Changes in nitrification and bacterial community structure upon
cross-inoculation of Scots pine forest soils with different initial nitrification rates
Rully A. Nugroho
a, Wilfred F.M. Ro¨ling
b,*, Nico M. van Straalen
a, Herman A. Verhoef
aaInstitute of Ecological Science, Faculty of Earth and Life Sciences, VU University Amsterdam, De Boelelaan 1085, 1081 HV Amsterdam, The Netherlands
bDepartment of Molecular Cell Physiology, Faculty of Earth and Life Sciences, VU University Amsterdam, De Boelelaan 1085, 1081 HV Amsterdam, The Netherlands
a r t i c l e
i n f o
Article history: Received 9 May 2008 Received in revised form 13 October 2008 Accepted 17 October 2008 Available online 12 November 2008
keywords: Cross-inoculation Bioaugmentation Net nitrification Bacterial communities Scots pine
a b s t r a c t
Nitrification occurs slowly in many acid Scots pine forest soils. We examined if bacterial community structure and interactions between members of the bacterial community in these forest soils prohibit growth of ammonia-oxidising microorganisms and their nitrifying activity. Native and gamma-irradiated Scots pine forest soils known to have low net nitrification rates were augmented with fresh soils or soil slurries from nitrifying Scots pine forest soil, andvice versa. Augmentation of native non-nitrifying soils with nitrifying soils induced net nitrification, although no significant changes in bacterial community structure, as measured by 16S rRNA gene-based denaturing gradient gel electrophoresis (DGGE), were observed. In sterilised soils, the inoculum, i.e. native nitrifying soil or non-nitrifying soil, determined the occurrence of net nitrification and bacterial community structure, and not the origin of the sterilised soils. Our results demonstrate that low net nitrification rates in acid Scots pine forest soils cannot be (solely) explained by unfavourable abiotic soil conditions, but that still uncaptured biotic factors contribute to suppression of nitrification.
Ó2008 Elsevier Ltd. All rights reserved.
1. Introduction
Nitrification tests using soil incubations have revealed that nitrification occurred readily in some acid pine forest soils, but slowly in others (Bottomley et al., 2004; Nugroho et al., 2005, 2007). Differences in the occurrence and nitrification rate can in part be explained by abiotic factors. Acid forest soils with low net nitrification rates are correlated with high C/N ratios (or low total N) and low atmospheric N depositions (Ba¨ckman et al., 2003; Compton et al., 2004; Nugroho et al., 2005, 2007; Persson and Wire´n, 1995; Tolli and King, 2005). Low net nitrification rates in acid forest soils cannot be explained solely by soil pH, since soil pH values did not differ significantly between soils with low and high net nitrification rates (Nugroho et al., 2005). N availability also does not constrain net nitrification in these soils as large amounts of NH4þ-N are produced during incubation of these soils in the labo-ratory (Booth et al., 2005; Nugroho et al., 2005; Persson and Wire´n, 1995). As in some acid soils with increased NH4þ-N concentrations net nitrification does not occur (Nugroho et al., 2007), other suppressive factors need to be considered.
Differences in nitrification could also be driven by biotic factors. Nitrification rates might be linked to the composition of the soil
bacterial community (Balser and Firestone, 2005; de Boer and Kes-ter, 1996; de Boer et al., 1996; Wheatley et al., 2003). Community members can have specific positive or negative effects on the growth and activity of the nitrifying bacteria. Chitinolytic soil bacteria can produce antibiotics against nitrifying bacteria (de Boer et al., 1996). Ammonia-oxidising bacteria (AOB) are poor competitors for NH4þ relative to ammonia-assimilating heterotrophs when ammonium is limiting (van Niel et al., 1993; Verhagen and Laanbroek, 1991; Ver-hagen et al., 1992, 1995).Wheatley et al. (2003), using a nested PCR with the not very specific CTO primer set, also indicated that nitri-fication rates might be linked to the composition of the soil bacterial community, rather than to the AOB community itself. They compared the structure of the bacterial community within and between three arable fields differing in potential nitrification rates but broadly similar in basic characteristics (soil pH, total C and total N contents). The bacterial community structure in each field differed significantly. In contrast, molecular analyses specific to AOB sug-gested that the populations in all three fields were similar in types and did not vary with time (Wheatley et al., 2003).
The relationships between overall bacterial community struc-ture and nitrification rates in acid Scots pine forest soils have not been studied thoroughly. Previous studies (Laverman et al., 2000; Nugroho et al., 2005), applying specific inhibitors of autotrophic nitrification, have revealed that heterotrophic nitrification does not play a significant role in such soils. Our aim was to examine if general bacterial community structure and interactions between *Corresponding author. Tel.:þ31 20 598 7192; fax:þ31 20 598 7229.
E-mail address:wilfred.roling@falw.vu.nl(W.F.M. Ro¨ling).
Contents lists available atScienceDirect
Soil Biology & Biochemistry
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / s o i l b i o
members of the bacterial community in acid Scots pine forest soils with low nitrification rate prohibit growth of ammonia oxidizers and their nitrifying activity. If so, we may expect that when native soils (showing either low or high nitrification rates) are inoculated, or augmented, with a small quantity of forest soil with contrasting nitrification activity, no significant changes in nitrification will occur. However, we would expect that when the soil microbial community is destroyed by sterilisation, the nitrifying potential of the inoculum will be able to establish itself in the sterilised soil, unless abiotic conditions in the sterilised soil prohibit this. To test these hypotheses, native and gamma-irradiated Schoorl (The Netherlands) soils, with low nitrification rates (Nugroho et al., 2005, 2007), were augmented with fresh soils or soil slurries from nitrifying Wekerom soil (The Netherlands) (Laverman et al., 2001; Nugroho et al., 2005, 2007), andvice versa. Nitrification rates were determined and related to changes in overall bacterial community profiles, established by 16S rRNA gene-based community profiling.
2. Materials and methods
2.1. Study sites and soil sampling
The forest floors of Scots pine stands were sampled from Schoorl (latitude 52430N; longitude 4400E) and Wekerom (latitude 52060N; longitude 5410E), The Netherlands. Previously,Nugroho et al. (2005)determined that net nitrification rates in these soils were 0.1 and 14.4
m
g NO3-N g1dry soil wk1, respectively, while net ammonification rates were similar (about 23m
g NH4þ-N g1dry soil wk1). Further details on the two forest sites used in this study are given inNugroho et al. (2005). At each sampling site, eighteen samples (1520 cm) of the forest floor (F layer) were randomly collected from a 55 m plot, then randomly pooled to give six composite samples and returned to the laboratory in cooling boxes.2.2. Laboratory incubation
Field-moist soils were immediately passed through a 4 mm sieve in the laboratory and homogenized by hand. Three composite samples were then stored at 5C, while three other composite samples were sterilised by 25 kGy gamma (
g
-) irradiation at Isotron Netherland B.V.Sub-samples of native and sterilised soils were brought to 68% moisture content (wet weight) by adding sterile demineralised water. Native or sterilised soil samples (10 g fresh weight) from Schoorl and Wekerom were put in 250 ml sterile bottles, using aseptic techniques, and amended with different inoculants (1 g fresh soil or 1 ml soil slurry from the same or different site of origin) as outlined inTable 1, each inoculation having 12 replicates. This inoculation procedure with fresh soil or soil slurry has also been applied in other studies on microbial-driven processes, including nitrification (Marschner and Rumberger, 2004; Meier et al., 2006). Soil slurry was chosen as a treatment in order to add a fraction of soil microorganisms separate from large soil materials, possibly containing abiotic factors affecting nitrification. Soil slurries were prepared by mixing unsterilised soil and sterile 0.1% sodium pyrophosphate (soil:solution ratio 1:1), shaken for 2 h on a shaker (100 rev min1) at room temperature: the mixture was allowed to settle for 15 min before the supernatant containing the desorbed bacterial cells and small soil particles was decanted into sterile Eppendorf tubes. Soils were thoroughly homogenized with a sterile spatula after addition of soil or soil slurry. Bottles were sealed with cotton plugs and incubated at 18C in the dark. Soil moisture was maintained by periodic addition of sterile demineralised water. Destructive samplings were conducted after 0, 1, 3 and 6 months. Three bottles were sampled per treatment and per sampling occasion. Extraction and determination of NH4þ-N and NO3-N
concentrations were carried out as described previously (Nugroho et al., 2005).
2.3. DNA extraction, PCR, DGGE and cloning
Samples for analysis of bacterial communities were taken at the end (6 months) of the experiment for each of the triplicate of each treatment. DNA was extracted from approximately 0.15 g (fresh weight) sub-samples of soil using the FastDNAÒ
SPIN Kit for Soil (Qbiogene, Carlsbad, CA, USA). The extracted DNA was cleaned with the Wizard DNA clean-up system (Promega, Madison, WI, USA).
Bacterial 16S rRNA gene fragments were amplified from DNA extracts in 50
m
l reactions containing 400 nM general eubacterial 357F-GC/518R primers (Muyzer et al., 1993), 0.2 mM dNTPs, 10m
g BSA, 2.5 units Taq DNA polymerase, the buffer conditions recom-mended by the manufacturer, and 5m
l template. The reaction conditions were 4 min at 94C followed by 35 cycles of 30 s at 94C, 1 min at 54C, 1 min at 72C, and 5 min at 72C for the last cycle. DGGE (BIO-RAD DcodeÔsystems, Hercules, California, USA) of 16S rRNA gene fragments was performed using polyacrylamide gel with a gradient of 30–55% denaturant and run for 4 h at 200 V in 1 TAE buffer at a constant temperature of 60C. DNA was visualised after SYBR Gold (Molecular Probes) staining by UV transilluminating and photographed with a digital camera. To aid statistical analysis of gels, a marker was added on the outsides and in the middle of the gels (Ro¨ling et al., 2001).2.4. Data analysis
[image:2.595.302.553.111.267.2]A general linear model with type IV sums of squares (Shaw and Mitchell-Olds, 1993) was used to test the effects of irradiation, site of origin and inoculation on the initial NH4þ-N and NO3-N concentrations and pH values using the software SPSS 11.5. This type of analysis allows for unbalanced design, which is necessary since there were ten treatment combinations (five levels of treat-ment factor inoculation and two levels of treattreat-ment factor site of origin) applied to sterilised soil while there were six treatment combinations (three levels of treatment factor inoculation and two levels of treatment factor site of origin) applied to native soil. When required, variables were log10 transformed to fulfill ANOVA assumptions. Cumulative net mineralisation and nitrification were calculated by subtracting the initial (NH4þþNO3)-N and NO3-N concentrations at the start of the experiment from the (NH4þþNO3)-N and NO3-N concentrations in the soil during the respective incubation period (1, 3 and 6 months).
Table 1
Treatments applied to soils in 6-month incubations. A 10 g fresh weight of native or sterilised soil samples from Schoorl and Wekerom was placed in 250 ml sterile bottles, using aseptic techniques, and amended with different inoculants (1 g fresh soil or 1 ml soil slurry from the same or different site of origin).
Soil condition Site of origin
Schoorl Wekerom
Native soil 1. Control 1. Control
2. Inoculated with 1 g fresh Wekerom soil
2. Inoculated with 1 g fresh Schoorl soil 3. Inoculated with 1 ml fresh
Wekerom soil slurry
3. Inoculated with 1 ml fresh Schoorl soil slurry
Sterilised soil 1. Control 1. Control
2. Inoculated with 1 g fresh Wekerom soil
2. Inoculated with 1 g fresh Schoorl soil 3. Inoculated with 1 ml fresh
Wekerom soil slurry
3. Inoculated with 1 ml fresh Schoorl soil slurry 4. Inoculated with 1 g fresh
Schoorl soil
4. Inoculated with 1 g fresh Wekerom soil 5. Inoculated with 1 ml fresh
Schoorl soil slurry
DGGE gel images were converted, normalized and analysed with the GelCompar 4.0 software package (Applied Maths, Kortrijk, Belgium) per gel. Similarities between lanes were calculated by using the Pearson product moment correlation coefficient derived from the same software package. As each of the triplicate per treatment was run in a separate DGGE, three similarity values were obtained for each comparison of two different treatments. These values were used to test the hypotheses that (i) the origin of inoculum determines the bacterial community in sterilised soil, or (ii) the origin of sterilised soil determines the bacterial community in sterilised soil. These hypotheses were tested by classifying the similarity coefficients into discrete groups, which were subse-quently tested statistically to determine whether their averages were significantly different (van Verseveld and Ro¨ling, 2004). As the similarity data were not normally distributed, a non-parametric analysis (Mann–WhitneyUtest) was performed using Systat 7.0 (SPSS Inc., Chicago, Illinois). Within-group similarity coefficients were assigned to testing variable 1 and between-group similarity coefficients testing variable 2. As an example, for hypothesis (i), all comparisons among sterilised treatments inoculated with the same soil were assigned to testing variable 1 while all similarity coeffi-cients comparing a sterilised soil inoculated with Wekerom soil to a sterilised soil inoculated with Schoorl soil were assigned to testing variable 2.
A composite matrix was created by averaging the DGGE-based similarity values of each pair of treatments being compared. The composite matrix was clustered with the unweighted pair-group averages (UPGMA) algorithm using PAST program (Hammer et al., 2001). The variability of DGGE profiles between replicates and the variability of relationships between treatments were calculated using coefficient of variation (CV) as standard deviation divided by the mean of the DGGE-based similarity values of all replicates and each pair of treatments.
3. Results
3.1. Initial NH4þ-N and NO3-N concentrations and soil pH
In our experiments, 25 kGy
g
-irradiation significantly (P<0.05) increased initial NH4þ-N concentration in Schoorl soil from 58.9m
g g1 to 124.4m
g g1, but did not affect the initial NH4þ-N concentration in Wekerom soil significantly (from 120.2m
g g1to 129.8m
g g1). The initial NO3-N concentration in Schoorl soil was not affected byg
-irradiation, but declined (P<0.05) from 43.6m
g g1to 8.1m
g g1in Wekerom soil (Table 2). Initial soil pH levels in the Schoorl and Wekerom soils were not affected byg
-irradiation (Table 2).Inoculations of the Schoorl and Wekerom soils with soil or soil slurry from the same site or the other site did not have a significant
effect on the initial NH4þ-N concentrations and initial pH values (Table 2). On the other hand, initial NO3-N concentrations for the Schoorl soils’ inoculation with Wekerom soil or soil slurry signifi-cantly (P<0.05) increased from 0.1 to 4.3 and 2.8
m
g g1, respec-tively (Table 2). Calculations (data not shown) revealed that these higher nitrate concentrations can be fully attributed to the fact that the initial NO3-N concentrations in native Wekerom soils are much higher than in Schoorl soils, and are introduced by the amendment of the Schoorl soil with native Wekerom soil. Because of the effects ofg
-irradiation and cross-inoculation on initial NH4þ-N and NO3-N concentrations, these values were subtracted from all subsequent measurements, in order to be able to compare the results of the different treatments.3.2. Effects of
g
-irradiation and inoculants on cumulative netmineralisation and nitrification in Schoorl soil
Cumulative net mineralisation over the 6-month period in native Schoorl control soil was 149.0
m
g (NH4þþNO3)-N g1dry soil (Fig. 1a). When this soil was inoculated with Wekerom soil or soil slurry, the cumulative net mineralisation was indistinguish-able from the native control soil (182.3 and 149.8m
g (NH4þþNO3 )-N g1dry soil, respectively (Fig. 1a)). Mineralisation of ammonia occurred during the first three months ing
-irradiated Schoorl control soil, the cumulative net mineralisation was lower (P<0.05)than in native Schoorl control soil, on average 93.8
m
g(NH4þþNO3)-N g1dry soil (Fig. 1b). When this soil was inoculated with soil or soil slurry from the same site of origin, this cumulative net mineralisation increased significantly (P<0.05) to 212.3 and 203.4
m
g (NH4þþNO3)-N g1 dry soil, respectively (Fig. 1b). The cumulative net mineralisation ing
-irradiated Schoorl soil inocu-lated with Wekerom soil or soil slurry was also significantly (P<0.05) higher (221.1 and 206.1m
g (NH4þþNO3)-N g1dry soil, respectively) than in theg
-irradiated Schoorl control soil (Fig. 1b) and comparable to the cumulative net mineralisation in native Schoorl control soil.Cumulative net nitrification in native Schoorl control soil was relatively low, less than 0.5
m
g NO3-N g1dry soil produced over the 6-month period (Fig. 1c). Inoculating the native soil with Wekerom soil or soil slurry significantly (P<0.05) and immediately increased nitrate concentrations, leading to the production of 33.9 and 16.3m
g NO3-N g1dry soil in six months, respectively (Fig. 1c). The cumulative net nitrification ing
-irradiated Schoorl control soil was comparable to the native Schoorl control soil (Fig. 1d). Inocu-lating thisg
-irradiated soil with Schoorl soil or soil slurry did not have significant effects on net nitrification, while inoculating this soil with Wekerom soil or soil slurry again resulted in significantly (P<0.05) higher concentrations of nitrate, 27.2 and 10.1m
g NO3 -N g1dry soil, respectively, after six months (Fig. 1d).Table 2
Initial NH4þ-N and NO3-N concentrations and pH values in Schoorl and Wekerom soils. Standard errors of the mean are shown in brackets,n¼3.
Soil condition Inoculum Schoorl Wekerom
NH4þ-N (mg g1) NO3-N (mg g1) pHKCl NH4þ-N (mg g1) NO3-N (mg g1) pHKCl
Native soil Control 58.9 (0.2) 0.3 (0.0) 2.8 (0.0) 120.2 (12.0) 43.6 (6.0) 2.9 (0.0)
Soil, allo-inoculateda 71.2 (0.9) 4.9 (0.7) 2.8 (0.0) 122.3 (9.7) 44.1 (4.6) 2.8 (0.0)
Soil slurry, allo-inoculateda 61.3 (0.3) 3.3 (0.2) 2.8 (0.0) 115.2 (9.5) 44.6 (5.9) 2.9 (0.0)
Sterilised soil Control 124.4 (2.4) 0.1 (0.0) 2.8 (0.0) 129.8 (1.2) 8.1 (0.5) 2.7 (0.0)
Soil, allo-inoculateda 138.3 (4.5) 4.3 (0.6) 2.8 (0.1) 136.9 (1.4) 8.2 (0.5) 2.7 (0.0)
Soil slurry, allo-inoculateda 23.0 (2.8) 2.8 (0.2) 2.9 (0.1) 134.0 (3.0) 8.5 (0.6) 2.7 (0.0)
Soil, auto-inoculatedb 127.6 (5.9) 0.2 (0.1) 2.8 (0.1) 149.2 (3.7) 11.4 (0.7) 2.7 (0.0)
Soil slurry, auto-inoculatedb 127.9 (5.6) 0.5 (0.0) 2.8 (0.1) 138.9 (0.5) 9.5 (0.2) 2.7 (0.0)
aSoils were inoculated with soil or soil slurry from the other location sampled in this study, sterilised or native Schoorl soil was inoculated with Wekerom soil or soil slurry,
while sterilised or native Wekerom soil was inoculated with Schoorl soil or soil slurry.
b Soils were inoculated with soil or soil slurry from the same site of origin, sterilised Schoorl soil was inoculated with Schoorl soil or soil slurry, while sterilised Wekerom soil
3.3. Effects of
g
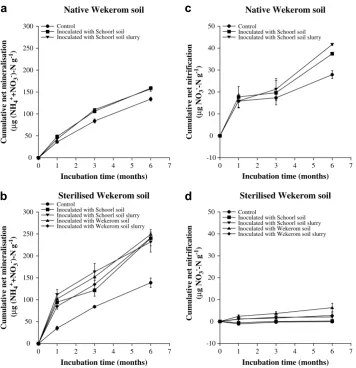
-irradiation and inoculants on cumulative net mineralisation and nitrification in Wekerom soilCumulative net mineralisation in native Wekerom control soil was 133.9
m
g (NH4þþNO3)-N g1 dry soil in six months (Fig. 2a). Inoculation with Schoorl soil or soil slurry significantly (P<0.05) increased the cumulative net mineralisation to 158.8 and 157.1m
g (NH4þþNO3)-N g1dry soil, respectively (Fig. 2a). The cumulative net mineralisation ing
-irradiated Wekerom control soil was 138.7m
g (NH4þþNO3)-N g1dry soil (Fig. 2b), comparable to native Wekerom control soil. When this soil was inoculated with Wekerom soil or soil slurry, the cumulative net mineralisation increased significantly (P<0.05) to 249.3 and 241.3m
g (NH4þþNO3)-N g1dry soil, respectively (Fig. 2b). The cumulative net mineralisation ing
-irradiated Wekerom soil inoculated with Schoorl soil or slurry also increased significantly (P<0.05) to 240.3 and 231.6m
g (NH4þþNO3)-N g1dry soil, respectively (Fig. 2b).Cumulative net nitrification in native Wekerom control soil was 27.9
m
g NO3-N g1 dry soil (Fig. 2c). Inoculating this soil with Schoorl soil or soil slurry significantly (P<0.05) increased cumu-lative net nitrification to 37.4 and 41.7m
g NO3-N g1 dry soil,respectively (Fig. 2c). The cumulative net nitrification in
g
-irradi-ated Wekerom control soil was relatively low, less than 0.5m
g NO3 -N g1dry soil (Fig. 2d). Inoculating this soil with soil from Wekerom significantly (P<0.05) increased cumulative net nitrification to 6.4m
g NO3-N g1dry soil, while inoculating this soil with soil slurry from Wekerom, or soil and soil slurry from Schoorl did not have significant effects on the cumulative net nitrification (Fig. 2d).The effects of
g
-irradiation and inoculation of Schoorl and Wekerom soils on soil pH values were not significant. Soil pH values increased slightly from 2.8 to 3.0 within three months in all microcosms, irrespective of whether they were sterilised or not, and stayed the same till the end of the experiment (data not shown).3.4. Effects of
g
-irradiation and inoculants on general bacterialcommunity profiles in Schoorl and Wekerom soils
Bacteria-specific PCR-DGGE fingerprinting for each of the trip-licates per treatment revealed very reproducible profiles, with the exception of the uninoculated sterilised soils which showed more variation (Fig. 3). The variability of DGGE profiles between 0 50 100 150 200 250 300
0 1 2 3 4 5 6 7
Control
Inoculated with Wekerom soil Inoculated with Wekerom soil slurry
Control
Inoculated with Wekerom soil Inoculated with Wekerom soil slurry
Incubation time (months) Incubation time (months)
C u m u la ti v e n e t m in e ra li s a ti o n ( µ g (N H4 ++N O3 -)-N g -1) C u m u la ti v e n e t m in e ra li s a ti o n ( µ g (N H4 ++N O3 -)-N g -1)
Native Schoorl soil
Native Schoorl soil
0 50 100 150 200 250 300
0 1 2 3 4 5 6 7
Control
Inoculated with Wekerom soil Inoculated with Wekerom soil slurry Inoculated with Schoorl soil Inoculated with Schoorl soil slurry
Control
Inoculated with Wekerom soil Inoculated with Wekerom soil slurry Inoculated with Schoorl soil Inoculated with Schoorl soil slurry
Incubation time (months) Incubation time (months)
Sterilised Schoorl soil
Sterilised Schoorl soil
-10 0 10 20 30 40 50
0 1 2 3 4 5 6 7
C u m u la ti v e n e t n itr ifi c a ti o n ( µ g N O3 --N g -1) C u m u la ti v e n e t n itr ifi c a ti o n ( µ g N O3 --N g -1) -10 0 10 20 30 40 50
0 1 2 3 4 5 6 7
a
b
c
d
replicates and relationships between treatments was low; the coefficient of variance (CV) in the percentage of similarity in
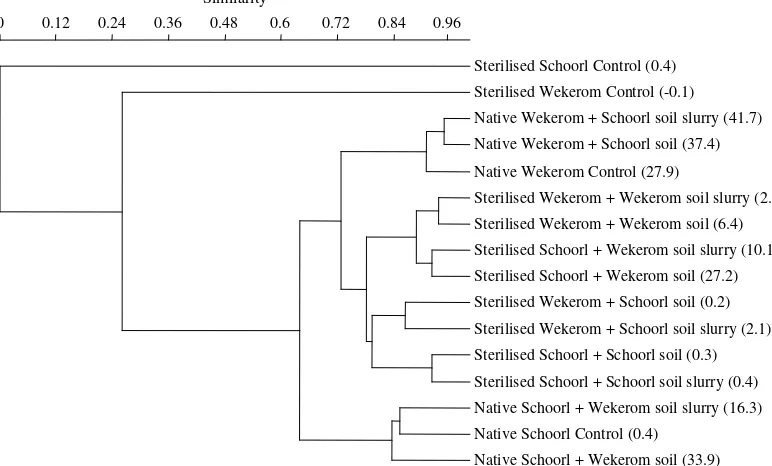
community profiles was around 10%. Cluster analysis of
a composite matrix, containing an averaged similarity value per comparison of two treatments, revealed that bacterial community structure at the end of the experiment (6 months) revealed a grouping based on the combination of origin of the soil and type of treatment (Fig. 4). Sterilisation plus subsequent inoculation had pronounced impacts on the bacterial community structure in both
soils, in comparison to the native soils (Figs. 3 and 4). The community fingerprints of sterilised Schoorl and Wekerom control soils, that did not receive an inoculum, differed most strongly (similarity of<26%) compared to all other treatments (Fig. 4). They revealed a low diversity, when judged in terms of visible DGGE bands (<6 bands,Fig. 3). The inoculated
g
-irradiated Schoorl and Wekerom soils generally clustered together, separately from native Schoorl and Wekerom soils (Fig. 4). For the sterile soils, the final community composition was significantly (Mann–WhitneyUtest0 50 100 150 200 250 300
0 1 2 3 4 5 6 7
0 1 2 3 4 5 6 7
Control
Inoculated with Schoorl soil Inoculated with Schoorl soil slurry
Control
Inoculated with Schoorl soil Inoculated with Schoorl soil slurry
Incubation time (months) Incubation time (months)
Incubation time (months) Incubation time (months)
Cumulative net mineralisation
(
µ
g (NH
4
++NO
3
-)-N g -1)
Cumulative net mineralisation
(
µ
g (NH
4
++NO
3
-)-N g -1)
Native Wekerom soil
Native Wekerom soil
0 50 100 150 200 250 300
Control
Inoculated with Schoorl soil Inoculated with Schoorl soil slurry Inoculated with Wekerom soil Inoculated with Wekerom soil slurry
Control
Inoculated with Schoorl soil Inoculated with Schoorl soil slurry Inoculated with Wekerom soil Inoculated with Wekerom soil slurry
Sterilised Wekerom soil
Sterilised Wekerom soil
-10 0 10 20 30 40 50
Cumulative net nitrification
(
µ
g NO
3
--N g -1)
Cumulative net nitrification
(
µ
g NO
3
--N g -1)
-10 0 10 20 30 40 50
0 1 2 3 4 5 6 7
0 1 2 3 4 5 6 7
a
b
c
[image:5.595.126.483.65.434.2]d
Fig. 2.Cumulative net mineralisation (a and b) and nitrification (c and d) in native (a and c) and sterilised Wekerom soils (b and d). Bars show standard errors of the mean,n¼3.
[image:5.595.136.468.581.705.2]on group similarity values,P<0.01) related to the origin of the inoculum and not (P>0.05) to the origin of the inoculated soil: community fingerprints of sterilised Schoorl and Wekerom soils inoculated with Wekerom soil or soil slurry clustered at 88% simi-larity and were clearly different from community fingerprints of the soils inoculated with Schoorl soil or soil slurry (Fig. 4). In contrast, the bacterial fingerprints of inoculated native soils clus-tered were determined by the origin of the soils and not on inoc-ulum. Bacterial communities in native Wekerom soils inoculated with Schoorl soil or soil slurry clustered with the native Wekerom control soil (similarity of 91%), while native Schoorl soils inoculated with Wekerom soil or soil slurry grouped at 84% with the native Schoorl soil (Fig. 4). A clear difference (clustering at less than 40%) was observed between the communities in native Schoorl and Wekerom soils.
4. Discussion
The effect of biotic factors on nitrification was studied by inoc-ulation of native and
g
-irradiated forest soils with soils or soil slurries from a forest with contrasting nitrifying activity. Augmentation of the non-nitrifying Schoorl soil with a small amount of Wekerom nitrifying soil or soil slurry induced nitrifica-tion. To manipulate soil microbial community, inoculation of native andg
-sterilised soils with soils or soil slurries is more appropriate than the methods of, for example,Bell et al. (2005)andGriffiths et al. (2000).Bell et al. (2005)sterilised soil and then re-inoculated it with increasing numbers of cultured species. Griffiths et al. (2000)reduced diversity by increasing the duration of fumigation. The former method has its limitations because only culturable microorganisms can be added to the soil, while the latter approach may select for species resistant to fumigation. It should be realised that no method is without disadvantages; inoculation in sterilised soils will likely select for organisms which are able to grow rapidly and which are good dispersers or only loosely attached to the inoculated soil particles.For soil sterilisation
g
-irradiation is recommended over other methods and widely used (e.g.Thompson, 1990; Griffiths et al.,2004; Jones et al., 2004); it causes minimum alteration in physical properties with minor effects on soil chemical properties such as soil pH (McNamara et al., 2003). Liming treatments to increase pH were previously found to induce nitrification in initially non-nitrifying Schoorl soils (Nugroho et al., 2007). However, the original pH values in Wekerom and Schoorl soils were comparable and
g
-irradiation did not change these pH values in our study. Therefore, we can exclude a change in pH due tog
-irradiation or cross-inoc-ulation as a factor inducing nitrification in Schoorl soils.Unexpectedly, toxic effects due to the sterilisation process may have inhibited the nitrification process in Wekerom soil, but not in the Schoorl soil. In the sterilised Wekerom soil, inoculated with soil slurry or soil from Wekerom, the amount of nitrification was clearly much less than in native Wekerom soils or sterilised Schoorl soil inoculated with soil slurry or soil from Wekerom.McLaren (1969) did not observe toxicity following soil irradiation. However, it should be noted that our most important experiment is in fact on the inoculations of the Schoorl soils (previously non-nitrifying) with Wekerom inoculum. In that case no toxicity is indeed observed. Furthermore, even though inhibition is observed in the Wekerom soil inoculated with Wekerom soil or soil slurry, clearly nitrification is occurring and thus it serves as a reasonable control versus sterilised Wekerom soil inoculated with Schoorl soil or soil slurry. The general bacterial community structure after 6 months of incubation of the sterilised soils was not comparable to the general bacterial community structure in the original soils. Using reciprocal transfers of soils between a grassland and coniferous forest ecosystem over a 2-year period,Balser and Firestone (2005)also found that microbial community composition may not equilibrate to an environmental change as readily or as rapidly as it has been often assumed.
g
-Irradiation of soils in general leads to an overall increase in NH4þ-N, while in almost all instances NO3-N declined afterg
-irra-diation (McNamara et al., 2003). We made similar observations, with the strongest increase in NH4þ-N content for the Schoorl soil. This increased NH4þ-N content cannot explain the onset of nitrifi-cation in sterilised Schoorl soils after inoculation with nitrifying Wekerom soil or soil slurries: in a previous study amending acidic, 0 0.12 0.24 0.36 0.48 0.6 0.72 0.84 0.96Similarity
Sterilised Schoorl Control (0.4)
Sterilised Wekerom Control (-0.1)
Native Wekerom + Schoorl soil slurry (41.7)
Native Wekerom + Schoorl soil (37.4)
Native Wekerom Control (27.9)
Sterilised Wekerom + Wekerom soil slurry (2.7)
Sterilised Wekerom + Wekerom soil (6.4)
Sterilised Schoorl + Wekerom soil slurry (10.1)
Sterilised Schoorl + Wekerom soil (27.2)
Sterilised Wekerom + Schoorl soil (0.2)
Sterilised Wekerom + Schoorl soil slurry (2.1)
Sterilised Schoorl + Schoorl soil (0.3)
Sterilised Schoorl + Schoorl soil slurry (0.4)
Native Schoorl + Wekerom soil slurry (16.3)
Native Schoorl Control (0.4)
[image:6.595.98.484.72.305.2]Native Schoorl + Wekerom soil (33.9)
non-nitrifying Schoorl soils with ammonium did not lead to nitri-fication (Nugroho et al., 2007). Net NH4þ-N production still occurred during the six-month incubation period in
g
-irradiated control soils. This might be due to the activity of proteolytic enzymes which can resistg
-irradiation (McNamara et al., 2003), or the survival and re-growth of some bacteria after sterilisation. Low-diversity bacterial communities, whose composition varied between replicates, were present in theg
-irradiated control soils (Fig. 3, data not shown). Observing changes in NO3-N concentrations was the main interest in our study, and nitrification was absent in any of the sterilised control soils. Therefore, we assume that methodological issues relating tog
-irradiation do not interfere strongly with drawing conclusions from our observations, also since the results on steri-lised soils are in line with those on native soils, as discussed below. Our results, taken together with our previous study (Nugroho et al., 2007), demonstrate that low nitrification rates in acid Scots pine forest soils cannot solely be explained by unfavourable abiotic soil conditions, such as C/N ratios and pH related factors other than the effect of pH on ammonia availability (Nugroho et al., 2007). The present study showed that the onset of nitrification was observed after native or sterilised Schoorl soil, which in its native state hardly showed nitrification, was inoculated with fresh soil or soil slurry from the nitrifying Wekerom location. The cumulative net nitrifi-cation in the augmented Schoorl soils was comparable to or even higher than those in native Wekerom soils. This indicates an enhanced growth and activity of ammonia oxidizers upon inocu-lation, since 10% (w/w) of fresh Wekerom soil or soil slurry was inoculated into Schoorl soils and the nitrification rates were much higher than just 10% of the rate found in native Wekerom soil. Our result is in contrast to another study where nitrification in native wet tropical sugarcane soils that exhibited high NH4þ-N concen-trations relative to NO3-N in the field was not significantly affected by inoculation with subtropical nitrifying soils in which mineral N in the field was dominated by NO3-N, although the properties of the soils clearly did not limit nitrification by wet tropical nitrifier populations (Meier et al., 2006). Support for a limited role of abiotic factors in the occurrence of nitrification and in the development of the structure of bacterial communities also comes from our experiments on the sterilised soils. Here, the occurrence of nitrifi-cation and community structure were determined mainly by the inoculum and not by the origin of the soil that was inoculated.Nugroho et al. (2007)suggested that pH related factors, other than the effect of pH on ammonia availability, had negative effects on the growth of AOB and their associative nitrifying activity in non-nitrifying soils. The results ofNugroho et al. (2007)and our results may appear to contradict each other however two factors determining occurrence of nitrification may simultaneously be active, or even relate to each other. For example, microorganisms transferred from Wekerom soil might be able to destroy the pH related factors in Schoorl soil, while the indigenous Schoorl community is not capable to do so. The pH related factor might in fact also be pH-sensitive microorganisms themselves that inhibit growth and activity of nitrifiers.
The onset of nitrification in Schoorl soils upon amendment with Wekerom soils or slurries is not simply due to the introduction of nitrifiers into Schoorl soil. AOB are known to be present in Schoorl soils; after these soils were limed to increase pH, nitrification occurred and AOB could be shown to be present by molecular detection ofamoAgenes (Nugroho et al., 2007). Thus, the fact that AOB are inoculated from Wekerom soil is not sufficient to explain the occurrence of nitrification as they were already present at very low levels. These AOB belonged toNitrosospiracluster II, similar AOB were observed for the Wekerom soils with high nitrifying activity. The AOB community in Wekerom is both spatially and temporally very stable (Laverman et al., 2001, 2005; Nugroho et al., 2005, 2007). The occurrence of nitrification in native Schoorl soils
upon augmentation with Wekerom soil or soil slurries appears to falsify our hypothesis that interactions within the non-nitrifying Schoorl bacterial community prevent the onset of the growth and substantial activity of AOB in Schoorl soils. However, we cannot exclude the possibility that diversity withinNitrosospiracluster II affects the interaction of cluster II type members with the non-nitrifying community members in Schoorl soil. For example, the
Nitrosospira indigenous to Schoorl might be sensitive for an
inhibitory compound produced by the Schoorl non-nitrifying microbial community while the Wekerom-derived Nitrosospira cluster II members are resistant for it. Despite the occurrence of nitrification the general bacterial community structure did not change to become similar to that of Wekerom.
Thus, nitrification can be induced in non-nitrifying soils by augmentation with soils or soil slurries from nitrifying forests. In our experiments, the reverse was not observed: when Wekerom soil was inoculated with Schoorl soils or soil slurries, nitrification was not inhibited. This indicates that abiotic factors or microorganisms that can inhibit nitrification could not establish themselves in the Wekerom soils or were not even present in the non-nitrifying Schoorl soil in the first place. In fact, we found significant higher cumulative net nitrification when native Wekerom soil was inocu-lated with Schoorl soil or soil slurry (>37.0
m
g g1dry soil month1) than in the native Wekerom control (27.9m
g g1dry soil month1), while the bacterial community structure remained highly similar to that in the native Wekerom control. Overall, this study indicates that biotic factors contribute to why some acidic Scots pine forest soils nitrify and some do not.Recent research has revealed that ammonia-oxidising Archaea (AOA) are widespread, abundant and active in grassland soil (Treusch et al., 2005) and in pristine and agricultural soils, with pH ranging from 5.5 to 7.3 (Leininger et al., 2006) thus in soils with higher pHs than the ones studied here. The assumption that AOA are important under extreme conditions such as acidic conditions (Valentine, 2007) is not supported by the absence of nitrification in the acidic Schoorl soil, which suggests the absence or low abun-dance of AOA, like we also observed for AOB (Nugroho et al., 2005, 2007). However, even if AOA were to be detected in acid soils, their presence cannot offer an explanation on why some forest soils nitrify, while others do not. AOB were detected in all forest soils that revealed high nitrification rates, while in forest soils with low nitrification AOB were not detectable (Nugroho et al., 2005). It might therefore be that the same factors that affect AOB and their nitrifying activity also affect AOA.
Acknowledgements
The authors gratefully acknowledge Frans Kuenen and Rudo Verweij for field assistance.
References
Balser, T.C., Firestone, M.K., 2005. Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 73, 395–415.
Ba¨ckman, J.S.K., Hermansson, A., Tebbe, C.C., Lindgren, P.-E., 2003. Liming induced growth of a diverse flora of ammonia-oxidising bacteria in acid spruce forest soil as determined by SSCP and DGGE. Soil Biology & Biochemistry 35, 1337–1347.
Bell, T., Newman, J.A., Silverman, B.W., Turner, S.L., Lilley, A.K., 2005. The contri-bution of species richness and composition to bacterial services. Nature 436, 1157–1160.
Compton, J.E., Watrud, L.S., Porteous, L.A., DeGrood, S., 2004. Response of soil microbial biomass and community composition to chronic nitrogen additions at Harvard forest. Forest Ecology and Management 196, 143–158.
de Boer, W., Gunnewiek, P.J.A.K., Parkinson, D., 1996. Variability of N mineralization and nitrification in a simple, simulated microbial forest soil community. Soil Biology & Biochemistry 28, 203–211.
de Boer, W., Kester, R.A., 1996. Variability of nitrification potentials in patches of undergrowth vegetation in primary Scots pine stands. Forest Ecology and Management 86, 97–103.
Griffiths, B.S., Kuan, H.L., Ritz, K., Glover, L.A., McCaig, A.E., Fenwick, C., 2004. The relationship between microbial community structure and functional stability, tested experimentally in an upland pasture soil. Microbial Ecology 47, 104–113.
Griffiths, B.S., Ritz, K., Bardgett, R.D., Cook, R., Ekelund, F., Sørensen, S.J., Bååth, E., Bloem, J., de Ruiter, P.C., Dolfing, J., Nicolardot, B., 2000. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: an examination of the biodiversity–ecosystem function relationship. Oikos 90, 279–294.
Hammer, Ø., Harper, D.A.T., Ryan, P.D., 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4 (1), 9. Jones, H.E., West, H.M., Chamberlain, P.M., Parekh, N.R., Beresford, N.A., Crout, N.M.J.,
2004. Effects of gamma irradiation onHolcus lanatus(Yorkshire fog grass) and associated soil microorganisms. Journal of Environmental Radioactivity 74, 57–71.
Laverman, A.M., Braster, M., Ro¨ling, W.F.M., van Verseveld, H.W., 2005. Bacterial community structure and metabolic profiles in a forest soil exhibiting spatially variable net nitrate production. Soil Biology & Biochemistry 37, 1581–1588.
Laverman, A.M., Speksnijder, A.G.C.L., Braster, M., Kowalchuk, G.A., Verhoef, H.A., van Verseveld, H.W., 2001. Spatiotemporal stability on an ammonia-oxidizing community in a nitrogen-saturated forest soil. Microbial Ecology 42, 35–45. Laverman, A.M., Zoomer, H.R., van Verseveld, H.W., Verhoef, H.A., 2000. Temporal
and spatial variation of nitrogen transformations in a coniferous forest soil. Soil Biology & Biochemistry 32, 1661–1670.
Leininger, S., Urich, T., Schloter, M., Schwark, L., Qi, J., Nicol, G.W., Prosser, J.I., Schuster, S.C., Schleper, C., 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442, 806–809.
Marschner, P., Rumberger, A., 2004. Rapid changes in the rhizosphere bacterial community structure during re-colonization of sterilized soil. Biology and Fertility of Soils 40, 1–6.
McLaren, A.D., 1969. Radiation as a technique in soil biology and biochemistry. Soil Biology & Biochemistry 1, 63–73.
McNamara, N.P., Black, H.I.J., Beresford, N.A., Parekh, N.R., 2003. Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Applied Soil Ecology 24, 117–132.
Meier, E.A., Thorburn, P.J., Probert, M.E., 2006. Occurrence and simulation of nitri-fication in two contrasting sugarcane soils from the Australian wet tropics. Australian Journal of Soil Research 44, 1–9.
Muyzer, G., de Waal, E.C., Uitterlinden, A.G., 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and Environ-mental Microbiology 59, 695–700.
Nugroho, R.A., Ro¨ling, W.F.M., Laverman, A.M., Verhoef, H.A., 2005. Presence of Nitrosospiracluster 2 bacteria corresponds to N transformation rates in nine acid Scots pine forest soils. FEMS Microbiology Ecology 53, 472–481. Nugroho, R.A., Ro¨ling, W.F.M., Laverman, A.M., Verhoef, H.A., 2007. Low nitrification
rates in acid Scots pine forest soils are due to pH-related factors. Microbial Ecology 53, 89–97.
Persson, T., Wire´n, A., 1995. Nitrogen mineralization and potential nitrification at different depths in acid forest soil. Plant and Soil 168/169, 55–65.
Ro¨ling, W.F.M., van Breukelen, B.M., Braster, M., Lin, B., van Verseveld, H.W., 2001. Relationships between microbial community structure and hydrochemistry in a landfill leachate-polluted aquifer. Applied and Environmental Microbiology 67, 4619–4629.
Shaw, R.G., Mitchell-Olds, T., 1993. ANOVA for unbalanced data: an overview. Ecology 74, 1638–1645.
Thompson, J.P., 1990. Soil sterilization methods to show VA-mycorrhizae aid P and Zn nutrition of wheat in vertisols. Soil Biology & Biochemistry 22, 229–240. Tolli, J., King, G.M., 2005. Diversity and structure of bacterial chemolithotrophic
communities in pine forest and agroecosystem soils. Applied and Environ-mental Microbiology 71, 8411–8418.
Treusch, A.H., Leininger, S., Kletzin, A., Schuster, S.C., Klenk, H.-P., Schleper, C., 2005. Novel genes for nitrite reductase andAmo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environmental Microbiology 7, 1985–1995.
Valentine, D.L., 2007. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nature Reviews Microbiology 5, 316–323.
van Niel, E.W.J., Arts, P.A.M., Wesselink, B.J., Robertson, L.A., Kuenen, J.G., 1993. Competition between heterotrophic and autotrophic nitrifiers for ammonia in chemostat cultures. FEMS Microbiology Ecology 102, 109–118.
van Verseveld, H.W., Ro¨ling, W.F.M., 2004. Cluster analysis and statistical compar-ison of molecular community profile data. In: Kowalchuk, G.A., de Bruijn, F.J., Head, J.M., Akkermans, A.D.L., van Elsas, J.D. (Eds.)Molecular Microbial Ecology Manual, second ed., vol. 1.7.4, pp. 1–24.
Verhagen, F.J.M., Duyts, H., Laanbroek, H.J., 1992. Competition for ammonium between nitrifying and heterotrophic bacteria in continuously percolated soil columns. Applied and Environmental Microbiology 58, 3303–3311.
Verhagen, F.J.M., Laanbroek, H.J., 1991. Competition for ammonium between nitri-fying and heterotrophic bacteria in dual energy-limited chemostats. Applied and Environmental Microbiology 57, 3255–3263.
Verhagen, F.J.M., Laanbroek, H.J., Woldendorp, J.W., 1995. Competition for ammo-nium between plant roots and nitrifying and heterotrophic bacteria and the effects of protozoan grazing. Plant and Soil 170, 241–250.