www.elsevier.nlrlocateraqua-online
Variations in lipid classes and fatty acid content in
tissues of wild Macrobrachium rosenbergii
ž
de Man females during maturation
/
Ronaldo O. Cavalli
a,), Montakan Tamtin
b, Patrick Lavens
a,
Patrick Sorgeloos
aa
Laboratory of Aquaculture and Artemia Reference Center, Ghent UniÕersity, Rozier 44,
9000 Ghent, Belgium
b
Petchaburi Coastal Aquaculture Station, Haad Chao Samran, Muang, 76100 Petchaburi, Thailand
Received 18 April 2000; received in revised form 9 August 2000; accepted 9 August 2000
Abstract
This study describes the variations in total lipid, lipid classes and fatty acids in the midgut
Ž .
gland MG , ovary, and muscle tissue of wild-caught Macrobrachium rosenbergii to elucidate the importance of these components during sexual maturation. Mature females were captured in the Mae Klong River, Thailand, from July to September 1998, and divided into five groups according to their ovarian development. Total lipid levels in the ovary increased with maturation, but no concomitant decrease in MG lipids was observed. Thus, the lipid requirements of the developing ovary are thought to be more dependent on the immediate ingestion of dietary lipid than on MG
Ž . Ž .
reserves. Higher proportions of neutral lipids NL , mainly triacylglycerols TG , were observed in
Ž .
both MG and ovary, whereas polar lipids PL predominated in muscle. In the ovary, total NL increased significantly along with maturation mainly due to an increase of TG and sterols.
Ž . Ž .
Phosphatidylcholine PC and phosphatidylethanolamine PE formed the bulk of ovarian PL and also increased significantly as maturation progressed. Major fatty acids in both MG and ovary were 14:0, 16:0, 18:0, 18:1ny9, 18:2 ny6, 18:3ny3, 20:4 ny6, 20:5ny3 and 22:6 ny3. Significant increases in the levels of saturated and mono-unsaturated fatty acids were observed in
Ž .
both MG and ovary. The levels of ny3 highly unsaturated fatty acids HUFA , particularly 20:5ny3, decreased in the MG as ovarian development proceeded. In contrast, an increase in the ovarian contents of ny3 HUFA was detected.q2001 Elsevier Science B.V. All rights reserved.
Keywords: Prawn; Macrobrachium rosenbergii; Lipids; Maturation
)Corresponding author. Tel.:q32-9-264-3754; fax:q32-9-264-4193.
Ž .
E-mail address: [email protected] R.O. Cavalli .
0044-8486r01r$ - see front matterq2001 Elsevier Science B.V. All rights reserved.
Ž .
1. Introduction
Knowledge of the biochemistry and metabolism of the processes that occur during maturation are essential for a complete understanding of crustacean reproduction
ŽMourente et al., 1994 . Lipid deposition during maturation is crucial to reproduction.
and early larval development because lipids are known to play several essential roles in
Ž
the metabolism of crustaceans Teshima 1972, 1997; Pillay and Nair, 1973; Middleditch
.
et al., 1980; Teshima and Kanazawa, 1983; Galois, 1984; Harrison, 1990 . Apart from being a major source of metabolic energy and the main form of energy storage, lipids also supply essential fatty acids needed for the maintenance and integrity of cellular
Ž
membranes, and serve as precursors of steroid and moulting hormones Teshima, 1972;
.
Harrison, 1990 .
For the freshwater prawn Macrobrachium rosenbergii, however, most studies that have investigated lipid metabolism and requirements have been restricted to larval and
Ž
juvenile stages Sandifer and Joseph, 1976; Chanmugam et al., 1983; Hilton et al., 1984; Briggs et al., 1988; Reigh and Stickney, 1989; Devresse et al., 1990; Sheen and D’Abramo, 1991; Teshima et al., 1992, 1997; D’Abramo and Sheen, 1993; Querijero et
.
al., 1997; Roustaian et al., 1999 . As a result, little is known about the specific function of the different lipid classes and their components in the maturation of this prawn.
The purpose of the present work is to describe the distribution and variation of total
Ž .
lipids, lipid classes and fatty acids in the midgut gland MG , ovary, and muscle tissue of wild-caught M. rosenbergii females at different stages of ovarian development. These data may lead to a better understanding of the relative importance of the different components of the lipid fraction in the reproduction of this economically important species.
2. Material and methods
Adult M. rosenbergii females were captured in the Mae Klong River, Amphur Muang, Province of Samut Songkhram, Thailand, from July to September 1998. Soon after capture, female prawns were divided into five groups according to their stage of ovarian development. Stages were identified according to the size, colour and general
Ž .
aspect of the ovary as presented by Chang and Shih 1995 :
v Stage I: no ovarian tissue can be observed. This absence is characteristic of both
non-developed and spent females;
v Stage II: an ovary with a small spot of yellow colour found near the posterior part of
the carapace;
v Stage III: the ovarian tissue, with an orange colour, can be observed from the posterior
part of the carapace to the area just in front of the epigastric tooth;
v Stage IV: the ovarian tissues have grown and extended to the area of the epigastric
tooth;
Ž . Ž
After blotting the prawns dry, wet weight to the nearest 0.1 g and total length from
.
the tip of the rostrum to the end of the telson were recorded. Females were then sacrificed and the ovary and MG were removed by dissection and weighed to the nearest
Ž .
0.01 g. Samples of muscle tissue were also collected. The gonado-somatic GSI and
Ž .
midgut gland somatic indices MSI were calculated as the percentage of the weight of the gonad or MG to total body weight, respectively. When tissues of one individual were insufficient for analysis, tissues were pooled from two to four individuals. All samples were maintained aty208C until analysis.
2.1. Lipid analysis
Ž .
The total lipid, lipid class composition and fatty acid methyl esters FAME of the ovary, MG, and muscle tissue were determined using standard analytical procedures.
Ž .
Total lipids were determined according to Folch et al. 1957 , following the
modifica-Ž .
tions of Ways and Hanahan 1964 . FAME composition was verified by gas
chromatog-Ž .
raphy according to Coutteau and Sorgeloos 1995 . Lipid classes were analysed using
Ž .
high-performance thin-layer chromatography HPTLC as proposed by Olsen and
Hen-Ž .
derson 1989 .
2.2. Data analysis
Differences between the stages of ovarian development were analysed by one-way
Ž .
analysis of variance ANOVA and, when pertinent, by Tukey’s honest significant
Ž .
difference HSD test. The homogeneity of the variances of means was checked by the univariate tests of Cochran, Hartley and Barlett. A minimum of three replicates for each
Ž .
tissue was analysed. Results are presented as means"standard deviation SD .
3. Results
The biometric data of prawn females are summarised in Table 1. No significant differences were found among wet weight, total length or MSI of females representing the different stages of maturation. GSI significantly increased during maturation.
Table 1
Weight, total length, GSI and MSI of wild M. rosenbergii females at different stages of ovarian development.
Ž .
The MG contained the highest levels of lipid among the tissues examined throughout the maturation period, except at stage V when similar levels were found in the ovaries
ŽTables 2 and 3 . Mean lipid content in the MG ranged from 41.5% to 67.0%, whereas.
levels that ranged from 18.2% to 55.8% were found in the ovary. Lipid content of the
Ž .
muscle tissue was generally stable at around 5% Table 4 . Lipid levels in the MG showed a large increase from stages I to III, and tended to decrease between stages III and V but these changes were not statistically significant due to a wide variance. In the ovary, a significant increase in the content of lipid occurred from stages I to II, but
Ž .
between stages II and IV no significant differences were detected Table 3 . From stages IV to V, a significant increase in ovarian lipid was observed.
The lipid class contents in the MG throughout the maturation period are presented in
Ž .
Table 2. Neutral lipids NL were predominant in the MG, comprising from 73.4%
Žstage I to 79.4% stage III of all lipids. The NL:PL ratio in the MG ranged from 2.8 to. Ž .
3.9. Total NL increased significantly from stages I to III, but decreased significantly from stage III to stage V. The overall increase in NL was due mainly to a significant
Ž .
increase in triacylglycerols TG , which presented variations similar to NL. At stage V, TG represented 53.8% of all NL in the MG. Significant differences in sterol and steryl
Ž .
ester SE content were also detected in the MG. SE levels were stable between stages I and II, and followed by ups and downs between stages II to V. Sterols were also stable
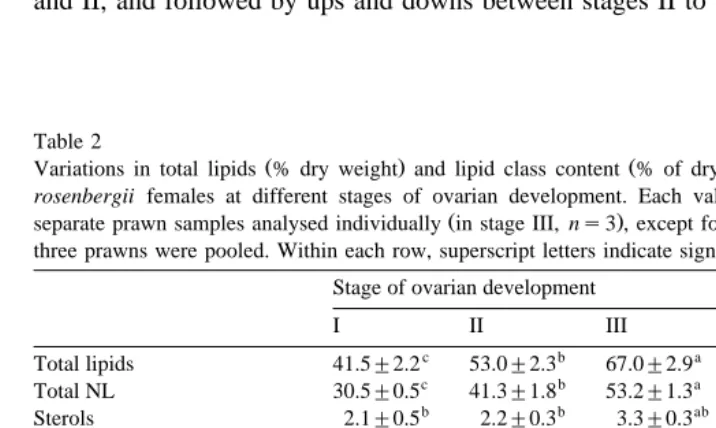
Table 2
Ž . Ž .
Variations in total lipids % dry weight and lipid class content % of dry weight in the MG of wild M.
Ž .
rosenbergii females at different stages of ovarian development. Each value is the mean "SD of four
Ž .
separate prawn samples analysed individually in stage III, ns3 , except for stages I and II where tissues of
Ž .
three prawns were pooled. Within each row, superscript letters indicate significant differences P-0.05 Stage of ovarian development
Triacylglycerol 12.6"3.8 16.2"1.8 26.9"2.0 16.9"6.3 21.2"3.0
b b a b a
Steryl esters 1.2"0.3 1.7"0.4 3.2"0.2 1.7"0.7 3.3"0.8 Monoacylglycerolqpigments 2.5"1.3 4.0"0.5 3.7"0.9 4.1"0.6 1.8"1.8
bc ab ab a c
Diacylglycerol 3.2"0.3 5.9"0.7 6.3"1.7 7.0"0.9 2.1"2.8 Total PL 11.0"0.5 11.7"1.8 13.8"1.3 13.6"4.9 12.3"4.9 Phosphatidylcholine 4.7"0.3 4.8"0.9 6.0"0.4 6.2"1.8 6.6"2.7 Phosphatidylserine 0.6"0.2 0.7"0.5 0.5"0.1 0.4"0.3 0.2"0.3 Phosphatidylinositol 1.1"0.2 1.1"0.6 1.7"0.2 1.4"0.8 0.9"0.4 Phosphatidylethanolamine 3.0"0.1 3.2"0.4 4.4"0.5 4.4"1.3 4.0"1.8 Lyso-phosphatidylcholine 0.9"0.9 1.0"0.7 0"0 0.2"0.1 0.1"0.1
ab ab a a b
Phosphatidic acid 0.6"0.2 0.7"0.2 1.1"0.2 1.0"0.6 0.2"0.0 Sphyngomyelin 0.1"0.1 0.2"0.1 0.1"0 0.1"0.1 0.1"0.2 Glycolipids 0"0 0.1"0.2 0"0 0"0 0.1"0.1
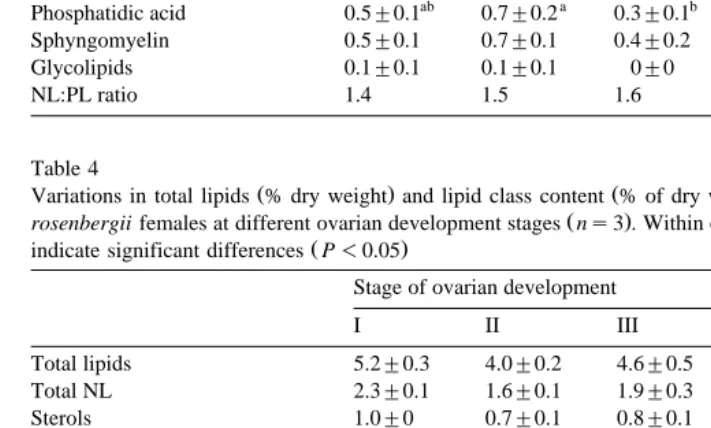
Table 3
Ž . Ž .
Variations in total lipids % dry weight and lipid class content % of dry weight in the ovary of wild M.
Ž .
rosenbergii females at different stages of maturation. Each value is the mean "SD of three separate prawn samples analysed individually, except for stages I, II and III, where tissues of four, three and two prawns were
Ž .
pooled, respectively. Within each row, superscript letters indicate significant differences P-0.05 Stage of ovarian development
Triacylglycerol 4.6"0.8 10.6"0.5 14.3"2.2 15.7"0.8 21.4"1.5
b a a a a
Steryl esters 0.8"0.3 1.8"0.1 1.7"0.3 1.6"0.1 2.1"0.1 Monoacylglycerolqpigments 0.8"0 0.7"0.1 0.6"0 0.5"0 0.6"0.4
c b b b a
Diacylglycerol 0.5"0 1.2"0.1 1.4"0.2 1.3"0 1.8"0
c b b b a
Total PL 7.5"0.3 13.8"0.5 15.3"2.2 14.0"1.4 22.8"1.2
c b b b a
Phosphatidylcholine 3.2"0.6 6.2"0.6 7.9"1.2 7.7"1.0 10.9"0.9
a a ab b ab
Phosphatidylserine 0.6"0.2 0.7"0.1 0.5"0.3 0.1"0 0.5"0.1
b a a b a
Phosphatidylinositol 0.6"0.1 1.3"0.1 1.2"0.3 0.5"0.2 1.4"0.1
d c bc b a
Phosphatidylethanolamine 2.0"0.3 4.0"0.4 5.0"0.5 5.1"0.3 9.0"0.3 Lyso-phosphatidylcholine 0.1"0.1 0"0 0"0 0"0 0"0
Variations in total lipids % dry weight and lipid class content % of dry weight in the muscle of wild M.
Ž .
rosenbergii females at different ovarian development stages ns3 . Within each given row, superscript letters
Ž . Monoacylglycerolqpigments 0.1"0 0.1"0 0.1"0 0.1"0 0.2"0.2 Diacylglycerol 0.1"0 0.1"0 0.1"0 0.1"0 0.1"0.1 Total PL 2.9"0.1 2.4"0.1 2.7"0.3 2.6"0 3.2"0.6 Phosphatidylcholine 1.1"0 1.0"0.1 1.1"0.2 1.0"0 1.2"0.3 Phosphatidylserine 0.4"0.1 0.3"0.1 0.3"0 0.4"0 0.5"0.1 Phosphatidylinositol 0.2"0.1 0.2"0 0.2"0 0.2"0 0.3"0 Phosphatidylethanolamine 0.9"0 0.7"0 0.8"0 0.9"0 0.8"0.3 Lyso-phosphatidylcholine 0"0 0"0 0"0 0"0 0"0 Phosphatidic acid 0.2"0 0.1"0 0.1"0 0.1"0 0.2"0.1 Sphyngomyelin 0.2"0 0.2"0 0.2"0 0.2"0 0.2"0.1
Glycolipids 0"0 0"0 0"0 0"0 0"0
Ž .
between stages I and II, but increased from stage II to stage V. Free fatty acids FFA and diacylglycerols followed similar up and down trends during the maturation period,
Ž .
but the lowest levels were found at stage V. Total polar lipid PL in the MG did not significantly vary throughout the maturation period. Phosphatidic acid was the only PL class to present significant variations, with stable levels from stages III to IV, but
Ž .
decreasing significantly towards stage V. Phosphatidylcholine PC , phosphatidylserine
ŽPS , phosphatidylinositol PI , phosphatidylethanolamine PE , lyso-phosphatidylcho-. Ž . Ž .
Ž .
line LPC , sphyngomyelin and glycolipids showed no significant variations.
Table 3 presents the changes in lipid class levels in the ovary during maturation. Total NL was more abundant in the ovary than PL. At stage I, NL:PL ratio was 1.4 and increased to 1.8 as GSI increased up to stage IV. Total NL levels doubled between stages I and II and continued to present significant increases throughout maturation.
Ž . Ž .
Most of the NL was TG, which ranged from 43% stage I to 64.8% stage V . Sterols were the second most abundant lipid class among NL in the ovary, increasing from 2.0% to 6.3% between stages I and V, respectively. SE levels increased significantly from stage I to stage II and remained stable thereafter. Levels of FFA fluctuated throughout maturation; the lowest level was found at stage V. Diacylglycerol levels increased significantly from stage I to II, were stable from stage II through IV, and reached a peak value at stage V. Total PL levels in the ovary at stages V were significantly greater than all other stages. PC and PE were quantitatively the main components of the PL fraction,
Ž . Ž .
collectively representing from 69.3% stage I to 91.4% stage IV of all PL in the ovary. Levels of both PC and PE were significantly higher at stage V of ovarian maturation. The level of PI also increased significantly during maturation, but was comparatively lower than either PC or PE. Other PL classes presented either negligible
Ž . Ž
up and down trends PS and phosphatidic acid or no significant variations LPC,
.
sphyngomyelin and glycolipids throughout the maturation period.
The lipid class composition of the muscle tissue is presented in Table 4. Unlike the MG and ovary, PL were more abundant than NL with the NL:PL ratio ranging between 0.7 and 0.8. No significant variation was observed for any of the lipid classes throughout maturation. Neither LPC nor glycolipids were detected in the muscles at any stage of ovarian development.
The fatty acid composition of the MG, ovary and muscle tissue throughout the maturation period is presented in Tables 5, 6 and 7, respectively. The predominant fatty
Ž . Ž . Ž . Ž .
acids in the MG were myristic 14:0 , palmitic 16:0 , stearic 18:0 , oleic 18:1ny9
Ž . Ž . Ž
and linoleic 18:2 ny6 acids, whereas linolenic 18:3ny3 , arachidonic 20:4 ny6;
. Ž . Ž .
ARA , eicosapentaenoic 20:5ny3; EPA and docosahexaenoic 22:6 ny3; DHA acids were present at intermediate levels. Saturated and mono-unsaturated fatty acids in the MG increased significantly as ovarian development proceeded. In contrast, ny3
Ž .
highly unsaturated fatty acids HUFA , particularly EPA, decreased. No significant
Ž .
variation in the relative amount of ny6 poly-unsaturated fatty acids PUFA was detected. Ovarian lipids were composed mainly of 16:0, 18:0, 18:1ny9 and 18:2 ny6. As in the MG, level of saturates and mono-unsaturates increased significantly in the ovary as gonadal development progressed. From stage I to V, the contents of saturates and mono-unsaturates in the ovary rose by 156.9% and 189.0%, respectively. Among
Ž .
Table 5
Ž y1 .
Principal fatty acid content mg g dry weight of the MG of wild M. rosenbergii females at different
Ž .
ovarian development stages ns4, except stage III, where ns3 . Different superscripts within rows represent
Ž . 18:1ny9 65.0"15.9 102.7"12.0 102.9"32.1 84.1"48.1 93.7"39.4 18:2 ny6 39.7"15.9 73.5"32.3 55.7"16.2 50.4"58.3 53.2"11.4
Saturates 143.5"44.9 177.3"35.5 234.6"32.4 213.2"22.3 268.0"29.6
b ab ab ab a
Mono-unsaturated 86.5"23.3 127.3"11.2 140.1"31.6 99.8"44.7 144.8"43.3 ny6 PUFA 49.0"15.6 80.2"31.9 63.1"15.4 55.4"58.1 58.2"12.0
a ab ab ab b
ny3 HUFA 12.9"2.3 9.5"4.6 8.3"2.0 9.3"4.4 6.0"1.8
ny6rny3 ratio 3.80 8.44 7.60 5.96 9.70
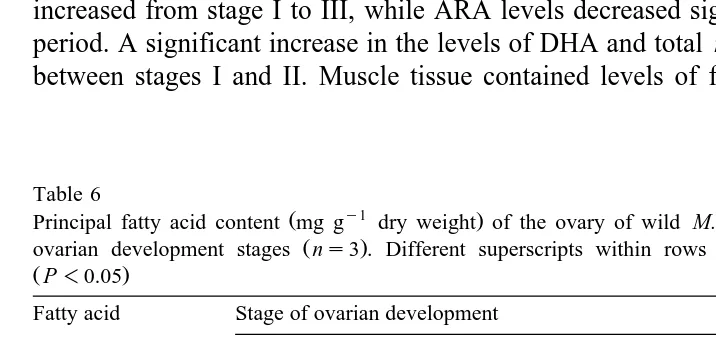
increased from stage I to III, while ARA levels decreased significantly during the same period. A significant increase in the levels of DHA and total ny3 HUFA was observed between stages I and II. Muscle tissue contained levels of fatty acids that were lower
Table 6
Ž y1 .
Principal fatty acid content mg g dry weight of the ovary of wild M. rosenbergii females at different
Ž .
ovarian development stages ns3 . Different superscripts within rows represent significant differences
ŽP-0.05.
Saturates 39.9"11.2 59.6"20.4 82.5"5.9 104.6"8.2 102.5"9.1
c b ab a a
Mono-unsaturated 32.6"9.6 56.5"11.3 76.5"15.8 89.8"12.8 94.2"10.7 ny6 PUFA 31.4"5.9 52.4"7.8 59.3"25.0 56.3"14.5 52.0"7.7
b a a a a
ny3 HUFA 12.3"3.3 20.1"5.1 17.2"5.0 18.1"5.7 17.3"5.1
Table 7
Ž y1 .
Principal fatty acid content mg g dry weight of the muscle tissue of wild M. rosenbergii females at
Ž .
different ovarian development stages ns3 . Different superscripts within rows represent significant
differ-Ž .
ences P-0.05
Fatty acid Stage of ovarian development
I II III IV V
14:0 0.6"0.3 0.5"0.3 0.9"0.3 0.6"0.2 0.5"0.3 16:0 5.6"1.2 5.9"1.3 6.0"0.4 4.6"0.6 4.9"0.5 18:0 3.5"0.9 3.2"0.7 3.2"0.2 3.1"0.3 3.0"0.5 18:1ny9 5.0"1.1 5.4"0.8 6.0"0.7 5.4"0.7 5.5"0.9 18:2 ny6 2.8"1.0 3.9"0.5 2.9"0.5 3.1"0.5 2.8"0.5 18:3ny3 0.3"0.1 0.3"0.2 0.3"0.2 0.1"0.1 0.4"0.2 20:4 ny6 3.5"0.2 3.2"0.6 2.9"0.3 2.7"0.5 2.8"0.3 20:5ny3 3.0"0.1 2.7"0.4 2.4"0.1 2.9"0.3 2.8"0.2 22:6 ny3 1.4"0.2 1.1"0.3 1.1"0.3 1.2"0.3 1.1"0.3
ÝSaturates 10.8"2.5 10.5"2.4 11.0"0.9 9.3"0.9 9.4"1.3
ÝMono-unsaturated 6.6"1.6 7.1"1.3 8.1"0.6 6.7"0.9 7.5"1.4 ny6 PUFA 7.0"1.5 7.6"1.2 6.2"0.8 6.0"0.9 6.0"0.5 ny3 HUFA 4.6"0.2 4.1"0.2 3.7"0.4 4.2"0.6 4.0"0.4
ny6rny3 ratio 1.52 1.85 1.68 1.43 1.50
than those of the other tissues examined. No significant variation in the levels of any fatty acid was detected.
4. Discussion
The analysis of the lipid profile of selected tissues has been a widely applied methodology in the study of lipid metabolism of crustaceans, particularly during sexual
Ž
maturation Pillay and Nair, 1973; Teshima and Kanazawa, 1983; Galois, 1984; Castille and Lawrence, 1989; Harrison, 1990; Millamena and Pascual, 1990; Mourente and
.
Rodriguez, 1991; Mourente et al., 1994 . In general, results from these studies have shown substantial increases in lipid levels with a corresponding increase in GSI. In the present study, an increase in lipids throughout ovarian maturation was also evident, but no corresponding significant decrease in the level of total lipids in the MG was observed. Significant decreases in some NL classes, particularly TG, FFA and diacyl-glycerols, occurred in the MG at the final stages of maturation. This decrease was followed by an increase in the levels of TG and diacylglycerols in the ovary at stage V. Our results therefore suggest that lipids from the MG may be, to some extent, transferred to the ovary of M. rosenbergii during maturation.
A low mobilisation of lipid reserves from the MG to the ovary during maturation has
Ž . Ž
been reported in Uca annulipes Pillay and Nair, 1973 , Chorismus antarcticus Clarke,
. Ž . Ž .
Ž
rosenbergii, the MG is the main lipid storage and processing organ D’Abramo and
. Ž .
Sheen, 1993 , but with a limited lipid-storage capability Cavalli et al., 1999 , its reserves only contribute partially to vitellogenesis. As a result, dietary lipids need to be rapidly processed through the MG and exported to the ovary. This conclusion is consistent with previous findings that lipid mobilisation to the developing ovary depends much more on the immediate ingestion of dietary lipids during maturation than on lipids
Ž .
stored in the MG Harrison, 1990 .
In contrast, the utilisation of MG lipid reserves during ovarian maturation has been
Ž .
described in Metapenaeus affinis and Portunus pelagicus Pillay and Nair, 1973 , P.
Ž . Ž .
japonicus Teshima and Kanazawa, 1983 , P. indicus Galois, 1984 , P. setiferus
ŽCastille and Lawrence, 1989 , P. monodon Millamena and Pascual, 1990 and U.. Ž .
Ž .
tangeri Mourente et al., 1994 . Differences in the patterns of mobilisation of lipid via
Ž
the MG may be explained by differences in nutritional or habitat characteristics Castille
.
and Lawrence, 1989 . For omnivorous species, when enough food is available through-out the year, the reciprocal relationship between the MG and ovary may not be observed
ŽClarke, 1982 . This suggestion is in line with the biology of M. rosenbergii. Ling. Ž1969 indicated that M. rosenbergii could be classified as an omnivore because it.
readily consumes a variety of food items, which include worms, insect larvae, adult aquatic insects, small molluscs, other crustaceans, fish, grain seeds, nuts, fruits, algae, leaves and stems of aquatic plants. Additionally, M. rosenbergii presents a rather
Ž .
constant growth rate under natural conditions Sahavacharin and Pongsuwan, 1974 , suggesting the lack of seasonal food shortages.
Ž .
Interestingly, at the end of the maturation period stage V the levels of lipid in the MG of M. rosenbergii females were relatively high and declined following spawning. This change suggests a possible storage of energy in stage V females necessary to cover
Ž
the metabolic demands for spawning, as proposed for P. monodon Millamena and
.
Pascual, 1990 . Furthermore, a decrease in the feeding activity by newly moulted, pre-spawning females may also contribute to the decline in MG lipids between stages V and I. The present authors have observed that M. rosenbergii females maintained in captivity usually cease feeding 1 to 3 days prior to spawning.
Levels of muscle lipid were comparatively low and remained nearly constant throughout the maturation period, demonstrating the absence of lipid mobilisation. Around 50% of muscle lipids are phospholipids, probably almost entirely due to the cellular membrane content, hence a significant mobilisation of muscle lipids is much limited.
Phospholipids, as emulsifying agents in biological systems, are important constituents of cell membranes, play an active role in the transport of lipids in the hemolymph and in
Ž .
the absorption of fatty acids within the body Teshima, 1997 . In crustaceans, phospho-lipids are considered the main components of hemolymph and tissues, except the MG
Ž
where lipids are composed mainly of TG Teshima and Kanazawa, 1983; Mourente and
. Ž .
Rodriguez, 1991 . In this study, however, the levels of NL mainly TG in both ovary and MG were higher than those of PL. Similar results have been reported in P.
Ž . Ž .
japonicus Teshima et al., 1989 and P. monodon Millamena and Pascual, 1990 . Also,
in U. tangeri the predominance of TG in the MG and ovary was considered an indicator
Ž .
Ž .
Results from this study are in agreement with Mourente et al. 1994 who reported PC and PE to be the predominant forms of PL in the MG and ovary of maturing females. No variation in the MG content of PC and PE was observed, whereas in the ovary there was an elevation of PC and PE concentrations as maturation progressed. Although results from feeding experiments suggest that PC and PI are the main
Ž
growth-promoting fractions in larval penaeids Kanazawa et al., 1985; Teshima et al.,
.
1986 , the relative importance of PC and PI is not necessarily the same for different
Ž .
species. For instance, in juvenile lobsters Homarus americanus the active component of dietary lecithin was identified as PC, because PE and PI could not substitute for the
Ž .
effect of PC to reduce mortality D’Abramo et al., 1981 . Nevertheless, as the biosyn-thetic pathways of phospholipids are closely related and thus their interconversion is
Ž .
easily carried out, Chapelle 1986 considered that the total amount of PL present in the tissues is more important than a change in the concentration of an individual PL class. Several studies have indicated that dietary phospholipids improve the reproductive
Ž .
performance Alava et al., 1993; Cahu et al., 1994 , and larval and early postlarval
Ž .
development of penaeid shrimps Coutteau et al., 1997; Teshima, 1997 . The require-ment of dietary phospholipids for early life stages is thought to be due to their limited ability to perform de novo synthesis. In contrast, the addition of dietary phospholipids
Ž
had no beneficial effect on growth or survival of M. rosenbergii Hilton et al., 1984;
.
Briggs et al., 1988; Devresse et al., 1990; Kanazawa, 1993; Querijero et al., 1997 . Similarly, we found that increases in the level of dietary phospholipids had no
Ž
significant effect on the performance of M. rosenbergii broodstock Cavalli et al.,
.
2000 . Based on these results, a possible conclusion is that the freshwater prawn may posses the ability to synthesise phospholipids at a rate sufficient to fulfil its require-ments. Nevertheless, the numerous evidence that penaeids require dietary phospholipids and the results of the present study, showing a sharp increase in ovarian PL levels during maturation, warrant further research.
Sterols are regarded as essential nutrients because of the role of cholesterol as a cell
Ž .
constituent, and as a precursor of steroid and moulting hormones Teshima, 1972 . As most crustaceans are incapable of synthesizing steroid ring compounds, dietary supple-mentation of cholesterol is known to promote the growth and survival of several species
ŽTeshima, 1972 , including M. rosenbergii Teshima et al., 1997 . These authors have. Ž .
recently suggested that, although M. rosenbergii possesses the ability for de novo synthesis of cholesterol, the rate of production is not sufficiently high to fulfill the metabolic requirements. In the present study, a significant increase in the concentrations of sterols and SEs was observed in the MG and particularly in the ovary. Therefore, cholesterol might be critically involved in the reproductive process, as demonstrated in
Ž .
P. japonicus Kanazawa et al., 1988 . The increase may be related to the role of
cholesterol as precursor of ecdysteroids, as these compounds are known to increase
Ž .
along with the gonadal maturation of M. rosenbergii Wilder et al., 1991 .
Ž
Similar to the fatty acid profiles described for M. rosenbergii eggs Tidwell et al.,
. Ž .
1998; Cavalli et al., 2000 , newly hatched larvae Roustaian et al., 1999 , juveniles
ŽChanmugam et al., 1983 , and MG and ovary of mature females Cavalli et al., 2000 ,. Ž .
rather than those of the ny3 series, predominated in the PUFA profile of M.
rosenbergii lipids.
Significant increases in the content of saturated and mono-unsaturated fatty acids in the MG and the maturing ovary were evident throughout ovarian development. The lower levels of saturated and mono-unsaturated fatty acids in the ovary compared to levels in the MG suggest their conversion to longer chain and unsaturated forms. Yet, saturated and mono-unsaturated fatty acids may also have been catabolised for the production of energy while ny6 PUFA and ny3 HUFA were conserved. Saturated and mono-unsaturated fatty acids are the major sources of energy during embryonic
ŽClarke et al., 1990 and early larval development of M. rosenbergii Roustaian et al.,. Ž .
1999 .
Ž .
The essentiality of dietary sources of linoleic acid 18:2 ny6 has been demonstrated
Ž .
in M. rosenbergii juveniles Reigh and Stickney, 1989; D’Abramo and Sheen, 1993 , as their ability to perform de novo synthesis is either absent or extremely limited. In a series of experiments with M. rosenbergii broodstock, the ny6rny3 ratio of the eggs was comparatively higher than those of the diets, suggesting a selective incorporation of
Ž . Ž .
ny6 particularly 18:2 ny6 over ny3 fatty acids Cavalli et al., 1999, 2000 . In our study, the ovarian concentration of 18:2 ny6 increased significantly during maturation, suggesting some important metabolic function or response. Relative to this apparent need, 18:2 ny6 has been shown to affect positively the fecundity of M. rosenbergii
ŽCavalli et al., 1999 ..
HUFA, especially the ny3 series, have been found to be important for the
Ž
maturation and reproduction of penaeid shrimp Middleditch et al., 1980; Teshima and
.
Kanazawa, 1983; Millamena and Pascual, 1990; Alava et al., 1993; Cahu et al., 1994 . ARA and EPA are important as structural components of cell membranes and as
Ž .
precursor of prostaglandins Lilly and Bottino, 1981 , while DHA may play an
impor-Ž
tant role in the development of the central nervous system of crustaceans Xu et al.,
.
1994 . Although the bioconversion of C18 to C20 fatty acids of the ny6 and ny3
Ž .
series is rather restricted D’bramo and Sheen, 1993 , evidence of the selective retention
Ž .
of ARA in the tissues of starved and unfed pond-reared M. rosenbergii juveniles have
Ž . Ž .
been reported by Reigh and Stickney 1989 and Tidwell et al. 1998 , respectively. Therefore, results of these studies agree with those of our study in that the relatively high content of ARA in the muscles denotes the important function of this fatty acid as a component in muscle cell membranes. Additionally, in spite of the decrease in ovarian levels of ARA during maturation, the remaining levels of this fatty acid in the ovary were still significant and provide further evidence of its metabolic importance.
In the present study, ovarian lipids contained higher proportions of ARA, EPA and DHA than those found in the MG. EPA was present in higher levels than DHA in all tissues examined. The concentration of EPA dropped significantly in the MG, but remained constant in the ovarian lipids during maturation. Radiotracer studies by
Ž .
Teshima et al. 1992 have shown that M. rosenbergii lacks the ability for
bioconver-Ž .
sion of EPA to DHA, but a feeding experiment by D’Abramo and Sheen 1993 suggested some synthesis of EPA from DHA. The experimental results of Xu et al.
Ž1994 indicate that dietary levels of EPA and DHA positively affected the fecundity.
in the eggs of M. rosenbergii improved not only hatchability but also quality of the
Ž .
larvae Cavalli et al., 1999 .
Aside from ovarian maturation, other factors might have contributed to the variability in the lipid composition of tissues. Food intake might be responsible for some variability
ŽCastille and Lawrence, 1989 . For instance, the large variance in the lipid composition.
of MG observed in our investigation may have been caused by differences in food availability andror composition at the sampling stations or perhaps due to minor variations caused by seasonality as the sampling campaign lasted for 3 months.
Acknowledgements
Sincere thanks are due to Mr. Nopadol Phuwapanish, Department of Fisheries, Thailand, for his assistance in the capture and sampling of the prawns, and to Dr. Greet Merchie, Inve Aquaculture, Thailand, for the transportation of the samples. We also thank Mathieu Wille and an anonymous reviewer whose comments and suggestions improved the quality of this manuscript. The technical support of Anita Dehaese, Christ Mahieu and Geert Vandewiele is also greatly appreciated. The Brazilian Council for
Ž .
Science and Technology CNPq supported this study through a grant to the first author
ŽProc. 200796r96-8 ..
References
Alava, V.R., Kanazawa, A., Teshima, S., Sakamoto, S., 1993. Effect of dietary phospholipids and ny3 highly unsaturated fatty acids on ovarian development of kuruma prawn. Nippon Suisan Gakkaishi 59, 345–351. Briggs, M.R.P., Jauncey, K., Brown, J.H., 1988. The cholesterol and lecithin requirements of juvenile prawn
ŽMacrobrachium rosenbergii fed semi-purified diets. Aquaculture 70, 121–129..
Cahu, C., Guillaume, J.C., Stephan, J., Chim, L., 1994. Influence of phospholipid and highly unsaturated fatty´ acids on spawning rate and egg and tissue composition in PenaeusÕannamei fed semi-purified diets.
Aquaculture 126, 159–170.
Castille, F.L., Lawrence, A.L., 1989. Relationship between maturation and biochemical composition of the
Ž .
gonads and digestive glands of the shrimps Penaeus aztecus Ives and Penaeus setiferus L. . J. Crustacean Biol. 9, 202–211.
Cavalli, R.O., Lavens, P., Sorgeloos, P., 1999. Performance of Macrobrachium rosenbergii broodstock fed diets with different fatty acid composition. Aquaculture 179, 387–402.
Cavalli, R.O., Menschaert, G., Lavens, P., Sorgeloos, P., 2000. Maturation performance, offspring quality and lipid composition of Macrobrachium rosenbergii females fed increasing levels of dietary phospholipids. Aquacult. Int. 8, 41–58.
Chang, C.F., Shih, T.W., 1995. Reproductive cycle of ovarian development and vitellogenin profiles in the freshwater prawn, Macrobrachium rosenbergii. Invertebr. Reprod. Dev. 27, 11–20.
Chanmugam, P., Donovan, J., Wheeler, C.J., Hwang, D.H., 1983. Differences in the lipid composition of fresh
Ž .
water prawn Macrobrachium rosenbergii and marine shrimp. J. Food Sci. 48, 1440–1443.
Chapelle, S., 1986. Aspects of phospholipid metabolism in crustaceans as related to changes in environmental temperatures and salinities. Comp. Biochem. Physiol. 84B, 423–439.
Clarke, A., Brown, J.H., Holmes, L.J., 1990. The biochemical composition of eggs from Macrobrachium rosenbergii in relation to embryonic development. Comp. Biochem. Physiol. 96B, 505–511.
Coutteau, P., Sorgeloos, P., 1995. Intercalibration exercise on the qualitative and quantitative analysis of fatty acids in Artemia and marine samples. ICES Cooperative Research Reports 211, 30 pp.
Coutteau, P., Geurden, I., Camara, M.R., Bergot, P., Sorgeloos, P., 1997. Review on the dietary effects of phospholipids in fish and crustacean larviculture. Aquaculture 155, 149–164.
D’Abramo, L.R., Sheen, S.S., 1993. Polyunsaturated fatty acid nutrition in juvenile freshwater prawn Macrobrachium rosenbergii. Aquaculture 115, 63–86.
D’Abramo, L.R., Bordner, C.E., Conklin, D.E., Baum, N.A., 1981. Essentiality of dietary phosphatidylcholine for the survival of juvenile lobsters. J. Nutr. 111, 425–431.
Devresse, B., Romdhane, M., Buzzi, M., Rasowo, J., Leger, P., Brown, J., Sorgeloos, P., 1990. Improved´ larviculture outputs in the giant freshwater prawn Macrobrachium rosenbergii fed a diet of Artemia enriched with ny3 HUFA and phospholipids. World Aquacult. 21, 123–125.
Folch, J., Lees, M., Stanley, G.H.S., 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 266, 497–509.
Galois, R., 1984. Variations de la composition lipidique tissulaire au cours de la vitellogenese chez la crevette Penaeus indicus Milne Edwards. J. Exp. Mar. Biol. Ecol. 84, 155–166.
Harrison, K.E., 1990. The role of nutrition in maturation, reproduction and embryonic development of decapod crustaceans: a review. J. Shellfish Res. 9, 1–28.
Hilton, J.W., Harrison, K.E., Slinger, S.J., 1984. A semi-purified test diet for Macrobrachium rosenbergii and the lack of need for supplemental lecithin. Aquaculture 37, 209–215.
Ž .
Kanazawa, A., 1993. Essential phospholipids of fish and crustaceans. In: Kaushik, S.F., Luquet, P. Eds. , Fish Nutrition in Practice. INRA Editions, Versailles, France, pp. 519–530.
Kanazawa, A., Teshima, S., Sakamoto, M., 1985. Effects of dietary lipids, fatty acids, and phospholipids on
Ž .
growth and survival of prawn Penaeus japonicus larvae. Aquaculture 50, 39–49.
Kanazawa, A., Chim, L., Laubier, 1988. Tissue uptake of radioactive cholesterol in the prawn Penaeus japonicus Bate during ovarian maturation. Aquat. Living Resour. 1, 85–91.
Lilly, M.L., Bottino, N.R., 1981. Identification of arachidonic acid in Gulf of Mexico shrimp and degree of biosynthesis in Penaeus setiferus. Lipids 16, 871–875.
Ling, S.W., 1969. The general biology and development of Macrobrachium rosenbergii. FAO Fish. Rep. 57, 607–619.
Middleditch, B.S., Missler, S.R., Hines, H.B., McVey, J.P., Brown, A., Ward, D.J., Lawrence, A.L., 1980. Metabolic profiles of penaeid shrimp: dietary lipids and ovarian maturation. J. Chromatogr. 195, 359–368. Millamena, O.M., Pascual, F.P., 1990. Tissue lipid content and fatty acid composition of Penaeus monodon
Fabricius broodstock from the wild. J. World Aquacult. Soc. 21, 116–121.
Mourente, G., Rodriguez, A., 1991. Variation in the lipid content of wild-caught females of the marine shrimp Penaeus kerathurus during sexual maturation. Mar. Biol. 110, 21–28.
Mourente, G., Medina, A., Gonzalez, S., Rodriguez, A., 1994. Changes in the lipid class and fatty acid´
Ž .
contents in the ovary and midgut gland of the female fiddler crab Uca tangeri Decapoda, Ocypodiadae during maturation. Mar. Biol. 121, 187–197.
Olsen, R.E., Henderson, R.J., 1989. The rapid analysis of neutral and polar marine lipids using double-devel-opment HPTLC and scanning densitometry. J. Exp. Mar. Biol. Ecol. 129, 189–197.
Pillay, K.K., Nair, N.B., 1973. Observations on the biochemical changes in the gonads and other organs of
Ž .
Uca annulipes, Portunus pelagicus, and Metapenaeus affinis Decapoda: Crustacea during the reproduc-tive cycle. Mar. Biol. 18, 167–198.
Querijero, B.V.L., Teshima, S., Koshio, S., Ishikawa, M., 1997. Utilization of monounsaturated fatty acid
Ž18:1ny9 by freshwater prawn Macrobrachium rosenbergii de Man juveniles. Aquacult. Nutr. 3,. Ž .
127–139.
Reigh, R.C., Stickney, R.R., 1989. Effects of purified dietary fatty acids on the fatty acid composition of freshwater shrimp Macrobrachium rosenbergii. Aquaculture 77, 157–174.
Roustaian, P., Kamarudin, M.S., Omar, H., Saad, C.R., Ahmad, M.H., 1999. Changes in fatty acid profile
Ž .
during larval development of freshwater prawn Macrobrachium rosenbergii de Man . Aquacult. Res. 30, 815–824.
in Lake Songkhla by tagging. Technical Report of Songkhla Marine Fishery Station, 1973–1974. Department of Fisheries, Bangkok, pp. 55–60, in Thai.
Sandifer, P.A., Joseph, J.D., 1976. Growth responses and fatty acid composition of juvenile prawns
ŽMacrobrachium rosenbergii fed a prepared ration augmented with shrimp head oil. Aquaculture 8,.
129–138.
Sheen, S.S., D’Abramo, L.R., 1991. Response of juvenile freshwater prawn, Macrobrachium rosenbergii, to different feeding levels of a cod liver oilrcorn oil mixture in a semi-purified diet. Aquaculture 93, 121–134.
Teshima, S., 1972. Sterol metabolism. Mem. Fac. Fish., Kagoshima Univ. 21, 69–147.
Ž .
Teshima, S., 1997. Phospholipids and sterols. In: D’Abramo, L.R., Conklin, D.E., Akiyama, D.M. Eds. , Crustacean Nutrition. World Aquaculture Society, Baton Rouge, pp. 85–107.
Teshima, S., Kanazawa, A., 1983. Variation in lipid composition during the ovarian maturation of the prawn. Bull. Jpn. Soc. Sci. Fish. 49, 957–962.
Teshima, S., Kanazawa, A., Kakuta, Y., 1986. Growth, survival and body lipid composition of the prawn larvae receiving several dietary phospholipids. Mem. Fac. Fish., Kagoshima Univ. 35, 17–27.
Teshima, S., Kanazawa, A., Koshio, S., Horinouchi, K., 1989. Lipid metabolism of the prawn P. japonicus during maturation: variation in lipid profiles of the ovary and hepatopancreas. Comp. Biochem. Physiol. 92B, 45–49.
Teshima, S., Kanazawa, A., Hitotsumatsu, K., Kim, K.S., Oshida, K., Koshio, S., 1992. Tissue uptake and bioconversion of icosapentaenoic acid and phosphatidylcholine in prawns, Penaeus and Macrobrachium. Comp. Biochem. Physiol. 102B, 885–890.
Teshima, S., Ishikawa, M., Koshio, S., Kanazawa, A., 1997. Necessity of dietary cholesterol for the freshwater prawn. Fish. Sci. 63, 596–599.
Tidwell, J.H., Webster, C.D., Coyle, S.D., Daniels, W.H., D’Abramo, L.R., 1998. Fatty acid and amino acid
Ž
composition of eggs, muscle and midgut glands of freshwater prawns, Macrobrachium rosenbergii de
.
Man , raised in fertilized ponds, unfertilized ponds or fed prepared diets. Aquacult. Res. 29, 37–45. Ways, P., Hanahan, D.J., 1964. Characterization and quantification of red cell lipids in normal man. J. Lipid
Res. 5, 318–328.
Wilder, M.N., Okumura, T., Aida, K., 1991. Accumulation of ovarian ecdysteroids in synchronization with gonadal development in the giant freshwater prawn, Macrobrachium rosenbergii. Zool. Sci. 8, 919–927. Xu, X.L., Ji, W.J., Castell, J.D., O’Dor, R.K., 1994. Influence of dietary lipid sources on fecundity, egg
Ž .