www.elsevier.comrlocateranireprosci
Local versus systemic effects of exogenous
estradiol-17b
on ovarian follicular dynamics in
heifers with progestogen implants
G.A. Bo
a,1, D.R. Bergfelt
b, G.M. Brogliatti
b, R.A. Pierson
c,
G.P. Adams
b, R.J. Mapletoft
a,)a
Departments of Herd Medicine and Theriogenology, UniÕersity of Saskatchewan, 52 Campus DriÕe,
Saskatoon, Saskatchewan, Canada S7N 5B4
b
Department of Veterinary Anatomy, Western College of Veterinary Medicine, UniÕersity of Saskatchewan,
Saskatoon, Saskatchewan, Canada S7N 5B4
c
Department of Obstetrics and Gynecology, College of Medicine, UniÕersity of Saskatchewan, Saskatoon,
Saskatchewan, Canada S7N 5B4
Received 19 August 1999; received in revised form 24 February 2000; accepted 3 March 2000
Abstract
Two experiments were designed to determine if the suppressive effect of estradiol treatment on ovarian follicles in progestogen-implanted heifers is mediated directly at the ovary or systemically, at a higher level. The purpose of Experiment 1 was to determine a minimal effective dose of
Ž .
estradiol-17b E-17b that would induce follicle regression in progestogen-implanted heifers. Beef
Ž .
heifers were implanted with progestogen on Day 2 Day 0sovulation and were assigned
Ž . Ž .
randomly to five groups: control sesame seed oil, ns9 ; 0.1 mg of E-17b ns8 ; 0.5 mg of
Ž . Ž . Ž . Ž .
E-17b ns8 ; 1 mg of E-17b ns8 ; or 5 mg of E-17b ns8 by intramuscular im injection
Ž .
on Day 3. Treatment with 5 and 1 mg of E-17b resulted in smaller P-0.05 day-to-day diameter profiles of the dominant follicle compared with controls, whereas 0.1 mg of E-17b did not have an apparent effect on follicle growth. The effect of a dose of 0.5 mg was intermediate
Ž .
and tended P-0.06 to result in a smaller diameter profile of the dominant follicle compared
Ž .
with control heifers. Experiment 2 was designed to utilize a subminimal dose of E-17b 0.1 mg , locally, to determine whether estradiol treatment induces follicle regression through a direct action
)Corresponding author. Tel.:q1-306-966-7149; fax:q1-306-966-7159.
Ž . Ž .
E-mail addresses: [email protected] G.A. Bo , [email protected] R.J. Mapletoft .
1 Ž . Ž .
Present address: Instituto de Reproduccion Animal Cordoba IRAC , J.L. de Cabrera 106 5000 Cordoba, Argentina.
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
on the ovary. Beef heifers received a progestogen ear implant on Day 2 and were assigned
horn ipsilateral to the dominant follicle intrauterine iu , ns8 ; or 0.1 mg of E-17b given into
Ž Ž . .
the stroma of the ovary, immediately adjacent to the dominant follicle intraovarian io , ns6 .
Ž .
Local iu and io treatments were given via a transvaginal ultrasound-guided needle injection. Treatment with 5 mg of E-17b im resulted in suppression of the dominant follicle of the first
Ž .
follicular wave and early emergence of the second follicular wave P-0.05 . Diameter profiles of the dominant follicle in heifers treated with 0.1 mg im or 0.1 mg iu differed from those of control heifers on Day 5, whereas diameter profiles of the dominant follicle in heifers treated with 0.1 mg io did not differ from the controls. Daily changes in diameter of the dominant follicle did
Ž .
not differ among the three groups treated with 0.1 mg of E-17b im, iu and io . Hourly changes in circulating concentrations of FSH and LH were not detected following estradiol treatment either before or after the results were combined for all estradiol-treated groups. Results are supportive of the hypothesis that the suppressive effect of estradiol in cattle is exerted indirectly through a systemic route rather than directly at the ovary. Although low plasma concentrations of FSH and LH were not detected, systemic treatments with high E-17b dosages resulted in follicular suppression whereas local treatments with subminimal dosages, within the ovary bearing the dominant follicle, were without effect.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Heifer; Follicle; Progestogen; Estradiol; Gonadotropins
1. Introduction
The dominant follicle of a follicular wave suppresses the growth of other follicles of
Ž .
the wave subordinates and prevents emergence of the next follicular wave until the Ž
dominant follicle of the preceding wave regresses or ovulates Ginther et al., 1989c; .
Adams et al., 1992a, 1993 . The mechanism by which follicular dominance occurs is not understood. However, it has been shown that the dominant follicle produces large
Ž . Ž
amounts of steroidal i.e., estrogen, androgens and non-steroidal factors i.e., inhibin, .
follistatin, growth factors of which some have been shown to suppress follicular growth Ž
by altering systemic concentrations of FSH Ginther et al., 1989c; Adams et al., 1992a; .
Findlay et al., 1992; Fortune, 1994; Bergfelt et al., 1994 . Intraovarian products may Ž
also act directly or locally on the ovary to alter follicular development Lobb and .
Dorrington 1992; Findlay, 1993 .
Experiments in which exogenous estradiol was administered to determine its effect on
Ž .
follicular development Hutz et al., 1988; Engelhart et al., 1989 have indicated follicle regression after treatment. Similarly, we have documented suppression of growth of the
Ž
dominant follicle after estradiol treatment to progestogen-implanted cattle Bo et al., .
1991, 1995a,b . In this regard, the dominant follicle of a follicular wave gains the Ž
capacity to produce larger quantities of estradiol than subordinate follicles Badinga et .
al., 1992; Bodensteiner et al., 1996 . Perhaps, the degree of estrogen production by the dominant follicle is part of a mechanism that facilitates dominance by suppressing
Ž . Ž
development of other follicles of the wave either directly i.e., locally or indirectly i.e., .
Ž
Exogenous estrogens have been shown to induce an LH surge Short et al., 1979; .
Kinder et al., 1991 and it was suggested that LH release may be associated with induction of follicular atresia. However, results from our laboratory do not support such a hypothesis; estradiol treatment to heifers with progestogen-ear implants or during the luteal phase of the cycle did not induce an LH surge yet growth of the dominant follicle
Ž .
was suppressed Bo et al., 1993, 1994 . In another laboratory, follicular atresia was detected prior to the estradiol-induced LH release in cows treated on Day 16 of the cycle ŽEngelhart et al., 1989 . Alternatively, estrogen may induce follicular atresia by altering.
Ž .
tonic LH secretion andror through FSH suppression Price and Webb, 1988 . Estradiol
Ž .
has been reported to decrease LH pulse amplitude in sheep Rawlings et al., 1984 and
Ž .
cattle Price and Webb, 1988 and, progesterone or progestogen implants in cattle have been reported to decrease LH pulse frequency and suppress maximal diameter of the
Ž
dominant follicle in a dose-dependent manner Ireland and Roche, 1982; Adams et al., .
1992a; Savio et al., 1993a,b; Stock and Fortune, 1993; Sanchez et al., 1995 . Estrogen-induced suppression of LH may be apparent only during a progestational phase, and estradiol and progesterone may have a synergistic effect on altering circulating
concen-Ž .
trations of FSH. Estradiol alone Butler et al., 1983; Wolfe et al., 1992 or in
Ž .
combination with progestogen Barnes et al., 1981 has been shown to suppress FSH; Ž however, suppression was more prolonged in the progestogen-implanted heifers Bolt et
.
al., 1990 . It has been suggested that estradiol and progesterone given in combination
Ž .
have an additive suppressive effect on both LH and FSH Price and Webb, 1988 . Treatment of rhesus monkeys with estradiol into the ovary bearing the largest Ždominant. follicle caused follicular atresia without apparently altering circulating
Ž . Ž .
concentrations of LH or FSH Hutz et al., 1988 . Dierschke et al. 1994 also reported that estrogen induced follicular atresia in monkeys and rats by an action directly within the ovary rather than through gonadotropin suppression. To our knowledge, there are no reports of a local effect of estrogen on follicular dynamics in cattle.
We hypothesized that the suppressive effect of exogenous estradiol, in combination with progestogen, on ovarian follicles in cattle is mediated systemically rather than locally. Experiment 1 was designed to determine a minimal effective systemic dose of estradiol for inducing follicular regression in progestogen-implanted heifers. Experiment 2 was designed to utilize a dose just below the minimal effective systemic dose of
Ž . Ž .
estradiol-17b E-17b subminimal dose; 0.1 mg locally, to determine whether estradiol treatment induces suppression of follicles through a direct action at the level of the ovary.
2. Materials and methods
2.1. Experiment 1
Ž .
Cross-bred beef heifers ns41 , 15 to 18 months of age and weighing 320 to 420 kg Ž . Ž
were treated with 500 mg of cloprostenol intramuscularly im Estrumate, Coopers .
Agropharm, Ajax, ON, Canada and monitored daily by ultrasonography to detect
Ž . Ž
.
Sanofi, Overland Park, KS, USA and were assigned randomly to one of five groups:
Ž . Ž . Ž .
control sesame seed oil, ns9 ; 0.1 mg of E-17b ns8 ; 0.5 mg of E-17b ns8 ; 1
Ž . Ž . Ž
mg of E-17b ns8 ; or 5 mg of E-17b ns8 . Each dose of E-17b Sigma, St. Louis, .
MO, USA was dissolved in 1 ml of sesame seed oil. Administration was done by a single im injection into the hind leg in the region of the semitendinosus muscle on Day 3. Day 3 was selected as the day of E-17b treatment because it corresponded to the mid-growing phase of the dominant follicle of the first follicular wave and the earliest day the dominant follicle could be consistently differentiated from subordinate follicles ŽGinther et al., 1989a ..
2.2. Experiment 2
Ž .
Cross-bred beef heifers ns38 from the same population as those in Experiment 1 were treated with cloprostenol, monitored for ovulation and received a progestogen ear implant on Day 2. On Day 3, heifers were assigned randomly to one of five treatment
Ž . Ž .
groups: control sesame seed oil, im; ns8 ; 5 mg of E-17b im ns8 ; 0.1 mg of
Ž .
E-17bim ns8 ; 0.1 mg of E-17b given into the wall of the uterus, near the tip of the
Ž .
horn ipsilateral to the dominant follicle iu; ns8 ; or 0.1 mg of E-17b into the ovarian
Ž .
stroma, immediately adjacent to the dominant follicle io; ns6 .
Ž .
Local treatments iu and io were administered with a 21-gauge needle soldered to a Ž 17-gauge, 60-cm-long barrel using transvaginal ultrasound-guided needle puncture 5.0
. MHz convex-array transducer; Corometrics Medical Systems, Wallingford, CT, USA .
Ž .
Intrauterine iu treatments were administered in 1 ml of sesame seed oil into the myometrium, near the ovarian end of the uterine horn. A local relationship between
Ž
venous drainage of the proximal portion of the uterine horn uterine branch of the
. Ž .
ovarian vein and the arterial supply of the ovary ovarian artery has been well
Ž .
documented Ginther, 1974 . Treatment into the uterine wall was intended to induce an elevation of E-17b locally in the ovary containing the dominant follicle. Intraovarian Ž .io treatment was administered in 0.2 ml of sesame seed oil into the ovarian stroma immediately adjacent to the dominant follicle. The io treatment was similar to that used
Ž .
in monkeys Hutz et al., 1988 . Progestogen ear implants were removed at the end of the observational period, 4 days after detection of the emergence of the second follicular wave.
2.3. Ultrasonography
Heifers were examined daily by transrectal ultrasonography using a 7.5-MHz
trans-Ž .
ducer Aloka SSD500, ISM, Edmonton, AB to individually identify and monitor the dominant and largest subordinate follicles of the first follicular wave and to detect the
Ž .
day of emergence of the second follicular wave Knopf et al., 1989 . Accordingly, ultrasound scanning commenced 2 or 3 days prior to the expected time of ovulation and continued until 4 days after the emergence of the second wave. Ultrasound data were collected without knowledge of treatment groups.
Ž .
same follicular pool as the dominant follicle Ginther et al., 1989a . Emergence of a follicular wave was defined as the day that the dominant follicle was retrospectively
Ž .
identified at a diameter of 4 to 5 mm Ginther et al., 1989a . Cessation of growth of the dominant follicle was defined as the day that the dominant follicle appeared to cease a
Ž .
progressive increase in diameter Ginther et al., 1989a . The first day that the follicle appeared to begin a progressive decrease in diameter was defined as the onset of
Ž .
regression of the dominant follicle Ginther et al., 1989a,b .
2.4. Blood sampling and hormone assays
In Experiments 1 and 2, changes in circulating concentrations of estradiol were determined by collecting blood samples via jugular venipuncture into heparinized tubes.
Ž .
Plasma was harvested within 30 min of collection and stored frozen y208C until assays were done. In Experiment 1, samples were taken every 2 h during the first 12 h after E-17b treatment and every 6 h for the next 36 h and, in Experiment 2, blood samples were collected at 0, 2, 6, 12, 24 and 48 h after E-17b treatment. Plasma concentrations of estradiol were measured in all samples using a validated
radioim-Ž .
munoassay Joseph et al., 1992 . Standards were prepared in charcoal-stripped bovine serum and the standard curve ranged from 6.25 to 1600 pgrml. Values greater than the standard curve were diluted in charcoal-stripped serum and re-analyzed. Sensitivity of the assay was 5 pgrml and coefficients of variation were 14% within-assay and 16% between-assays.
In Experiment 2, changes in circulating concentrations of FSH and LH were Ž
determined by collecting blood samples via an indwelling jugular catheter SV-70 vinyl tubing, i.d. 1.0 mm and o.d. 1.5 mm; Dural Plastics and Engineering, Dural, NSW,
.
Australia . Heifers were fitted with a catheter on Day 2 and held in stalls overnight. On Day 3, E-17btreatments were given at 0600 h. The first serial bleeding period began 2 h later at 0800 h and ended 10 h later at 1800 h. The second serial bleeding period began 16 h after E-17b treatment at 2200 h and ended 10 h later at 0800 h. Blood samples were taken once every hour for each of the serial bleeding periods. An initial 1-ml sample was withdrawn and discarded, a second 4-ml sample was withdrawn and transferred to a centrifuge tube. All samples were allowed to clot at room temperature Ž12–18 h ; the clots were removed, the tubes were centrifuged and the serum was.
Ž .
poured-off into storage vials and frozen y208C until assays were done. Serum concentrations of FSH and LH were determined using a validated radioimmunoassay ŽBolt and Rollins, 1983; Bolt et al., 1990 which has been modified and described. ŽAdams et al., 1992a . Reagents were obtained from the USDA Animal Hormone. Program. For the FSH assay, USDA-bFSH-I-2 was used for iodination and reference
Ž .
2.5. Statistical analyses
Ž
Single-point measurements e.g., day of wave emergence, duration of growing, static . and regressing phases, onset of regressing phase, maximum follicle diameter were compared among groups by analysis of variance. End points involving repeated
mea-Ž .
surements over time e.g., follicle diameter and hormonal profiles were compared among groups by split-plot analysis of variance to examine the main effects of group
Ž . Ž .
and time hour or day , and their interactions Gill and Hafs, 1971 . Variation due to sequential data was accounted for by using heifer within group as the error term to test the effect of group. If main effects or interactions were significant, multiple comparisons
Ž .
among groups were made by the method of protected least significant difference LSD .
3. Results
3.1. Experiment 1
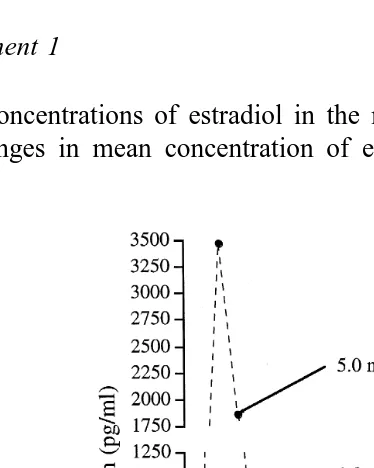
Plasma concentrations of estradiol in the respective treatment groups are shown in Fig. 1. Changes in mean concentration of estradiol in all groups given E-17b were
Ž .
Fig. 1. Mean plasma E-17bconcentrations in heifers implanted with progestogen on Day 2 Day 0sovulation
Ž
and treated intramuscularly with different dosages of E-17bon Day 3. Mean concentrations for control no
. Ž .
Table 1
Ž .
Mean "S.E.M. follicular characteristics in heifers implanted with progestogen on Day 2 and treated
Ž .
intramuscularly with different doses of E-17bon Day 3 ovulationsDay 0 . Experiment 1
End points Control E-17b
Žno E-17b. 0.1 mg 0.5 mg 1.0 mg 5.0 mg
n 9 8 8 8 8
First waÕe Ž .
Emergence day y0.2"0.1 y0.2"0.2 0.0"0.0 y0.1"0.2 y0.5"0.8
Dominant follicle
Ž .
Maximum diameter mm 12.5"0.7 12.4"0.5 11.1"0.4 11.2"0.4 11.2"0.5
Ž .
Cessation of growth day 6.1"0.4 5.6"0.4 5.1"0.6 5.5"0.6 5.3"0.7
ab b ab ab a
Ž .
Onset of regression day 10.4"0.5 12.0"0.5 11.0"0.7 10.4"0.4 9.8"0.9
Largest subordinate follicle
Ž .
Maximum diameter mm 8.1"0.3 7.8"0.4 8.6"0.4 8.1"0.3 8.4"0.5
Ž .
Cessation of growth day 3.0"0.2 2.6"0.4 3.4"0.5 2.9"0.4 3.1"0.4
Ž .
Onset of regression day 4.3"0.2 5.1"0.5 6.4"0.3 5.0"0.6 5.1"0.4
Second waÕe
ab b ab a a
Ž .
Emergence day 7.5"0.3 8.6"0.4 7.9"0.3 7.4"0.4 7.2"0.4
a,b Ž .
Means within a row with superscripts not in common are different P-0.05 .
Ž .
characterized by a dramatic increase P-0.05 2 h after treatment and a gradual
Ž .
decrease thereafter. Peak concentrations mean"S.E.M. were 118"8, 559"76,
Fig. 2. Mean diameter profiles of the dominant follicles of the first follicular wave in heifers implanted with
Ž .
progestogen on Day 2 Day 0sovulation and treated intramuscularly with different dosages of E-17bon Day 3. Diameter profiles of the dominant follicles in heifers treated with 5 and 1 mg of E-17b were smaller
ŽP-0.05 compared with heifers in the 0.1-mg and control no E-17. Ž b.groups. Diameter profiles of the
Ž .
903"184 and 3471"595 pgrml for heifers treated with 0.1, 0.5, 1 or 5 mg of E-17b,
Ž .
respectively. Mean concentrations in control heifers were lower P-0.05 than those of estradiol-treated heifers between 2 and 10 h after 0.1 mg of E-17b, 2 to 12 h after 0.5 mg, or 1 mg of E-17b, and between 2 and 24 h after 5 mg of E-17b.
The effects of E-17b on follicular characteristics and wave emergence in progesto-gen-implanted heifers are shown in Table 1. In the first follicular wave, mean day of emergence, cessation of growth and maximum diameter of the dominant follicle were not different among groups. However, the mean day of onset of regression of the
Ž .
dominant follicle was earlier P-0.05 in heifers treated with 5 mg of E-17b than in heifers treated with 0.1 mg of E-17b. Mean day of emergence of the second follicular
Ž .
wave was earlier P-0.05 in heifers treated with 1 or 5 mg than in those treated with 0.1 mg of E-17b. The day of emergence of the second wave in the control group and the 0.5 mg of E-17b group was intermediate and not different from other E-17b treated groups.
The effects of E-17b treatment on diameter profiles of the dominant follicle in Ž progestogen-implanted heifers are shown in Fig. 2. A group-by-day interaction P
-. Ž .
0.05 in the mean profiles of the dominant follicles was attributed to larger P-0.05
Ž .
Fig. 3. Mean plasma E-17bconcentrations in heifers implanted with progestogen on Day 2 Day 0sovulation
Ž .
and treated with 5 or 0.1 mg of E-17bintramuscularly im , or 0.1 mg of E-17binto the myometrium near the
Ž .
tip of the uterine horn ipsilateral to the dominant follicle iu , or 0.1 mg of E-17b into the ovarian stroma,
Ž . Ž .
immediately adjacent to the dominant follicle io on Day 3. Mean concentrations for control no E-17b
Ž .
heifers were different P-0.05 from those heifers treated with 5 mg of E-17bbetween 2 and 24 h and from
Ž .
those treated with 0.1 mg im, iu and io at 2 and 6 h after treatment. Concentrations in heifers treated with 0.1
Ž .
follicle diameters in control heifers or those treated with 0.1 mg of E-17b compared
Ž . Ž
with heifers treated with 5 mg of E-17b Days 4 to 10 or 1 mg of E-17b Days 8 to .
10 . The mean diameter profile of the dominant follicle in heifers treated with 0.5 mg of E-17b was intermediate and did not differ from those of the 1- and 5-mg groups, but
Ž .
tended P-0.06 to be smaller than those of the 0.1 mg of E-17b and control groups. Mean diameter profiles of the largest subordinate follicle did not differ among groups.
3.2. Experiment 2
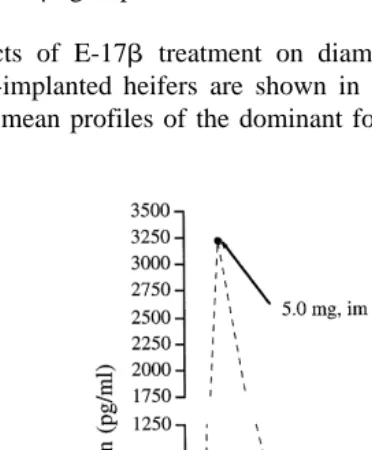
Plasma concentrations of E-17b in each of the experimental groups are shown in Fig. 3. Changes in mean concentrations in all treated groups were characterized by a
Ž .
dramatic increase P-0.01 2 h after treatment and a gradual decrease thereafter. Peak
Ž .
concentrations mean"S.E.M. were highest in heifers treated with 5 mg of E-17b im Ž3228.3"305.6 pgrml . Mean peak concentrations in heifers treated with 0.1 mg, im.
Ž .
and 0.1 mg, iu were not different 197.2"38.3 and 203.3"39.1 , but both were higher ŽP-0.05 compared with heifers treated with 0.1 mg, io 102.6. Ž "8.9 . Mean concen-.
Ž . Ž
trations in the control group were lower P-0.05 than in the 0.1 mg groups im, io or .
iu from 2 to 6 h and the 5 mg group from 2 to 24 h after E-17b treatment.
The effects of E-17b treatment on follicular characteristics and wave emergence in progestogen-implanted heifers are shown in Table 2. Mean day of emergence of the first follicular wave did not differ among groups. The dominant follicle of the first follicular wave in heifers treated with 5 mg of E-17b had a smaller maximum diameter, ceased
Table 2
Ž .
Mean "S.E.M. follicular characteristics in heifers implanted with progestogen on Day 2 and treated with 5.0
Ž .
or 0.1 mg of E-17bim, or 0.1 mg into the wall of the uterine horn ipsilateral to the dominant follicle iu , or
Ž . Ž .
0.1 mg into the ovary, adjacent to the dominant follicle io on Day 3 Day 0sovulation . Experiment 2
End points Control E-17b
Ž .
growth and regressed earlier compared with control heifers P-0.05 . Correspond-ingly, the mean day of emergence of the second follicular wave was also earlier ŽP-0.05 . Mean characteristics of the dominant follicle of the first follicular wave and. the emergence of the second follicular wave in the three groups treated with 0.1 mg of
Ž .
E-17b im, iu and io and the control group were not different.
The effects of E-17b treatment on diameter profiles of the dominant follicle in Ž progestogen-implanted heifers are shown in Fig. 4. A group-by-day interaction P
-.
0.05 was attributed to a smaller mean follicle diameter in the treated heifers. Differ-ences from the control group were detected from Days 5 to 12 in heifers treated with 5 mg im of E-17b, and on Day 5 in those heifers treated with the 0.1 mg im and iu. Mean diameter of the dominant follicle in heifers treated with 5 mg of E-17b was smaller ŽP-0.05 compared with heifers in the control no E-17b. Ž .group or those treated with 0.1 mg E-17b io on Days 5 to 12 and those treated with 0.1 mg E-17b im or iu on Days 7 to 12. Diameter profiles of heifers treated with 0.1 mg of E-17b im, iu and io were not different. Diameter profiles of the largest subordinate follicles did not differ among groups.
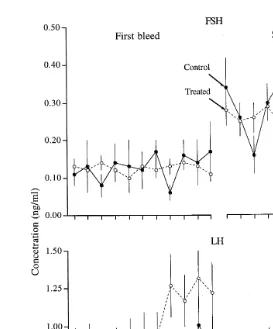
The effects of E-17b treatment on systemic concentrations of gonadotropins are shown in Fig. 5. As the mean hourly changes in FSH and LH were not significantly different among groups in the first or second serial bleeding periods, data for the E-17b-treated groups were combined and compared with the control group. Again, there was no significant difference between groups for FSH or LH in either the first or second
Fig. 4. Mean diameter profiles of the dominant follicles of the first follicular wave in heifers implanted with
Ž . Ž .
progestogen on Day 2 Day 0sovulation and treated with 5 or 0.1 mg of E-17bintramuscularly im , or 0.1
Ž .
mg of E-17binto the wall of the uterine horn near the tip ipsilateral to the dominant follicle iu , or 0.1 mg of
Ž .
E-17binto the ovarian stroma, immediately adjacent to the dominant follicle io on Day 3. Mean diameter of
Ž .
the dominant follicle in heifers treated with 5 mg of E-17bwas smaller P-0.05 compared with heifers in
Ž .
the control no E-17b group and those heifers treated with 0.1 mg E-17bio on Days 5 to 12 and those treated with 0.1 mg E-17bim or iu on Days 7 to 12. Mean diameter of the dominant follicle in heifers treated with 0.1
Ž .
Ž .
Fig. 5. Mean "S.E.M. plasma FSH and LH concentrations in heifers implanted with progestogen on Day 2
ŽDay 0sovulation and treated with 5 or 0.1 mg of E-17. bintramuscularly im , or 0.1 mg of E-17Ž . binto the
Ž .
wall of the uterine horn near the tip ipsilateral to the dominant follicle iu , or 0.1 mg of E-17b into the
Ž .
ovarian stroma, immediately adjacent to the dominant follicle io on Day 3. Since there was no significant difference among the treatment groups for either FSH or LH, the treated group represents combined data from all estradiol-treated heifers. Subsequent analyses indicated mean FSH concentrations were lower in the control
Ž . Ž . Ž
group P-0.03 and the combined-treatment group P-0.0007 in the first serial bleeding period Hours 2
. Ž .
to 12 compared with the second Hours 16 to 26 . Mean LH concentrations were similar in the control group
Ž .
between the two serial bleeding periods but were higher P-0.0004 for the first compared with the second serial bleeding period in the combined-treatment group.
serial bleeding period. However, comparison of concentrations of gonadotropin between the serial bleeding periods within groups indicated significant changes. The mean
Ž .
concentrations of FSH were lower in the control group P-0.03 and the
estradiol-Ž . Ž .
treated group P-0.0007 in the first serial bleeding period Hours 2 to 12 compared
Ž .
Ž .
higher P-0.0004 in the first than in the second serial bleeding period in the estradiol-treated group.
4. Discussion
Ž .
In Experiment 1, a single systemic im injection of supraphysiologic doses of E-17b in progestogen-implanted heifers resulted in a dose-dependent increase in circulating concentrations of estradiol. Concentrations after treatment were higher than expected. However, the first blood sample was taken 2 h after treatment in the present study,
Ž
whereas sampling began 6 h after E-17b treatment in the previous study Bo et al., .
1994 . Values at 6 h in the present study were similar to those reported previously. The
Ž . Ž
dynamics of E-17babsorption peak at 2 h after treatment and metabolism basal levels .
at 24 to 50 h depending on the dosage used are in agreement with reports from other
Ž .
laboratories Rexroad et al., 1977; Randel et al., 1979 . It would seem that increased
Ž .
circulating concentrations of E-17bfor a period of 12 h 0.5 or 1.0 mg of E-17b to 24
Ž .
h 5.0 mg of E-17b is adequate to cause suppression of the dominant follicle at this stage of development of the first follicular wave. Elevated concentrations of E-17b for 10 h, as was seen in the 0.1 mg of E-17b group, did not seem to be sufficient to induce follicular suppression. We have shown previously that the administration of 5.0 mg of
Ž
E-17bto progestogen-implanted heifers causing significantly elevated circulating E-17b .
concentrations for approximately 42 h will induce follicular regression and emergence of a new follicular wave, regardless of the stage of development of the dominant follicle
Ž .
of the first follicular wave Bo et al., 1994, 1995a,b . Therefore, greater doses or longer-acting forms of estrogen would not seem to be necessary, and may even result in
Ž
delayed andror asynchronous emergence of the new follicular wave Bo et al., 1993, .
1994 .
Experiment 2 utilized a subminimal dose of E-17b chosen from Experiment 1 to examine the local versus systemic effects of estradiol on follicular development. The io Žlocal treatment dose of 0.1 mg of E-17b. raised systemic concentrations of estradiol Ž-125 pgrml for about 6 h and follicle growth was not suppressed. Conversely, the.
Ž .
systemic treatment iu or im with 0.1 mg of E-17b resulted in a greater increase in
Ž .
systemic concentrations of estradiol -250 pgrml for a similar period of time Žapproximately 6 h and a transient suppression of follicular growth. The intraovarian. dose of 0.1 mg, E-17b and volume of sesame seed oil used as a vehicle was similar to
Ž .
that previously used in monkeys Hutz et al., 1988 , but contrary to findings in monkeys, this treatment did not result in follicular regression in cattle. The dosage used for the local treatment is considerably greater than the E-17b present in the fluid in the
Ž .
dominant follicle, which has been reported to be about 1500 ngrml Singh et al., 1998 . Therefore, the amount given adjacent to the dominant follicle would have been expected to induce profound follicular suppression compared with systemic treatments if the effects of E-17b were mediated locally.
Ž .
uterine horn and supply to the ovary Ginther, 1974 , E-17b treatment was expected to elevate estradiol concentrations in the arterial blood supplying the ovary containing the dominant follicle. Results indicated a slightly greater increase in systemic concentrations of estradiol compared with the intraovarian injection. Furthermore, results indicated a
Ž .
transient suppression of follicular growth for 1 day Day 5 before the diameter of the dominant follicle increased similar to that in the control group. In Experiment 1, systemic treatment with 0.1 mg of E-17b im did not appear to suppress follicular growth, whereas, in Experiment 2, there was a transient suppression of follicular growth similar to that following local treatment into the uterine wall. Although the difference between the two experiments with respect to systemic treatment is unknown, the intrauterine treatment may have been more representative of a systemic than a local treatment.
Differences in the concentrations of circulating E-17b in the 0.1 mg groups may have been due to the volume of sesame seed oil in which E-17b was injected into the ovary Ž0.2 ml versus 1.0 ml for im and iu sites or its absorption from the site of injection. In. any case, results support an indirect or systemic route of action. When systemic concentrations of E-17b were higher, follicular growth was affected, at least transiently,
Ž .
and as dose of E-17b and circulating concentrations increased, follicular growth was more profoundly affected.
The effects of 0.5 and 1 mg of E-17b on dominant follicle growth in Experiment 1 further support the notion that the suppressive effect of estradiol is synergistic with progestogenrprogesterone. Similar dosages of estradiol benzoate have been used to increase synchrony of estrus andror ovulation and fertility when given after
prosta-Ž .
glandin treatment Peters et al., 1977 or 24 to 48 h after the removal of progesterone-re-Ž
leasing vaginal devices in cattle McDougall et al., 1992; Day et al., 1997; Fike et al., .
1997; Hanlon et. al., 1997; Martinez et al., 1998 . If estrogens given alone had a direct suppressive effect on ovarian follicles, such treatments would have been expected to result in decreased fertility.
Although results support an indirect or systemic route of follicular suppression by estradiol, hourly changes in circulating concentrations of FSH and LH were not detected following estradiol treatment. Additional analyses for changes in FSH between serial bleeding periods within each group indicated significantly higher concentrations during
Ž .
the second serial bleeding period Hours 16 to 26 compared with the first serial
Ž .
bleeding period Hours 2 to 12 for both control and treated groups. The change in FSH between the two serial bleeding periods may be attributable to low amplitude surges of FSH that have been detected in plasma samples collected at 4-h intervals during the
Ž .
early luteal phase in cattle Bergfelt et al., 1997 . Regardless, the present results do not
Ž .
agree with those reported previously Bo et al., 1994 in which treatment of progesto-gen-implanted heifers with 5.0 mg of E-17bsuppressed plasma FSH for about 24 h and resulted in regression of the dominant follicle. Although comparable results with respect to alterations in development of the dominant follicle following different doses of estradiol and routes of administration are indicated in the present study, the lack of any detectable changes in FSH do not allow for a similar conclusion. The difference in FSH results between the previous and present studies may be attributable to the time of
Ž .
study, samples were collected at a time when systemic FSH would be expected to be at
Ž .
basal concentrations Adams et al., 1992a,b . Therefore, the FSH values in the control group and at the time of treatment with E-17b would have been near the sensitivity of
Ž .
the assay, an area of great variability Adams et al., 1992a,b . Furthermore, a recent report indicated that the bovine antiserum, used herein, was inferior to ovine antiserum
Ž .
when used in radioimmunoassays for measuring FSH Crowe et al., 1997 .
Changes in circulating concentrations of LH were similar between the two serial bleeding periods for the control group; however, LH concentrations were significantly
Ž .
higher during the first serial bleeding period Hours 2 to 12 compared with the second ŽHours 16 to 26 in the estradiol-treated groups. Bolus administration of estradiol that. resulted in supraphysiological concentrations in circulating estradiol has been reported to
Ž .
exert a biphasic effect on LH secretion Kesner et al., 1981; Butler et al., 1983 ; secretion was initially inhibited and this was followed by a surge release. In a previous
Ž
study, treatment of heifers on Day 1 with 5.0 mg of E-17b without exogenous
. Ž
progestogenrprogesterone was followed by a surge release of LH 16 to 17 h later Bo .
et al., 1994 . Conversely, when the same treatment was done in progestogen-implanted heifers no surge in LH was detected. The progestogen ear implant may have partially blocked the estradiol-induced LH release, resulting in apparent higher concentrations of LH between 9 and 12 h after treatment. Furthermore, considering that LH was apparently lower in both estradiol-treated and control groups during the second serial bleeding period, the increasing concentrations of progestogen from the ear implant and progesterone from the CL may have suppressed LH release. Although increases in progestogenrprogesterone concentrations have been shown to result in decreased LH
Ž .
pulse frequency and follicular suppression Savio et al., 1993b , this effect was not apparent in the present study.
5. Conclusion
The results of the present study confirm those of previous studies that exogenous E-17binduces follicular suppression and results in emergence of a follicular wave 3 to 5
Ž .
Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council of Canada and the University of Saskatchewan. We thank Sanofi, Overland Park, KS, USA for Syncro-Mate-B and Coopers Agropharm, Ajax, ON, Canada for Estrumate. We also thank B. Kerr and the Goodale Research Farm staff for animal handling and feeding.
References
Adams, G.P., Matteri, R.L., Kastelic, J.P., Ko, J.C.H., Ginther, O.J., 1992a. Association between surges of follicle stimulating hormone and the emergence of follicular waves in heifers. J. Reprod. Fertil. 94, 177–188.
Adams, G.P., Matteri, R.L., Ginther, O.J., 1992b. Effect of progesterone on ovarian follicles, emergence of follicular waves and circulating follicle-stimulating hormone in heifers. J. Reprod. Fertil. 95, 627–640. Adams, G.P., Kot, K., Smith, C.A., Ginther, O.J., 1993. Effect of the dominant follicle on regression of its
subordinates. Can. J. Anim. Sci. 73, 267–275.
Badinga, L., Driancourt, M.A., Savio, J.D., Wolfenson, D., Drost, M., de la Sota, R.L., Thatcher, W.W., 1992. Endocrine and ovarian responses associated with the first-wave dominant follicle in cattle. Biol. Reprod. 47, 871–883.
Barnes, M.A., Kazmer, G.W., Bierley, S.T., 1981. Gonadotropic and ovarian response in dairy cows treated with norgestomet and estradiol valerate. Theriogenology 16, 13–25.
Bergfelt, D.R., Plata-Madrid, H., Ginther, O.J., 1994. Counteraction of the inhibitory effect of follicular fluid by administration of FSH in heifers. Can. J. Anim. Sci. 74, 633–639.
Bergfelt, D.R., Smith, C.A., Adams, G.P., Ginther, O.J., 1997. Surges of FSH during the follicular and early luteal phases of the estrous cycle in heifers. Theriogenology 48, 757–768.
Bo, G.A., Pierson, R.A., Mapletoft, R.J., 1991. The effect of estradiol valerate on follicular dynamics and superovulatory response in cows with Syncro-Mate-B implants. Theriogenology 36, 169–183.
Bo, G.A., Nasser, L.F., Adams, G.P., Pierson, R.A., Mapletoft, R.J., 1993. Effect of estradiol valerate on ovarian follicles, emergence of follicular waves and circulating gonadotropins in heifers. Theriogenology 40, 225–239.
Bo, G.A., Adams, G.P., Pierson, R.A., Caccia, M., Tribulo, H., Mapletoft, R.J., 1994. Follicular wave dynamics after estradiol-17btreatment of heifers with or without a progestogen implant. Theriogenology 41, 1555–1569.
Bo, G.A., Adams, G.P., Pierson, R.A., Mapletoft, R.J., 1995a. Exogenous control of follicular wave emergence in cattle. Theriogenology 43, 31–40.
Bo, G.A., Adams, G.P., Caccia, M., Martinez, M., Pierson, R.A., Mapletoft, R.J., 1995b. Ovarian follicular wave emergence after treatment with progestogen and estradiol in cattle. Anim. Reprod. Sci. 39, 193–204. Bolt, D.J., Scott, V., Kiracofe, G.H., 1990. Plasma LH and FSH after estradiol, norgestomet and Gn-RH
treatment in ovariectomized beef heifers. Anim. Reprod. Sci. 23, 263–271.
Bodensteiner, K.J., Wiltbank, M.C., Bergfelt, D.R., Ginther, O.J., 1996. Alterations in follicular estradiol and gonadotropin receptors during development of bovine antral follicles. Theriogenology 45, 499–512. Butler, W.R., Katz, L.S., Arriola, J., Milvae, R.A., Foote, R.H., 1983. On the negative feed-back regulation of
gonadotropins in castrate and intact cattle with comparison of two FSH radioimmunoassays. J. Anim. Sci. 56, 919–929.
Crowe, M.A., Padmanabhan, V., Hynes, N., Sunderland, S.J., Enright, W.J., Beitins, I.Z., Roche, J.F., 1997. Validation of a sensitive radioimmunoassay to measure serum follicle-stimulating hormone in cattle: correlation with biological activity. Anim. Reprod. Sci. 48, 123–136.
Day, M.L., Burke, C.R., Taufa, V.K., Day, A.M., Macmillan, K.L., 1997. The strategic use of estradiol benzoate to enhance fertility and submission rates of progestin-based synchronization programs in lactating dairy cows. Proc. Aust. Soc. Reprod. Biol. 28, 101, abstr.
Engelhart, H., Walton, J.S., Miller, R.B., King, G.J., 1989. Estradiol induced blockade of ovulation in the cow: effect of luteinizing hormone release and follicular fluid steroids. Biol. Reprod. 40, 1287–1297. Fike, K.E., Day, M..L., Inskeep, E.K., Kinder, J.E., Lewis, P.E., Short, R.E., Hafs, H.D., 1997. Estrus and
luteal function in suckled beef cows that were anestrus when treated with an intravaginal device containing progesterone with without a subsequent injection of estradiol benzoate. J. Anim. Sci. 75, 2009–20015. Findlay, J.K., 1993. An update of the roles of inhibin, activin and follistatin as local regulators of
folliculogenesis. Biol. Reprod. 48, 15–23.
Findlay, J.K., Robertson, D.M., Clarke, I.J., Klein, R., Doughton, B.W., Xiao, S., Russel, D.L., Shukovski, L., 1992. Hormonal regulation of reproduction: general concepts. Anim. Reprod. Sci. 28, 319–328. Fortune, J.E., 1994. Ovarian follicular growth and development in mammals. Biol. Reprod. 50, 225–232. Gill, J.L., Hafs, H.D., 1971. Analysis of repeated measurements in animals. J. Anim. Sci. 33, 331–336. Ginther, O.J., 1974. Internal regulation of physiological processes through local venoarterial pathways: a
review. J. Anim. Sci. 39, 550–564.
Ginther, O.J., Kastelic, J.P., Knopf, L., 1989a. Temporal associations among ovarian events in cattle during estrous cycles with two and three follicular waves. J. Reprod. Fertil. 87, 223–230.
Ginther, O.J., Kastelic, J.P., Knopf, L., 1989b. Composition and characteristics of follicular waves during the bovine estrous cycle. Anim. Reprod. Sci. 20, 187–200.
Ginther, O.J., Kastelic, J.P., Knopf, L., 1989c. Intraovarian relationships among dominant and subordinate follicles and the corpus luteum in heifers. Theriogenology 32, 787–795.
Hanlon, D.W., Willamson, N.B., Witchel, J.J., Steffert, I.J., Craigie, A.L., Pfeiffer, D.U., 1997. Ovulatory responses and plasma luteinizing hormone concentrations in dairy heifers after treatment with exogenous progesterone and estradiol benzoate. Theriogenology 47, 963–976.
Hutz, R.J, Dierschke, D.J., Wolf, R.C., 1988. Induction of atresia of the dominant follicle in rhesus monkeys by the local application of estradiol-17b. Am. J. Primatol. 15, 69–77.
Ireland, J.J., Roche, J.F., 1982. Effect of progesterone on basal LH and episodic LH and FSH secretion in heifers. J. Reprod. Fertil. 64, 295–302.
Joseph, I.B.J.K., Currie, W.D., Rawlings, N.C., 1992. Effects of time after ovariectomy, season and estradiol on luteinizing hormone and follicle stimulating hormone secretion in ovariectomized ewes. J. Reprod. Fertil. 94, 511–523.
Kinder, J.E., Garcia-Winder, M., Imakawa, K., Day, M.L., Zalesky, D.D., D’Occhio, M.L., Stumpf, T.T., Kittok, R.J., Schanbacher, B.D., 1991. Circulating concentrations of 17b-estradiol influence pattern of LH in circulation in cows. Domest. Anim. Endocrinol. 8, 463–469.
Knopf, L., Kastelic, J.P., Schallenberger, E., Ginther, O.J., 1989. Ovarian follicular dynamics in heifers: test of two-wave hypothesis by ultrasonically monitoring individual follicles. Domest. Anim. Endocrinol. 6, 111–120.
Lobb, D.K., Dorrington, J., 1992. Intraovarian regulation of follicular development. Anim. Reprod. Sci. 28, 343–354.
Martinez, M.F., Kastelic, J.P., Adams, G.P., Janzen, E., Olson, W., Mapletoft, R.J., 1998. Alternative methods of synchronizing estrus and ovulation for fixed-time insemination in cattle. Theriogenology 49, 350, abstr. McDougall, S., Burke, C.R., Macmillan, K.L., Williamson, N.B., 1992. The effect of pretreatment with progesterone on the estrous response to estradiol benzoate in the postpartum dairy cow. Proc. N. Z. Soc. Anim. Prod. 52, 157–160.
Peters, J.B., Welch, J.A., Lauderdale, J.W., Inskeep, E.K., 1977. Synchronization of estrus in beef cattle with PGF2aand estradiol benzoate. J. Anim. Sci. 45, 230–235.
Price, C.A., Webb, R., 1988. Steroid control of gonadotropin secretion and ovarian function in heifers. Endocrinology 122, 2222–2231.
Randel, R.D., Short, R.E., Bellows, R.A., 1979. Steroid levels after intramuscular injection of radioactive estradiol-17b, estrone, progesterone and testosterone in the bovine. Theriogenology 11, 185–195. Rawlings, N.C., Jeffcoate, I.A., Rieger, D.L., 1984. The influence of estradiol-17b and progesterone on
Rexroad, C.E. Jr., 1977. Plasma estradiol-17bconcentrations in ewes and cows after estradiol-17b administra-tion. Theriogenology 8, 83–91.
Sanchez, T., Wehrman, M.E., Bergfeld, E.G., Petters, K.E., Kojima, F.N., Cupp, A.S., Mariscal, V., Kittok, R.J., Rasby, R.J., Kinder, J.E., 1995. Pregnancy rate is greater when the corpus luteum is present during the period of progestin treatment to synchronize time of estrus in cows and heifers. Biol. Reprod. 49, 1102–1107.
Savio, J.D., Thatcher, W.W., Badinga, L., De la Sota, R.L., Wolfenson, D., 1993a. Regulation of dominant follicle turnover during the oestrous cycle in cows. J. Reprod. Fertil. 97, 197–203.
Savio, J.D., Thatcher, W.W., Morris, G.R., Entwistle, K., Drost, M., Mattiacci, M.R., 1993b. Effects of induction of low plasma progesterone concentrations with a progesterone-releasing intravaginal device on follicular turnover and fertility in cattle. J. Reprod. Fertil. 98, 77–84.
Short, R.E., Randel, R.D., Staigmiller, R.B., Bellows, R.A., 1979. Factors affecting estrogen-induced LH release in the cow. Biol. Reprod. 21, 683–689.
Singh, J., Pierson, R.A., Adams, G.P., 1998. Ultrasound image attributes of bovine ovarian follicles and endocrine and functional correlates. J. Reprod. Fertil. 112, 19–29.
Stock, A.E., Fortune, J.E., 1993. Ovarian follicular dominance in cattle: relationship between prolonged growth of the ovulatory follicle and endocrine parameters. Endocrinology 132, 1108–1114.