extreme elongation of the internodes, until the photosynthetic struc-tures are carried into the open, or until stored food reserves are used up (cf. Smith 1986).
Increased SWR and decreased LWR in response to reduced R/Fr ratio (Table 5) presumably reflect a shift in biomass allocation from leaf to stem associated with increased stem extension (Corré 1983a, Smith 1986). We conclude, therefore, that increased stem extension in J. procera at low R/Fr ratio was more the result of a change in dry matter allocation from leaf to stem than of a reduction in stem thickness. The finding that increasing irradiance had no effect on LWR and decreased SWR, whereas a reduced R/Fr ratio increased SWR and de-creased LWR indicates that there are important differences between the effects of light quality and light quantity. Because SSL mainly depends on the amount of energy fixed by the plants, SSL depends more on the total absorption of photons than on the spectral distribution (Smith 1981).
We conclude that differences in the response of seedlings to light and nutrient availability are detectable but small, and insufficient to justify the characterization of J. procera and A. gracilior as light-demanding and shade-tolerant species, respectively. It seems more likely that their differing distribu-tions reflect differences in the condidistribu-tions they require for seed germination or seedling establishment.
Acknowledgments
References
Aphalo, P.J. and C.L. Ballaré. 1995. On the importance of informa-tion-acquiring systems in plant-plant interactions. Funct. Ecol. 9:5--14.
Ballaré, C.L., A.L. Scopel and R.A. Sánchez. 1990. Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247:329--332.
Björkman, O. 1981. Responses to different quantum flux densities. In
Physiological Plant Ecology. Eds. O.I. Lange, P.S. Nobel, C.B. Osmond and H. Ziegler. Encyclopaedia of Plant Physiology, New Series, Vol. 12A, Springer-Verlag, pp 57--107.
Boardman, N.K. 1977. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 28:355--377.
Chaffey, D.R. 1979. South-west Ethiopia forest inventory project: A reconnaissance inventory of forest in south-west Ethiopia. Project Report No. 31. Land Resources Development Centre, Tolworth Tower, Surbiton, Surrey, England, 312 p.
Child, R. and H. Smith. 1987. Phytochrome action in light-grown mustard: kinetics, fluence-rate compensation and ecological sig-nificance. Planta 172:219--229.
Corré, W.J. 1983a. Growth and morphogenesis of sun and shade plants
I. The influence of light intensity. Acta Bot. Neerl. 32:49--62. Corré, W.J. 1983b. Growth and morphogenesis of sun and shade plants
II. The influence of light quality. Acta Bot. Neerl. 32:185--202. Corré, W.J. 1983c. Growth and morphogenesis of sun and shade plants
III. The combined effects of light intensity and nutrient supply. Acta Bot. Neerl. 32:277--294.
Dale, I.R. and P.J.J. Greenway. 1961. Kenya trees and shrubs. Bucha-nan’s Kenya Estates, Nairobi, 654 p.
Evans, J.R. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9--19.
Eliáš, P. and E. Masarovicová. 1980. Chlorophyll content in leaves of plants in an oak--hornbeam forest. Photosynthetica 14:604--610. Farjon, A. 1992. The taxonomy of multiseed junipers (Juniperus Sect.
Sabina) in Southwest Asia and East Africa (taxonomic notes on Cupressaceae I). Edinburgh J. Bot. 49:251--283.
Fetcher, N., B.R. Strain and S.F. Oberbauer. 1983. Effects of light regime on growth, leaf morphology, and water relations of seed-lings of two species of tropical trees. Oecologia 58:314--319. Field, C. and H.A. Mooney. 1986. The photosynthesis--nitrogen
rela-tionships in wild plants. In On the Economy of Plant Form and
Function. Ed. T.J. Givnish. Cambridge University Press, pp 25--55. Gardner, H.M. 1926. East African pencil cedar. Imp. For. J. 5:39--53. Gaussen, H. 1974. Les gymnospermes actuelles et fossiles
Podocar-paces. Trav. Lab. For. Toulouse Tome 2, Vol. 1. fasc. 13.
Grubb, P.J. 1977. Control of forest growth and distribution on wet tropical mountains. Annu. Rev. Ecol. Syst. 8:83--107.
Hall, J.B. 1984. Juniperus excelsa in Africa: a biogeographical study of an Afromontane tree.J. Biogeogr. 11:47--61.
Ingestad, T. 1979. Mineral nutrient requirements of Pinus sylvestris
and Picea abies seedlings. Physiol. Plant. 45: 373--380.
Kamaluddin, M. 1991. The growth and physiology of tropical forest tree seedlings in relation to light. Ph.D. Diss., Univ. Edinburgh, U.K., 142 p.
Kamaluddin, M. and J. Grace. 1993. Growth and photosynthesis of tropical forest tree seedlings (Bischofia javanica Blume) as influ-enced by a change in light availability. Tree Physiol. 13:189--201. Kerfoot, O. 1966. Distribution of the Coniferae: the Cupressaceae in
Africa. Nature 212:961.
Kigomo, B.N. 1985. Growth characteristics of natural regenerates of African cedar (Juniperus procera). East African Agric. For. J.
50:54--60.
Kwesiga, F.R. 1984. Aspects of the growth and physiology of tropical tree seedlings in shade. Ph.D. Diss., Univ. Edinburgh, U.K., 248 p. Kwesiga, F.R. and J. Grace. 1986. The role of the red/far-red ratio in the response of tropical tree seedlings to shade. Ann. Bot. 57:283--290.
Lee, D.W. 1988. Simulating forest shade to study the developmental ecology of tropical plants: juvenile growth in three vines in India. J. Tropic. Ecol. 4:281--292.
Lewandowska, M. and P.G. Jarvis. 1977. Changes in chlorophyll and carotenoid content, specific leaf area and dry weight fraction in Sitka spruce, in relation to shading and season. New Phytol. 79:247--256.
Melville, R. 1954. The podocarps of East Africa. Kew Bulletin 4:563--578.
Morales, D., M.S. Jimenez, J. Iriarte and F. Gil. 1982. Altitudinal effects on chlorophyll and carotenoid concentrations in gymno-sperms leaves. Photosynthetica 16:362--372.
Morgan, D.C., D.A. Rook, I.J. Warrington and H.L. Turnbull. 1983. Growth and development of Pinus radiata D. Don: the effect of light quality. Plant Cell Environ. 6:691--701.
Morgan, D.C. and H. Smith. 1981. Non-photosynthetic responses to light quality. In Physiological Plant Ecology. Eds. O.I. Lange, P.S. Nobel, C.B.Osmond and H. Ziegler. Encyclopaedia of Plant Physi-ology, New Series., Vol. 12A, Springer-Verlag, Berlin, pp 108--134. Popma, J. and F. Bongers. 1988. The effect of canopy gaps on growth and morphology of seedlings of rain forest species. Oecologia 75:625--632.
Porra, R.J., W.A. Thompson and P.E. Kriedemann. 1989. Determina-tion of accurate extincDetermina-tion coefficients and simultaneous equaDetermina-tions for assaying chlorophylls a and b extracted with four different
solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975:384--394.
Riddoch, I., T. Lehto and J Grace. 1991. Photosynthesis of tropical tree seedlings in relation to light and nutrient supply. New Phytol. 119:137--147.
Robson, M.J. and A.J. Parsons. 1978. Nitrogen deficiency in small closed communities of S24 ryegrass. I. Photosynthesis, respiration, dry matter production and partition. Ann. Bot. 42:1185--1197. Schmitt, J. and R.D. Wulff. 1993. Light spectral quality, phytochrome
and plant competition. Trends Ecol. Evol. 8:46--51.
Šesták, Z. 1971. Determination of chlorophylls a and b. In Plant
Photosynthetic Production: Manual of Methods. Eds. Z. Šesták, J. Èatsky and P.G. Jarvis. Junk, The Hague, pp 672--701.
Sharew, H. 1994. Regeneration of Juniperus procera and Afrocarpus gracilior in the Afromontane forests of Ethiopia. Ph.D. Diss., Univ. Edinburgh, U.K., 287 p.
Smith, H. 1981. Light quality as an ecological factor. In Plants and
their Atmospheric Environment. 21st Symposium of the British Ecological Society. Eds. J. Grace, E.D. Ford and P.G. Jarvis. Black-well Scientific, Oxford, pp 93--110.
Smith, H. 1986. The perception of light quality. In Photomorphogene-sis in Plants. Eds. R.E. Kendrick and G.H.M. Kronenberg. Njhoff, Dordrecht, pp 187--216.
Steel, R.G.D. and J.H. Torrie. 1981. Principles and procedures of statistics, 2nd. Edn. McGraw-Hill, New York, 633 p.
Thompson, W.A., G.C. Stocker and P.E Kriedemann. 1988. Growth and photosynthetic response to light and nutrients of Flindersia brayleyana F. Muell., a rainforest tree with broad tolerance to sun and shade. Aust. J. Plant Physiol. 15:299--315.
Tinoco-Ojanguren, C. and R.W. Pearcy. 1995. A comparison of light quality and quantity effects on the growth and steady state photo-synthetic characteristics of three tropical tree species. Funct. Ecol. 9: 222--230.
Vázquez-Yánes, C. and H. Smith. 1982. Phytochrome control of seed germination in the tropical rain forest pioneer trees Cecropia ob-tusifolia and Piper auritum and its ecological significance. New Phytol. 92:477--485.
Waring, R.H. and G.B Pitman. 1985. Modifying lodgepole pine stands to change susceptibility to mountain pine beetle attack. Ecology 66:889--897.
Warrington, I.J., D.A. Rook, D.C. Morgan and H.L. Turnbull. 1989. The influence of simulated shadelight and daylight on growth, development and photosynthesis of Pinus radiata, Agathis australis