Summary Dryobalanops aromatica Gaertn. f. is a major tropical canopy species in lowland tropical rain forests in Peninsular Malaysia. Diurnal changes in net photosynthetic rate (A) and stomatal conductance to water vapor (gs) were measured in fully expanded young and old leaves in the upper-most canopy (35 m above ground). Maximum A was 12 and 10
µmol m−2 s−1 in young and old leaves, respectively; however, because of large variation in A among leaves, mean maximum A in young and old leaves was only 6.6 and 5.5 µmol m−2 s−1, respectively. Both gs and A declined in young leaves when
Tleaf exceeded 34 °C and leaf-to-air vapor pressure deficit (∆W)
exceeded 0.025, whereas in old leaves, gs and A did not start to decline until Tleaf and ∆W exceeded 36 °C and 0.035,
respec-tively. Under saturating light conditions, A was linearly related to gs. The coefficient of variation (CV) for the difference between the CO2 concentrations of ambient air and the leaf intercellular air space (Ca−Ci) was smaller than the CV for A or gs, suggesting that maximum gs was mainly controlled by mesophyll assimilation (A/Ci). Minimum Ci/Ca ratios were relatively high (0.72--0.73), indicating a small drought-induced stomatal limitation to A and non-conservative water use in the uppermost canopy leaves.
Keywords: drought, irradiance, stomatal conductance, photo-synthesis, tropical rain forest, uppermost canopy, water deficit.
Introduction
Tropical rain forest trees are generally shallow rooted (Doley 1981) and are easily damaged by relatively small water deficits (Grubb 1977, Buckley et al. 1980). Because the regulation of stomatal conductance is an important trait in drought avoid-ance, several studies have examined stomatal responses in rain forest tree species. In some tropical species, stomatal conduc-tance remains high throughout the midday period (Grace et. al. 1982, Aylett 1985, Oberbauer et al. 1987), whereas in other tropical species, stomatal conductance shows a midday depres-sion when the leaves are exposed to a high leaf-to-air water
vapor deficit (∆W) (Aylett 1985, Roberts et al. 1990, Koch et al. 1994).
Zotz and Winter (1993) found that maximum net photosyn-thetic rate (A) was linearly related to diurnal CO2 uptake among canopy plants in a tropical rain forest in Panama. However, several published works have reported substantial variation in A among individual leaves in a canopy. These variations are caused by either differences in light intensity at the leaf surface (Doley et al. 1988), or leaf-age-related changes in intrinsic biochemical capacity and stomatal limitation to CO2 fixation (Reich and Borchert 1982, Witkowski et al. 1992, Sobrado 1994). To obtain a more detailed understanding of how environmental variables limit primary production at the single leaf level, we examined diurnal changes in microclimate and leaf gas exchange characteristics in uppermost canopy leaves of a tropical canopy tree, Dryobalanops aromatica Gaertn. f. (Dipterocarpaceae) during the rainy season.
Materials and methods Plant species
Dryobalanops aromatica is one of the canopy tree species in lowland (elevation 70--400 m) dipterocarp forests in southeast Asia and sometimes forms mono-specific dominant (mono-dominant) forests in its natural state (Foxworthy 1932, Vincent 1961, Kachi et al. 1993). Some trees exceed 60 m in height (Symington 1974).
Study site and access to the uppermost canopy
The study site was a forest stand at the Forest Research Insti-tute of Malaysia (FRIM) in Kuala Lumpur (3°13′ N, 101°37′ E). Mean annual air temperature is about 26 °C and annual precipitation is about 2400 mm at Kuala Lumpur (Taka-hashi and Arakawa 1981). There are two rainy seasons, March to April and October to December, caused by seasonal mon-soons. However, because the mean monthly precipitation in the rainy and other seasons is about 260 and 150 mm, respec-tively, there is no well-defined dry season.
Diurnal changes in leaf gas exchange characteristics in the uppermost
canopy of a rain forest tree,
Dryobalanops aromatica
Gaertn. f.
ATSUSHI ISHIDA,
1TAKESHI TOMA,
1YOOSUKE MATSUMOTO,
1SON KHEONG YAP
2and
YUTAKA MARUYAMA
31 Forest Environment Division, Forestry and Forest Products Research Institute, P.O. Box 16, Tsukuba Norin Danchi, Ibaraki 305, Japan
2
Forest Research Institute of Malaysia, Kepong 52109, Kuala Lumpur, Malaysia
3 Japan International Research Center for Agricultural Science, Tsukuba, Ibaraki 305, Japan
Received October 13, 1995
In 1994, the forest stand consisted mainly of 60-year-old D. aromatica (Appanah and Weinland 1993, Kachi et al. 1993) and the average height of the uppermost canopy in the stand was about 35 m. Although leaf litter was thin, there was little bare soil on the forest floor. Soil was of laterite origin.
To measure micrometeorogical parameters just above the closed forest canopy, a 40-m-tall scaffolding tower (UP-RIGHT Co., Republic of Ireland) was installed. Gas exchange measurements were made on foliage accessible from the tower at a height of 35 m.
Chlorophyll content
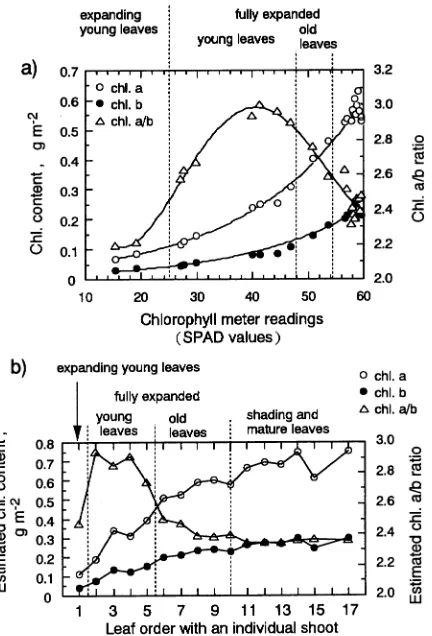
Leaf color, leaf chlorophyll content and leaf order were used to determine a relative leaf age scale (Sobrado and Medina 1980, Sobrado 1994). Leaf chlorophyll content was measured at 650 and 940 nm with a chlorophyll meter (Model SPAD-502, Minolta Co. Ltd., Tokyo, Japan). Twenty-two leaves were sampled to determine the relationships between leaf chloro-phyll content and the chlorochloro-phyll meter readings (SPAD val-ues). After a SPAD value was determined for a leaf, chlorophyll was extracted with 80% (v/v) acetone and the amounts of chlorophyll a and b were determined by the method of Arnon (1949).
Diurnal changes in microclimate and leaf gas exchange characteristics
Measurements were made on October 20 and 21 and Novem-ber 11, 1994. Photosynthetically active photon flux density (PPFD, µmol m−2 s−1) at the leaf surface was determined with a quantum sensor attached to the leaf chamber (PLC-4, Ana-lytical Development Company (ADC), Hoddeson, UK). An additional quantum sensor (IKS-25, Koito-Kogyo Co., Tokyo, Japan), set at the top of the tower, was used on November 11, 1994. Relative humidity of ambient air (RH, %) was deter-mined from wet and dry bulb temperatures on October 20 and 21, 1994, and with a thin-film capacitance sensor (CHS-APS XD3, TDK Co., Tokyo, Japan) on November 11, 1994. Ambi-ent air temperature (Tair, °C) was measured with a platinum
resistance thermometer probe (Pt 100 Ohm, Model KDC-S3, KONA system Co., Tokyo, Japan). An aluminum roof was installed above the humidity and air temperature sensors to prevent exposure to direct solar radiation. Leaf temperature (Tleaf, °C) was measured with fine-wire copper constantan
thermocouples (diameter 0.1 mm, Hayashi-Denko Co., Tokyo, Japan) attached to the abaxial leaf surface by adhesive tape.
Gas exchange rates were measured in fully expanded old leaves on October 20 and 21, 1994, and in just fully expanded young leaves on November 11, 1994. Net photosynthetic rate (A, µmol m−2 s−1) and stomatal conductance to water vapor (gs, mol m−2 s−1) per unit leaf area were measured with a portable H2O/CO2 analyzer (LCA-4, ADC, Hoddeson, UK) equipped with a portable leaf chamber (PLC-4 ADC, Hoddeson, UK) in an open system. There are technical difficulties associated with measuring concentrations of CO2 based on infrared absorption properties; however, the error in our estimates of CO2 concen-tration was small because the differences between ambient air RH and RH reference readings of the LCA-4 were small. The
error was further minimized by use of the computerized cor-rection routine supplied with the LCA-4 analyzer.
Leaf gas exchange characteristics were measured three to four times an hour. Dew on the leaf surface was dried with paper in the early morning. Six or seven fully expanded, sun-exposed leaves near the tips of twigs were randomly se-lected for each measurement. Because the longevity of canopy leaves was about 1 year, measured leaves were younger than 1 year old. The leaves of D. aromatica are hard and repeated clipping of a leaf by the leaf chamber often caused the leaf to split into two laminae from the midrib. To ensure that leaves did not split during measurement, we used a new leaf for each measurement. The total number of leaves measured was about 200 for both young and old leaves.
To examine whether variation in A among leaves was a result of differences in gs or in assimilation capacity, the coefficients of variation (CVs) for A, gs and (Ca − Ci) (the difference between ambient air and leaf intercellular space CO2 concen-trations) were compared.
Transpiration rate per unit leaf area (E, mmol m−2 s−1) was calculated based on leaf-to-air vapor pressure deficit (∆W), stomatal conductance to water vapor (gs, mol m−2 s−1) and boundary layer conductance (gb, mol m−2 s−1), as follows:
E=∆W(gsgb/(gs+gb))103, (1)
where ∆W was calculated using the Goff-Gratch formulation for saturated water vapor pressure. Boundary layer conduc-tance was determined as follows (Jones 1992, Pearcy et al. 1989):
The calibrated relationships between chlorophyll content per unit leaf area and chlorophyll a/b ratio and the SPAD values are shown in Figure 1a. Chlorophyll content and chlorophyll a/b ratio differed with leaf order in a single shoot (Figure 1b). The leaves selected for gas exchange measurements were grouped as young or old leaves, based on leaf chlorophyll content and leaf order. The young leaves were light green and had a low chlorophyll content and a high chlorophyll a/b ratio (> 2.7). The old leaves were green and had a high chlorophyll content and a low chlorophyll a/b ratio (< 2.7). The SPAD values were about 40 and 50 in the young and old leaves, respectively.
Microenvironments
air, maximum PPFDs on October 20 and 21, 1994 were rela-tively low----about 1000 µmol m−2 s−1 (Figure 2), whereas on November 11, 1994 when there was little dry haze, maximum PPFD reached 2000 µmol m−2 s−1. On November 11, diurnal PPFD and Tleaf fluctuated widely because of frequent cloud
cover. Direct solar radiation caused Tleaf to increase 6--8 °C
above Tair and ∆W to increase to 0.04. Diurnal Tair seldom
exceeded 32 °C and RH decreased to 65--50% during the daytime.
Diurnal changes in gas exchange
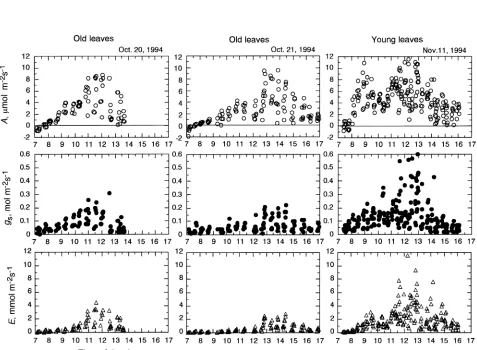
When uppermost canopy leaves were exposed to high solar radiation, A, gs and E were especially variable among leaves (Figure 3). In young and old leaves, maximum A was 12 and 10 µmol m−2 s−1, respectively, and the corresponding values of maximum gs were 0.6 and 0.3 mol m−2 s−1. However, mean maximum A was only 6.6 and 5.5 µmol m−2 s−1 in young and old leaves, respectively, and mean maximum gs was 0.25 and 0.14 mol m−2 s−1 in young and old leaves, respectively (Fig-ure 4). During the day, the CO2 concentration of ambient air
(Ca) at the uppermost canopy level (35 m height) decreased from about 380 to 310 µmol mol−1, mean CO2 concentration of the leaf intercellular space (Ci) decreased to 220 µmol mol−1 and mean Ci/Ca ratio decreased to 0.72--0.73. Mean water use efficiency (WUE, A/E) decreased to 1.7 and 2.3
µmol mmol−1 in young and old leaves, respectively, during the day. (under near light-saturating conditions), we observed positive relationships between A and gs (Figure 6a) in both young and old leaves with low gs (< 0.3 mol m−2 s−1); however, some and 0.286, respectively. When PPFD at the leaf surface was less than 500 µmol m−2 s−1 (Figure 6b), A declined with increasing Ci because low PPFD resulted in reduced assimila-tion capacity.
Discussion
Diurnal changes in leaf gas exchange characteristics
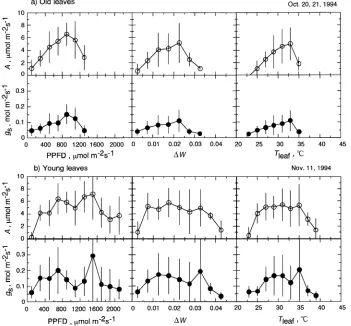
Stomatal conductance (gs) is sensitive to environmental factors such as light, CO2 concentration and ∆W (e.g., Körner 1994, Lee and Bowling 1995). In the morning (before 1100 h), both gs and A increased and Ci decreased as PPFD increased (Fig-ure 4). Because ∆W was low (< 0.025), gs and A were mainly controlled by PPFD in the morning.
In young leaves, both gs and A declined when ∆W exceeded 0.025 and Tleaf exceeded 34 °C, whereas gs and A of old leaves
did not decline until ∆W and Tleaf exceeded 0.035 and 36 °C,
respectively (Figure 5). Similar trends have been observed in other lowland rain forest trees such as Dialium pachyphyllum Harms (Caesalpinaceae) in southwest Cameroon, in which gs decreases in the dry season when ∆W and Tleaf are above 0.025
and 36 °C, respectively (Koch et al. 1994). After 1400 h on November 11, A was limited by reduced Ci as a result of low gs (Figure 4). The reduction in gs was the result of a high ∆W (above 0.025--0.035). Such severe ∆W accounted for only 5--15% of all values recorded during the daytime on the three measurement days.
Variation in gas exchange characteristics among canopy leaves
Maximum A in the uppermost canopy leaves reached 10--12
µmol m−2 s−1 (Figure 3). Maximum A in primary tropical forest tree species ranges from 4 to 15 µmol m−2 s−1, whereas maximum A in fast-growing secondary tropical trees ranges from 13 to 16 µmol m−2 s−1 (Koyama 1981, Aylett 1985). Thus, maximum A in D. aromatica was relatively high compared Figure 1. (a) Calibrated relationships between chlorophyll a content
Figure 2. Diurnal changes in photo-synthetic photon flux density (PPFD), ambient air temperature (Tair, solid line), relative humidity
(RH, broken line), Tleaf−Tair, and
leaf-to-air vapor pressure deficit (∆W). Measurements were made just above the canopy, at 35 m.
Figure 4. Diurnal changes in mean net photosynthetic rate (A, s), mean stomatal conductance to water vapor (gs, d), mean ambient air CO2 concentration (Ca, n), mean leaf intercellular CO2 concentration (Ci, m), mean Ci/Ca ratio (u), and water use efficiency (WUE, j) among the uppermost canopy leaves of Dryobalanops aromatica. Measurements were made on old leaves on October 20 and 21, 1994 and on young leaves on November 11, 1994.
Figure 5. Relationships of mean net photosynthetic rate (A, s) and mean stomatal con-ductance to water vapor (gs, d) to photosynthetic photon flux density (PPFD), leaf-to-air va-por vava-por pressure deficit (∆W), and leaf temperature (Tleaf) in the uppermost canopy
with maximum A values for other primary tropical tree species. However, because of large variation in A among leaves, the mean value of A was only about 5--7 µmol m−2 s−1 (Figure 4). Large variation in A has also been found in the uppermost canopy leaves of the rain forest tree, Dialium pachyphyllum Harms (Koch et al. 1994).
The smaller CV for (Ca−Ci) compared with the CVs for A and gs indicates that Ci is conservative. This finding supports the hypothesis of Wong et al. (1979) that mesophyll assimila-tion (A/Ci) controls maximum gs in each leaf by maintaining a constant Ci in full sunlight. The large variation in A at a given Ci (Figure 6b) suggests that variation in A was caused by variation in the mesophyll assimilation capacity of the canopy leaves rather than by variation in gs.
The Ci/Ca ratio is maintained at a constant or near-constant value in many plant species (e.g., Ehleringer and Cerling 1995), although it varies within leaves of various terrestrial biome types (Lloyd and Farqhar 1994). For example, the Ci/Ca ratio of Citrus and Eucalyptus species (Australian evergreen trees) is about 0.6--0.7 (Lloyd et al. 1992, Sheriff 1992), and is about 0.6 in Arbutus unedo L. (a Mediterranian evergreen sclerophyll shrub) (Beyschlag et al. 1987). The minimum Ci/Ca ratio in uppermost canopy leaves of D. aromatica was 0.72--0.73 (Figure 4), which is larger than that of both xero-phytic and dry tropical tree species. The Ci/Ca ratios estimated from carbon isotope discriminations are also relatively high in tropical rain forest species (Lloyd and Farqhar 1994), suggest-ing a relatively small stomatal limitation to A and non-conser-vative water use.
Acknowledgments
We thank Prof. Y. Morikawa, Dr. A. Furukawa, Dr. N. Kachi, and Dr. K. Hikosaka for their valuable suggestions and comments on an earlier
draft of the paper. The present study is part of a joint research project among Forest Research Institute of Malaysia (FRIM), Universiti Per-tanian Malaysia (UPM), National Institute for Environmental Studies of Japan (NIES), Japan International Research Center for Agricultural Science (JIRCAS), and Forestry and Forest Products Research Insti-tute (FFPRI) (Global Environment Research Program granted by Japan Environment Agency, Grant No. E-2 (2)).
References
Appanah, S. and G. Weinland. 1993. Planting quality timber trees in Peninsular Malaysia----a review. Malayan Forest Record No. 38, Forest Research Institute of Malaysia (FRIM), Kuala Lumpur, 221 p.
Arnon, D.I. 1949. Copper enzymes in isolated chloroplast. Polyphe-noloxidase in Beta vulgaris. Plant Physiol. 24:1--15.
Aylett, G.P. 1985. Irradiance interception, leaf conductance and pho-tosynthesis in Jamaican upper mountain rain forest trees. Photosyn-thetica 19:323--337.
Beyschlag, W., O.L. Lange and J.D. Tenhunen. 1987. Diurnal patterns of leaf internal CO2 partial pressure of the sclerophyll shrub Arbu-tus unedo growing in Portugal. In Plant Response to Stress. Func-tional Analysis in Mediterranean Ecosystems. Eds. J.D. Tenhunen, F.M. Catarino, O.L. Lange and W.C. Oechel. Springer-Verlag, Ber-lin, pp 355--368.
Buckley, R.C., R.T. Corlett and P.J. Grubb. 1980. Are the xeromorphic trees of tropical upper montane rain forests drought resistant? Biotropica 12:124--136.
Doley, D. 1981. Tropical and subtropical forests and woodlands. In Water Deficits and Plant Growth, Vol. VI. Ed. T.T. Kozlowski. Academic Press, New York, pp 209--323.
Doley, D., G.L. Unwin and D.J. Yates. 1988. Spatial and temporal distribution of photosynthesis and transpiration by single leaves in a rainforest tree, Argyrodendron peralatum. Aust. J. Plant Physiol. 15:317--326.
Ehleringer, J.R. and T.E. Cerling. 1995. Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiol. 15:105--111.
Foxworthy, F.W. 1932. Dipterocarpaceae of the Malay Peninsula. Malayan Forest Record No. 10, Printers Ltd, Singapore, 289 p. Grace, J., D.U.U. Okali and F.E. Fasehun. 1982. Stomatal conductance
of two tropical trees during the wet season in Nigeria. J. Appl. Ecol. 19:659--670.
Grubb, P.J. 1977. Control of forest growth and distribution on wet tropical mountains: with special reference to mineral nutrition. Annu. Rev. Ecol. Syst. 8:83--107.
Jones, H.G. 1992. Plants and microclimate. 2nd Edn. Cambridge University Press, Cambridge, 428 p.
Kachi, N., T. Okuda and S.K. Yap. 1993. Seedling establishment of a canopy tree species in Malayan tropical rain forests. Plant Species Biol. 8:167--174.
Koch, G.W., S.A. Jeffrey and M.L. Goulden. 1994. Diurnal patterns of leaf photosynthesis, conductance and water potential at the top of a lowland rain forest canopy in Cameroon: measurements from the Radeau des Cimes. Tree Physiol. 14:347--360.
Körner, Ch. 1994. Leaf diffusive conductances in the major vegetation types of the globe. In Ecophysiology of Photosynthesis. Ecological Studies, Vol. 100. Eds. E.-D. Schulze and M.M. Caldwell. Springer-Verlag, Berlin, pp 463--490.
Koyama, H. 1981. Photosynthetic rates in lowland rain forest trees of Peninsular Malaysia. Jpn. J. Ecol. 31:361--369.
Lee, J.S. and D.J.F. Bowling. 1995. Viewpoint: influence of the meso-phyll on stomatal opening. Aust. J. Plant Physiol. 22:357--363. Lloyd, J. and G.D. Farquhar. 1994. 13C Discrimination during CO2
assimilation by the terrestrial biosphere. Oecologia 99:201--215. Lloyd, J., J.P. Syvertsen, P.E. Kriedemann and G.D. Farquhar. 1992.
Low conductances for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant Cell Environ. 15:873--899.
Oberbauer, S.F., B.R. Strain and G.H. Riechers. 1987. Field water relations of a wet-tropical forest tree species, Pentaclethra macroloba (Mimosaceae). Oecologia 71:369--374.
Pearcy, R.W., E.-D. Schulze and R. Zimmermann. 1989. Measurement of transpiration and leaf conductance. In Plant Physiological Ecol-ogy: Field Methods and Instrumentation. Eds. R.W. Pearcy, J. Ehleringer, H.A. Mooney and P.W. Rundel. Chapman and Hall, New York, pp 137--160.
Reich, P.B. and R. Borchert. 1982. Phenology and ecophysiology of the tropical tree, Tabebuia neochrysantha (Bignoniaceae). Ecology 63:294--299.
Roberts, J., O.M.R. Cabral and L.F. de Aguiar. 1990. Stomatal and boundary-layer conductances in an Amazonian terra firme rain forest. J. Appl. Ecol. 27:336--353.
Sheriff, D.W. 1992. Roles of carbon gain and allocation in growth at different nitrogen nutrition in Eucalyptus camaldulesis and Euca-lyptus globulus seedlings. Aust. J. Plant Physiol. 19:637--652. Sobrado, M.A. 1994. Leaf age effects on photosynthetic rate,
transpi-ration rate and nitrogen content in a tropical dry forest. Physiol. Plant. 90:210--215.
Sobrado, M.A. and Medina, E. 1980. General morphology, anatomical structure, and nutrient content of sclerophyllous leaves of the ‘‘Bana’’ vegetation of Amazonas. Oecologia 45:341--345. Symington, C.F. 1974. Malayan forest records. No. 16 Foresters’
manual of dipterocarps. Penerbit Universiti Malaya, Kuala Lumpur, pp 194--196.
Takahashi, K. and H. Arakawa. 1981. Climates of Southern and West-ern Asia. World Survey of Climatology, Vol. 9. Elsevier Scientific Publishing Co., New York, 333 p.
Vincent, A.J. 1961. A survey of the Kapur (Dryobalanops aromatica Gaertn. f.): Silvicultural treatment research plots in naturally and artificially regenerated forest, Malaya. Forest Research Institute Research Pamphlet, No. 36. Kepon, Kuala Lumpur, 96 p. Witkowski, E.T.F., B.B. Lamont, C.S. Walton and S. Radford. 1992.
Leaf demography, sclerophylly and ecophysiology of two Banksias with contrasting leaf life spans. Aust. J. Bot. 40:849--862. Wong, S.C., I.R. Cowan and G.D. Farquhar. 1979. Stomatal