Foreign proteins synthesised by plants are now in the marketplace, and clinical trials for plant-derived therapeutic proteins are underway. Economic analysis of plant production systems has helped identify the types of protein that would be most suitable for manufacture using tissue culture methods. The major advantages associated with in vitroplant systems include the ability to manipulate environmental conditions for better control over protein levels and quality, the rapidity of production compared with agriculture, and the use of simpler and cheaper downstream processing schemes for product recovery from the culture medium.
Addresses
Department of Biotechnology, University of New South Wales, Sydney, NSW 2052, Australia; e-mail: [email protected]
Current Opinion in Biotechnology2000, 11:199–204 0958-1669/00/$ — see front matter
© 2000 Elsevier Science Ltd. All rights reserved.
Abbreviations
ER endoplasmic reticulum
GMP Good Manufacturing Practice
Introduction
Foreign proteins can be synthesised using microbial cell culture, animal cell culture, plant tissue culture, transgenic animals and transgenic plants. Choosing the most appro-priate method for commercial production requires case-by-case analysis. A wide range of factors must be con-sidered, including cost of production, market volume, the efficacy, safety and stability of the product, and whether the foreign protein has different biochemical or pharmaco-logical properties compared with material from existing sources. Although most interest during the past 15–20 years has been focused on microbial and animal cell cul-tures, plants and plant cells are now considered as viable and competitive expression systems for large-scale protein production. The development of transgenic plants and plant viral vectors for foreign protein synthesis has been reviewed extensively [1•,2–6].
Factors in favour of plant systems as sources of animal-derived proteins include: the potential for large-scale, low-cost biomass production using agriculture; the low risk of product contamination by mammalian viruses, blood-borne pathogens, oncogenes and bacterial toxins; the capacity of plant cells to correctly fold and assemble mul-timeric proteins; low downstream processing requirements for proteins administered orally in plant food or feed; the ability to introduce new or multiple transgenes by sexual crossing of plants; and the avoidance of ethical problems associated with transgenic animals and the use of animal materials. There are, however, potential issues of concern for plant protein production: containment of genetically
modified plants in the environment; allergic reactions to plant protein glycans and other plant antigens; product contamination by mycotoxins, pesticides, herbicides and endogenous plant secondary metabolites; and regulatory uncertainty, particularly for proteins requiring approval for human drug use.
An important indicator of the cost competitiveness of plant-based foreign protein synthesis is the increasing involvement of industrial groups. Commercial processes for the production of avidin, β-glucuronidase and aprotinin from transgenic corn have already been developed [7,8•,9•]. Plant-derived non-injectable immunotherapeutic
and vaccine proteins are also progressing through human clinical trials; these include a secretory IgA/G antibody against oral bacterial colonisation [10•], an edible vaccine
against an Escherichia colienterotoxin [11•] and an edible
vaccine against hepatitis B [12•]. Details of company
activ-ity in selected areas, including plant production of monoclonal antibodies, vaccines and industrial enzymes, are provided in recent reviews [2,3,5,13••].
An alternative but less well-developed technology for pro-ducing foreign proteins is plant tissue culture. Using this approach, plant cells in differentiated or dedifferentiated states are grown axenically in nutrient medium in bioreac-tors under controlled conditions, with foreign protein harvested from either the biomass or culture liquid, or a combination of both. Although plant tissue culture may not be suitable for all applications of foreign protein syn-thesis, such as food-based production of edible vaccines, it offers a number of advantages for the manufacture of pro-teins that are extracted and purified after synthesis. The purpose of this review is to outline the potential advan-tages and limitations associated with plant tissue culture for foreign protein production, and to summarise recent developments in the area. The review focuses on the pro-duction of proteins of direct commercial value. Application of foreign proteins in plants for metabolic modulation or to improve pathogen resistance or nutritional value are dis-cussed elsewhere [14–16].
Recent applications of plant tissue cultures for
production of foreign proteins
Suspended plant cells have been used in several recent studies as a means of producing a variety of foreign pro-teins. These include recombinant antibodies and antibody fragments [17–23], enzymes such as β-glucuronidase [24] and invertase [25], and proteins of therapeutic value such as human interleukin (IL)-2 and IL-4 [26], ribosome-inac-tivating protein [27], ricin [28], and human α1-antitrypsin [29•,30•]. The most commonly used host species for
pro-tein synthesis in suspension cultures is tobacco [17–28], although rice cell cultures have also been tested [29•,30•].
Several stirred bioreactor studies at volumes up to 40 L have been reported, using batch [22,23], continuous [25] and two-stage [29•] modes of operation. Hairy root cultures
of transgenic tobacco have also been tested for production of recombinant IgG1antibody [18,31], including in a 2 L sparged bioreactor [18]. In other work, bacterial xylanase and human placental alkaline phosphatase were produced using hydroponic culture of transgenic tobacco plants in sterile, sucrose-containing medium in flasks [32].
Large-scale plant tissue culture versus
agriculture
For bulk manufacture of proteins such as those used in industrial, analytical and diagnostic applications, most pro-duction systems will find it hard to compete with the low cost of agriculture. A detailed economic evaluation of β-glucuronidase extraction and purification from trans-genic corn yielded a production cost of only US$43 g–1for
an initial seed protein concentration of 0.015% dry weight, a product purity of 83%, and an annual production volume of 137 kg [33••]. Costs of research and development,
royal-ties and sales were not included in the analysis. In comparison, the cost of producing 100 kg of protein from transgenic goats’ milk has been estimated at US$105 g–1,
whereas manufacture using mammalian (Chinese hamster ovary) cell culture costs US$300–3000 g–1 depending on
the product yield and other operating factors [34]. Agricultural production of protein has been reported to be 10–50 times cheaper than E. coli fermentation, even though product levels in bacteria are higher than those in plants [35]. Foreign protein production using greenhouse-cultivated plants is considerably more expensive than with field-grown crops; recent estimates are US$500–600 g–1
based on operations in a 250 m2greenhouse and including
heating, labour and consumables for protein extraction and purification [36].
The cost of transgenic corn seed for extraction of foreign protein has been estimated as only US$0.20 kg–1 [33••].
This includes a premium of US$0.09 kg–1 to provide
incentive to farmers and grain handlers, and to cover any additional costs associated with growing the transgenic plants and keeping them and their seeds isolated from other plants and grain products. Although specific cost analyses for protein production using plant tissue culture have not been provided in the literature, it would be diffi-cult to produce a kilogram of plant biomass in a reactor at such a low price. Despite plant culture medium being sub-stantially cheaper than animal culture medium, the cost of the medium alone for generation of 1kg of plant cells is substantially greater than US$0.20, without factoring in other costs associated with bioreactor operation, such as labour, utilities, waste treatment, equipment depreciation, and so on.
Plant cell cultures are likely to be uncompetitive for the majority of proteins required in the large quantities deliver-able by agricultural production, unless they are deliver-able to
accumulate protein levels at least an order of magnitude higher than those achievable in agricultural systems. This would reduce the costs associated with product recovery to offset the substantially higher cost of biomass production. Plant cell cultures provide greater opportunity for manipu-lation of foreign protein levels, as culture conditions can be altered much more readily in reactors than in the field. For example, supplementation of suspended plant cells with amino acids prior to harvest resulted in a transient threefold increase in recombinant antibody levels [21], while accu-mulation of recombinant ricin in tobacco culture medium was sensitive to the concentration of auxin provided [28]. In other work, exposure of suspended plant cells to dark/light cycles was used to control foreign gene expression and pro-tein titre [24]. Whereas these examples demonstrate how protein production might be enhanced in plant cell cul-tures, some studies have shown that recombinant protein levels are significantly lower in plant cell suspensions than in whole plants or organ cultures such as hairy roots [19,20]. The reasons for this are unknown at present, but could relate more to post-synthesis product stability rather than to expression levels per se. Technology to improve foreign pro-tein accumulation in culture systems must be developed if product levels significantly greater than those achievable in whole plants are required for economic feasibility.
In vitro cell culture offers intrinsic advantages, or could even be a necessity, for foreign protein synthesis in certain situations. Plant tissue culture may be the most suitable production system when small-to-medium quantities of specialty, high-price, high-purity proteins are needed, such as in some therapeutic applications. The time involved for production using plant cell culture is significantly shorter than the growth cycle of whole plants; therefore, compared with the months needed for agriculture, proteins could be manufactured in days or weeks on a time-scale compatible with clinical demands. Because bioreactors provide a much more controlled and reproducible environment than the open field, regulatory requirements for therapeutic pro-teins can be met more easily using reactor-grown cells. In addition, when the protein of interest is secreted into the medium of plant cell cultures, product recovery and purifi-cation could be greatly simplified and much cheaper than from plant biomass, while eliminating the need to destroy the cells. However, if the most probable niche for plant cell cultures is the production of therapeutic proteins, an important issue is post-translational processing of proteins and the effects of plant glycans on the human immune sys-tem. These factors, together with recent developments influencing the choice of plant cell culture as a production vehicle for foreign proteins, are discussed below.
Good Manufacturing Practice, process
reproducibility and product quality
The regulatory issues relevant to Good Manufacturing Practice (GMP) for production of therapeutic proteins in plants have been outlined in recent papers [37,38••].
the production of therapeutic proteins using plants grown in open fields, even though this production system is sub-ject to the vagaries of weather, insect activity, soil variability and other factors that influence product yield and quality (Z Nikolov, personal communication). If, as seems likely, regulatory acceptance of the product will depend mainly on its compliance with purity, efficacy and biocomparability standards at the end of the production chain, field-grown protein will be able to satisfy require-ments if sufficient safeguards are designed into the process so that undesirable components are either prevented from forming or removed during purification. Reactor culture could make regulatory compliance easier by reducing vari-ation in production conditions and increasing average batch quality; whether or not this is a crucial advantage will depend on the properties of the particular system and their susceptibility to environmental influences. Although vari-ation in the level of protein accumulvari-ation has been reported for transgenic plants in different growing loca-tions [7], lot-to-lot consistency of purified plant vaccine proteins and conformity with regulatory specifications have also been demonstrated [39].
Developments in the area of inducible promoters may lead to future alleviation of problems with environment-related variability in field-grown plants by separating the biomass growth and protein synthesis steps. For example, using a recently patented post-harvest expression system [P1], plant tissues harvested from the field can be induced to produce foreign protein during storage in laboratory or GMP facili-ties. Because conditions during actual product synthesis are controlled to a greater extent than is possible with constitu-tive promoters and outdoor plants, better product yield and less variation in product quality can be expected. Such improvements in agriculture-based production systems could reduce the potential benefits associated with plant tis-sue culture with respect to regulatory compliance.
The ability of plant cells and organs to propagate indefinite-ly in tissue culture without the need for sexual reproduction offers a solution to other problems relating to gene segrega-tion and long-term transgene stability in agricultural crops. Variations in foreign protein expression within and between successive generations of corn have been reported [7,9•], as
well as loss of fertility and/or pollen transmission of the trans-gene [7] and loss of the original selection marker [7,9•].
Greater stability may develop with increasing number of generations [9•]; however, it is probable that breeding
pro-grams with continuous selection of elite lines will be necessary for consistent high-level protein production using whole plants. This situation could be improved by the use of perennial host species [36]. In contrast, there are several reports of stable, long-term foreign protein synthesis in plant tissue cultures, such as carrot invertase in tobacco suspen-sions over four years [25] and IgG1antibody in tobacco hairy root cultures over 19 months [18]. Gene silencing occurs in plant tissue cultures as well as in whole plants (EJ Finnegan, personal communication); however, if transmission of signals
for systemic post-transcriptional silencing occurs via plas-modesmata and the vascular system [40], the conditions of minimal cell–cell contact existing in plant suspensions could be advantageous for stable foreign protein production. On the other hand, dedifferentiated plant cells, such as those in suspension cultures, are subject to a range of genetic modifi-cations through the mechanisms of somaclonal variation. Further studies are needed to verify whether better long-term stability of protein production can be achieved in vitro compared with whole-plant systems.
Downstream processing, protein secretion
and stability
Recovery and purification of proteins from plant biomass is an expensive and technically challenging business, requir-ing multiple separation steps such as precipitation, adsorption, chromatography and diafiltration. In a recent cost analysis of β-glucuronidase production from transgenic corn seed, milling, protein extraction and purification oper-ations accounted for ~94% of the production cost [33••]. As
the corn itself contributed only 6%, the importance of down-stream processing in determining the economic feasibility of plant protein production is very clear. Costs of protein recov-ery from seed could be reduced if most of the protein is localised in a separable component such as the embryo [8•,41•], or by targeting protein expression to seed oil bodies
for easier extraction and purification [13••].
Because plant nutrient media are relatively simple solu-tions with no added proteins, if a foreign protein is produced in tissue culture and secreted into the medium rather than stored inside the cells, product recovery and purification could be carried out in the absence of large quantities of contaminating proteins. Economic analysis of protein recovery from spent plant culture medium has not been reported in the literature. Yet, significant cost advan-tages may be derived from this type of production system, despite the protein being diluted to low concentrations in the relatively large liquid volume. Extracellular protein secretion is effected in plant cells using signal peptides that direct transport of peptides into the lumen of the endoplasmic reticulum (ER). After folding and assembly in the ER, unless the protein is carrying a signal that tar-gets it for retention in the ER or transport to another organelle such as the vacuole, proteins leaving the ER enter the default secretory pathway to the Golgi apparatus and finally to the extracellular space [42].
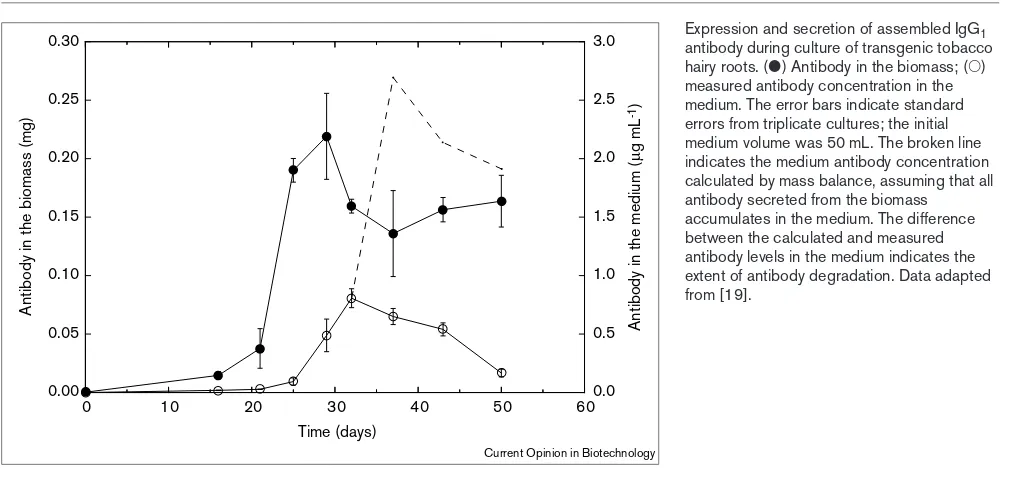
full-length antibodies and antibody heavy chains are degrad-ed in suspenddegrad-ed plant cell and hairy root cultures [17–19]. As indicated in Figure 1, a mass balance of assembled antibody during culture of transgenic tobacco hairy roots shows the extent to which product is lost from the system. Use of pro-tein stabilising agents such as polyvinylpyrrolidone (PVP) improved antibody titres in the culture fluid by up to 35-fold [17,18], suggesting that the medium is the site of antibody degradation. Application of bacitracin, a broad-spectrum pro-tease inhibitor, did not prevent antibody loss [19]. Further work is required to identify the mechanisms of extracellular antibody instability in plant culture medium. In other stud-ies, degradation of human α1-antitrypsin in the medium of transgenic rice cultures was attributed to protease release from disrupted cells; adjusting the medium osmotic pressure inhibited cell disruption and improved the active protein titre [30•]. Although secreted product levels of up to 3% of
total secreted protein have been reported in plant cultures [32], in some systems, concentrations of foreign protein are very low relative to other secreted proteins, for example, 0.05% of total medium protein [28].
For foreign proteins secreted into the apoplastic spaces of organs such as leaves and roots, methods are available for extraction of the protein-rich intercellular fluid without homogenisation of the biomass. Based on vacuum infiltra-tion of the tissue with buffer followed by mild centrifugation [32,44], these methods have been demon-strated mainly at the laboratory scale. Whether such techniques can be scaled up readily to handle high through-puts of biomass is unclear, although large-scale application for purification of human α-galactosidase A from plant leaves has been reported [39]. Protein harvesting from the apoplast might also be feasible for in vitrosystems such as hairy root cultures. Significant enrichment of recombinant
protein can be achieved using this approach: intercellular fluids in which the foreign protein accounted for 30% of the total protein present have been recovered [45].
Glycosylation
There are several differences in the biosynthesis and struc-ture of protein glycans between plants and mammals [46,47••]. Although differences in glycosylation may not
alter the activity of a protein, other properties, such as fold-ing, stability, solubility, susceptibility to proteases, blood clearance rate and antigenicity, can be affected profoundly [48,49]. Addition in the ER of high-mannose glycans at specific asparagine (N) sites on proteins is identical in mammalian and plant cells; however, subsequent trim-ming of the sugar residues in the ER and Golgi generate complex N-glycans with very different structures and prop-erties. Plant glycoproteins exhibit a high degree of heterogeneity of N-glycosylation, suggesting that many variables can affect the processes involved [46,47••].
The xylosyl and fucosyl residues on plant N-linked complex glycans have been demonstrated to be the key epitopes responsible for the allergenicity of plant glycoproteins in humans [50]. The possibility that plant-derived therapeutic proteins could elicit an immune or sensitisation response in patients is of concern, although clinical application might still be possible depending on the dose requirements and blood clearance rate [37]. Glycosylation differences are not an issue for non-glycosylated proteins and peptides, such as those used as antigens in some vaccine applications, and may only be a problem for therapeutic glycoproteins administered to patients by injection. Plant-derived antibodies given orally have been shown in human trials not to elicit abnormally high titres of serum IgG, IgA or IgM antibodies capable of binding to the foreign protein [10•]. This result is consistent
Figure 1
Expression and secretion of assembled IgG1 antibody during culture of transgenic tobacco hairy roots. (䊉) Antibody in the biomass; (䊊)
measured antibody concentration in the medium. The error bars indicate standard errors from triplicate cultures; the initial medium volume was 50 mL. The broken line indicates the medium antibody concentration calculated by mass balance, assuming that all antibody secreted from the biomass accumulates in the medium. The difference between the calculated and measured antibody levels in the medium indicates the extent of antibody degradation. Data adapted from [19].
0.00 0.05 0.10 0.15 0.20 0.25 0.30
0 10 20 30 40 50 600.0
0.5 1.0 1.5 2.0 2.5 3.0
Antibody in the biomass (mg)
Antibody in the medium (
µ
g mL
-1)
Time (days)
with our repeated exposure to plant oligosaccharides in foods, and the lack of novelty of plant glycans to the mucos-al immune system.
Strategies to avoid the formation of immunogenic plant gly-cans may be necessary for plant proteins administered systemically. Two possible approaches include the reten-tion of recombinant glycoproteins in the ER to prevent plant-specific modifications in the Golgi, and the modifica-tion of Golgi activity by removing or adding specific enzymes [46]. For application in plant cell cultures, the first strategy prevents extracellular protein secretion and puts the associated cost benefits for downstream processing out of reach; the second may be possible in the future as our understanding of plant glycosylation improves. Gross mod-ifications, such as complete inhibition of glycosylation or the removal of glycosylation sites from peptide chains, can result in protein folding defects, higher levels of aggrega-tion, and increased protease susceptibility [49,51]. Just as culture conditions such as pH, hormone levels, ammonium ion concentration and reactor configuration have been shown to affect N-linked glycosylation in mammalian cell systems [48,51], it is also possible that some control over plant glycan composition and structure may be achieved in plant tissue culture by manipulation of environmental vari-ables. As yet, however, this has not been demonstrated.
Conclusions
Plant cell and root cultures have been demonstrated to pro-duce a wide range of recombinant proteins. The role of plant tissue culture as a means for commercial protein production is not clear at present, but is likely to depend on the ability to express high levels of proteins in vitro by manipulating culture conditions. Extracellular localisation and the stability of secreted proteins in the medium of plant cultures may be important keys to economic feasibility, allowing easier prod-uct recovery without destroying the biomass. Because the controlled environment in plant cultures offers advantages for GMP and regulatory compliance, a potential niche for reactor-scale plant tissue culture is the rapid production of low-to-medium volume therapeutic proteins. Technology for modifying the glycans of plant glycoproteins may be required before plant systems can be fully exploited for the manufacture of injectable drugs and vaccines.
Acknowledgements
The author’s research was supported by the Australian Research Council (ARC).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. Conrad U, Fiedler U: Compartment-specific accumulation of • recombinant immunoglobulins in plant cells: an essential tool for
antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol Biol1998, 38:101-109. This paper summarises current knowledge about the influence of ER reten-tion, secretion and cytosolic expression on stability and accumulation levels of single-chain Fv antibodies in plant tissues.
2. Larrick JW, Yu L, Chen J, Jaiswal S, Wycoff K: Production of antibodies in transgenic plants. Res Immunol1998, 149:603-608.
3. Fischer R, Liao Y-C, Hoffmann K, Schillberg S, Emans N: Molecular farming of recombinant antibodies in plants. Biol Chem1999, 380:825-839.
4. Hammond J, McGarvey P, Yusibov V (Eds): Plant Biotechnology: New Products and Applications. Berlin: Springer-Verlag; 1999.
5. Hood EE, Jilka JM: Plant-based production of xenogenic proteins.
Curr Opin Biotechnol1999, 10:382-386.
6. Ma JK-C, Vine ND: Plant expression systems for the production of vaccines. In Defense of Mucosal Surfaces: Pathogenesis, Immunity and Vaccines. Edited by Kraehenbuhl J-P, Neutra MR. Berlin: Springer-Verlag; 1999:275-292.
7. Hood EE, Witcher DR, Maddock S, Meyer T, Baszczynski C, Bailey M, Flynn P, Register J, Marshall L, Bond D et al.: Commercial production of avidin from transgenic maize: characterization of transformant, production, processing, extraction and purification. Mol Breed
1997, 3:291-306.
8. Witcher DR, Hood EE, Peterson D, Bailey M, Bond D, Kusnadi A, • Evangelista R, Nikolov Z, Wooge C, Mehigh R et al.: Commercial production of ββ-glucuronidase (GUS): a model system for the production of proteins in plants. Mol Breed1998, 4:301-312. Outlines important practical considerations for commercial production of recombinant proteins in plants, including the effect of product localisation on the procedures used for downstream processing, and the steps required for physical and functional characterisation of the protein.
9. Zhong G-Y, Peterson D, Delaney DE, Bailey M, Witcher DR, Register JC, • Bond D, Li C-P, Marshall L, Kulisek E et al.: Commercial production
of aprotinin in transgenic maize seeds. Mol Breed1999, 5:345-356. The authors discuss the use of a crop species to manufacture a foreign pro-tein with potential therapeutic value. They provide information about the vari-ability of protein expression in successive generations of corn.
10. Ma JK-C, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, Hein MB, • Lehner T: Characterization of a recombinant plant monoclonal
secretory antibody and preventive immunotherapy in humans. Nat Med1998, 4:601-606.
This paper presents the results of the first human clinical trial of a plant monoclonal secretory antibody for the prevention of bacterial colonisation of the mouth and teeth. The antibody preparation was given orally and pro-vided protection over a period of four months without eliciting an adverse immunological response.
11. Tacket CO, Mason HS, Losonsky G, Clements JD, Levine MM, • Arntzen CJ: Immunogenicity in humans of a recombinant bacterial
antigen delivered in a transgenic potato. Nat Med1998, 4:607-609. An antigen derived from a bacterial enterotoxin was administered in human trials as an edible vaccine in uncooked potato. The results showed that mucosal and systemic immune responses were elicited.
12. Kapusta J, Modelska A, Figlerowicz M, Pniewski T, Letellier M, Lisowa O, • Yusibov V, Koprowski H, Plucienniczak A, Legocki AB: A
plant-derived edible vaccine against hepatitis B virus. FASEB J1999, 13:1796-1799.
Human volunteers were fed with transgenic lettuce plants expressing a hepati-tis B virus surface antigen. A specific serum IgG response was measured.
13. Cramer CL, Boothe JG, Oishi KK: Transgenic plants for therapeutic •• proteins: linking upstream and downstream strategies. In Plant
Biotechnology: New Products and Applications. Edited by Hammond J, McGarvey P, Yusibov V. Berlin: Springer-Verlag; 1999:95-118. The authors outline a range of molecular biological approaches for improv-ing post-translational processimprov-ing and product recovery and purification options for foreign proteins in plants. Several examples of commercial inter-est are provided.
14. Dempsey DA, Silva H, Klessig DF: Engineering disease and pest resistance in plants. Trends Microbiol1998, 6:54-61.
15. Tabe L, Higgins TJV: Engineering plant protein composition for improved nutrition. Trends Plant Sci1998, 3:282-286.
16. Herbers K, Sonnewald U: Production of new/modified proteins in transgenic plants. Curr Opin Biotechnol1999, 10:163-168.
17. LaCount W, An G, Lee JM: The effect of polyvinylpyrrolidone (PVP) on the heavy chain monoclonal antibody production from plant suspension cultures. Biotechnol Lett1997, 19:93-96.
19. Sharp JM, Doran PM: Effect of bacitracin on growth and monoclonal antibody production by tobacco hairy roots and cell suspensions. Biotechnol Bioprocess Eng1999, 4:253-258.
20. Fischer R, Schumann D, Zimmermann S, Drossard J, Sack M, Schillberg S: Expression and characterization of bispecific single-chain Fv fragments produced in transgenic plants. Eur J Biochem
1999, 262:810-816.
21. Fischer R, Liao Y-C, Drossard J: Affinity-purification of aTMV-specific recombinant full-size antibody from a transgenic tobacco suspension culture. J Immunol Methods1999, 226:1-10.
22. Fischer R, Emans N, Schuster F, Hellwig S, Drossard J: Towards molecular farming in the future: using plant-cell-suspension cultures as bioreactors. Biotechnol Appl Biochem1999, 30:109-112.
23. Liu F, Lee JM: Effect of culture conditions on monoclonal antibody production from genetically modified tobacco suspension cultures. Biotechnol Bioprocess Eng1999, 4:259-263.
24. Kurata H, Takemura T, Furusaki S, Kado CI: Light-controlled expression of a foreign gene using the chalcone synthase promoter in tobacco BY-2 cells. J Ferment Bioeng1998, 86:317-323.
25. Verdelhan des Molles D, Gomord V, Bastin M, Faye L, Courtois D: Expression of a carrot invertase gene in tobacco suspension cells cultivated in batch and continuous culture conditions.J Biosci Bioeng1999, 87:302-306.
26. Magnuson NS, Linzmaier PM, Reeves R, An G, HayGlass K, Lee JM: Secretion of biologically active human interleukin-2 and interleukin-4 from genetically modified tobacco cells in suspension culture. Protein Expr Purif1998, 13:45-52.
27. Francisco JA, Gawlak SL, Miller M, Bathe J, Russell D, Chace D, Mixan B, Zhao L, Fell HP, Siegall CB: Expression and
characterization of bryodin 1 and bryodin 1-based single-chain immunotoxin from tobacco cell culture. Bioconjugate Chem1997, 8:708-713.
28. Sehnke PC, Ferl RJ: Processing of preproricin in transgenic tobacco. Protein Expr Purif1999, 15:188-195.
29. Terashima M, Murai Y, Kawamura M, Nakanishi S, Stoltz T, Chen L, • Drohan W, Rodriguez RL, Katoh S: Production of functional human
α
α1-antitrypsin by plant cell culture. Appl Microbiol Biotechnol1999,
52:516-523.
Foreign protein expression was induced in rice suspension cultures by depletion of sucrose from the medium.
30. Terashima M, Ejiri Y, Hashikawa N, Yoshida H: Effect of osmotic • pressure on human αα1-antitrypsin production by plant cell culture.
Biochem Eng J1999, 4:31-36.
The low biological activity of recombinant protein secreted into the medium of a suspended plant cell culture was attributed to decomposition by pro-teases after secretion. Activity was improved by adjusting the osmotic pres-sure of the medium to prevent cell disruption and protease release.
31. Wongsamuth R, Doran PM: Hairy roots as an expression system for production of antibodies. In Hairy Roots: Culture and Applications. Edited by Doran PM. Amsterdam: Harwood Academic; 1997:89-97. 32. Borisjuk NV, Borisjuk LG, Logendra S, Petersen F, Gleba Y, Raskin I:
Production of recombinant proteins in plant root exudates. Nat Biotechnol1999, 17:466-469.
33. Evangelista RL, Kusnadi AR, Howard, JA, Nikolov ZL: Process and •• economic evaluation of the extraction and purification of
recombinantββ-glucuronidase from transgenic corn. Biotechnol Prog1998, 14:607-614.
An important and detailed economic analysis of recombinant protein duction from corn. The contributions of raw materials and downstream pro-cessing operations to the overall production cost are calculated explicitly. The sensitivity of production cost to levels of protein expression in the bio-mass is also determined.
34. Young MW, Okita WB, Brown EM, Curling JM: Production of biopharmaceutical proteins in the milk of transgenic dairy animals. Bio Pharm1997, 10(6):34-38.
35. Kusnadi AR, Nikolov ZL, Howard JA: Production of recombinant proteins in transgenic plants: practical considerations. Biotechnol Bioeng1997, 56:473-484.
36. Khoudi H, Laberge S, Ferullo J-M, Bazin R, Darveau A, Castonguay Y, Allard G, Lemieux R, Vézina L-P: Production of a diagnostic monoclonal antibody in perennial alfalfa plants. Biotechnol Bioeng
1999, 64:135-143.
37. Miele L: Plants as bioreactors for biopharmaceuticals: regulatory considerations. Trends Biotechnol1997, 15:45-50.
38. Russell DA: Feasibility of antibody production in plants for human •• therapeutic use. In Plant Biotechnology: New Products and
Applications. Edited by Hammond J, McGarvey P, Yusibov V. Berlin: Springer-Verlag; 1999:119-138.
Discusses regulatory, technical and commercial issues relating to the pro-duction of proteins in plants for use as human pharmaceuticals.
39. Turpen TH: Tobacco mosaic virus and the virescence of biotechnology. Phil Trans R Soc Lond B 1999, 354:665-673.
40. Crawford KM, Zambryski PC: Plasmodesmata signaling: many roles, sophisticated statutes. Curr Opin Plant Biol1999, 2:382-387.
41. Kusnadi AR, Evangelista RL, Hood EE, Howard JA, Nikolov ZL: • Processing of transgenic corn seed and its effect on the recovery
of recombinant ββ-glucuronidase. Biotechnol Bioeng1998, 60:44-52. The authors investigate various options for the recovery and purification of foreign protein from corn seed. Important practical issues, such as product stability during storage and processing, are also discussed.
42. Kermode AR: Mechanisms of intracellular protein transport and targeting in plant cells. Crit Rev Plant Sci1996, 15:285-423.
43. Conrad U, Fiedler U, Artsaenko O, Phillips J: High-level and stable accumulation of single-chain Fv antibodies in plant storage organs. J Plant Physiol1998, 152:708-711.
44. McCormick AA, Kumagai MH, Hanley K, Turpen TH, Hakim I, Grill LK, Tusé D, Levy S, Levy R: Rapid production of specific vaccines for lymphoma by expression of the tumor-derived single-chain Fv epitopes in tobacco plants. Proc Natl Acad Sci USA1999, 96:703-708.
45. Shivprasad S, Pogue GP, Lewandowski DJ, Hidalgo J, Donson J, Grill LK, Dawson WO: Heterologous sequences greatly affect foreign gene expression in tobacco mosaic virus-based vectors. Virology
1999, 255:312-323.
46. Lerouge P, Cabanes-Macheteau M, Rayon C, Fitchette-Lainé A-C, Gomord V, Faye L: N-glycosylation biosynthesis in plants: recent developments and future trends. Plant Mol Biol1998, 38:31-48.
47. Cabanes-Macheteau M, Fitchette-Lainé A-C, Loutelier-Bourhis C, •• Lange C, Vine ND, Ma JKC, Lerouge P, Faye L: N-glycosylation of a
mouse IgG expressed in transgenic tobacco plants. Glycobiology
1999, 9:365-372.
A comprehensive investigation of the glycoforms present on plant-pro-duced IgG1monoclonal antibodies, including comparison with the corre-sponding IgG1of murine origin. The N-linked glycans on the plant protein exhibited a high degree of structural diversity, with 60% of the oligosac-charides possessing β(1, 2)-xylose and α(1, 3)-fucose residues character-istic of plant glycans.
48. Jefferis R, Lund J: Glycosylation of antibody molecules: structural and functional significance. Chem Immunol Antibody Eng1997, 65:111-128.
49. Ceriotti A, Duranti M, Bollini R: Effects of N-glycosylation on the folding and structure of plant proteins. J Exp Bot1998, 49:1091-1103.
50. Garcia-Casado G, Sanchez-Monge R, Chrispeels MJ, Armentia A, Salcedo G, Gomez L: Role of complex asparagine-linked glycans in the allergenicity of plant glycoproteins. Glycobiology1996, 6:471-477.
51. Wright A, Morrison SL: Effect of glycosylation on antibody function: implications for genetic engineering. Trends Biotechnol1997, 15:26-32.