Modeling transient organic vapor transport in
porous media with the dusty gas model
Brent E. Sleep
Department of Civil Engineering, University of Toronto, Toronto, Canada M5S 1A4
(Received 2 June 1997; revised 6 April 1998; accepted 8 April 1998)
The dusty gas model constitutive relationships were incorporated into a numerical model for three-phase, multicomponent flow and transport in porous media. The dusty gas model properly accounts for interactions between all gas-phase species in multicomponent gas mixtures. The model also included Knudsen diffusion, which becomes important in very fine grained soils. The dusty gas model results were compared to predictions based on Fick’s law. For the cases studied, Fick’s law overpredicted flux rates of organic compounds, the effect becoming more pronounced as the permeability of the soil and the Knudsen coefficient were reduced. Increasing moisture content also appeared to increase the difference between predictions based on the dusty gas model and those based on Fick’s law. Hypothetical field-scale simulations were performed to show the impact of multicomponent effects and Knudsen diffusion in a sandy soil and in a clay soil. Results showed that remediation times were significantly underpredicted if Fick’s law was used for gas-phase diffusion. q1997 Elsevier Science Limited. All rights reserved
Keywords: dusty gas law, Knudsen diffusion, vapor transport, porous media.
1 INTRODUCTION
In recent years a number of numerical models have been developed for predicting the movement of organic vapors in soils. These models have ranged from phase single-component models8,11,17that include dispersion and advec-tion to three-phase (water–organic–gas) multicomponent compositional models that include advection, dispersion, capillary forces, and interphase partitioning of organic spe-cies between any of the phases present1,10,20. All of these models are based on the assumption that Fick’s law is an adequate representation of gas-phase diffusion. Thorstenson and Pollock21presented an extensive review of the chemical engineering literature related to gas-phase diffusion, much of which was developed for gas-phase diffusion in porous catalyst pellets. They applied the dusty gas model of Mason and Malinauskas15and the simplified version of this model, the Stefan–Maxwell equations to one-dimensional steady-state multicomponent gas-phase transport. Thorstenson and Pollock21 showed that for stagnant gases Fick’s law did not accurately predict the fluxes in multicomponent systems. For non-stagnant gases, Thorstenson and Pollock21 concluded that Fick’s law provided an accurate estimate of
the molecular diffusive fluxes relative to the mean molar velocity.
Baehr and Bruell6developed a steady-state one-dimen-sional model for multicomponent transport, based on the Stefan–Maxwell equations. They analysed several column experiments and concluded that Fick’s law did not ade-quately represent the physics of gas-phase diffusion. They did find that Fick’s law could be used as an empirical equa-tion for single-component diffusion if the tortuosity was used as a calibration parameter. Greater tortuosities were required for compounds with greater saturated vapor con-centrations to match experimental results. Abriola et al.2 developed a one-dimensional one-phase model for vapor transport in a binary component system based on the dusty gas model. They found that fluxes predicted with a model based on Fick’s law were always less than those predicted by the dusty gas model for a sandy soil. For finer soils, such as a clay soil, Knudsen diffusion became important and the Fickian model substantially overpredicted the gas fluxes compared to the dusty gas model. Abu-El-Sha’r and Abriola4 reported experiments on single and binary gases designed to measure Knudsen diffusion coeffi-cients. Massmann and Farrier16examined flux rates using a
Printed in Great Britain. All rights reserved 0309-1708/98/$ - see front matter
PII: S 0 3 0 9 - 1 7 0 8 ( 9 8 ) 0 0 0 1 1 - 6
steady-state form of the dusty gas model for three-compo-nent systems. They concluded that the Fickian and dusty gas models gave similar steady-state fluxes for permeabilities greater than 10¹10cm2. At lower permeabilities the Fickian model overpredicted the flux rates. Arnost and Schneider5 compared data for transient gas diffusion in porous catalysts to the predictions of a one-dimensional, transient, dusty gas model, and found good agreement between experimental data and model predictions.
The vapor transport models developed to date that incor-porate the dusty gas model or the Stefan–Maxwell equa-tions are all one-dimensional, single-phase models, and with the exception of the binary models of Abriola et al.2 and Arnost and Schneider5 are steady state. Thorstenson and Pollock21pointed out the need to compare Fick’s law pre-dictions with dusty gas model prepre-dictions under transient conditions. At most sites there are significant changes in source concentrations of organic components as the more volatile and soluble components are preferentially removed. The comparisons between Fick’s law and the Stefan– Maxwell equations made by Thorstenson and Pollock21 and Baehr and Bruell6 neglected gas-phase advection and the impacts of gas pressure gradients. Massmann and Farrier16showed that the Stefan–Maxwell equations gave erroneous results relative to the dusty gas model for low permeability soils, and for systems with pressure gradients. Pressure gradients frequently develop due to variations in atmospheric pressures as well as a result of remediation processes such as soil vacuum extraction.
There is a need to incorporate the dusty gas model into a comprehensive simulator to determine the situations where Fick’s law is inadequate for gas-phase diffusion. Of parti-cular importance are the predicted rates of spread of organic vapors, and the rates of diffusion of vapors to the atmo-sphere at the ground surface, and the predicted rates of dis-appearance of volatile organic compounds immobilized in the vadose zone, particularly in fine grained soils.
In this article the formulation of the dusty gas model is reviewed, and the incorporation of the model into the com-positional simulator previously presented by Sleep and Sykes20 is described. Example simulations of multiphase multicomponent systems are presented to highlight the implications of using either the dusty gas model or Fick’s law for gas-phase diffusion.
2 THE DUSTY GAS LAW MODEL FOR GASEOUS DIFFUSION IN POROUS MEDIA
The macroscopically averaged equation for the movement of a component i in a phaseb20is
]
]t[(fSbþKib,drb)rbxib]þ=·(rbxibqb)
þ[=·fSb(JibþNib)]¹Gib¹rib¼0 ð1Þ
wherefis the porosity, Sbis the phase saturation, Kib,dis
the linear adsorption coefficient,rbis the bulk soil density,
rbis the phase molar density, xib is the species mole
frac-tion, qbis the Darcy velocity vector, Jibis the mechanical
dispersion flux, Nib is the molecular diffusion flux, rib
represents interphase transfer of species i to, or from, phase b, andGib represents sinks and sources of species i
to phase b due to injection or pumping, or biological or chemical transformations. An overall molar balance for species i is derived by summing eqn (1) over the water, gas and organic phases. In this case, the interphase transfer terms, rib, for the different phases sum to zero20. As
described in Sleep and Sykes20, the model is based on assumptions of equilibrium interphase mass transfer and ideal phase behavior described by Henry’s and Raoult’s laws.
The Darcy velocity vector for phasebis given by
qb¼ ¹
kkrb mb
(=pbþgbg=z) (2)
where k is the intrinsic permeability tensor, krbis the
rela-tive permeability of phase b,mbis the viscosity, pbis the
pressure of phaseb, g is the gravitational acceleration,gbis
the mass density, and z is the elevation.
It should be noted that the assumption that Darcy’s law gives a molar-averaged velocity is consistent with develop-ments of the theory of flow and diffusion of gases in porous media in the chemical engineering literature7,15.
The mechanical dispersive molar flux, Jib, for the water
phase is given by
Jib¼ ¹rbDmechib ·=xib (3)
The mechanical dispersion tensor, Dmechib , is defined22 as
Dmechibkl ¼aTlnbldklþ(aL¹aT)
nbknbl lnbl
(4)
where aL andaT are the longitudinal and transverse dis-persivities, respectively,nbkandnblare the components of the linear porewater velocity, anddklis the Kronecker delta. Dispersivities are assumed to be independent of phase saturation.
The diffusive molar flux for the water phase is given by Fick’s law:
Niw¼ ¹rwtwDiw·=xiw (5)
wheretwis a tortuosity factor and Di wis the aqueous-phase molecular diffusion coefficient for species i.
The diffusive molar fluxes for the gas phase, in the pre-sence of a gravitational field, are given by the dusty gas model22:
components i and j, DKig is the Knudsen coefficient for component i, R is the gas constant, and T is the temperature (K). Knudsen diffusion becomes important when the pore diameters are small enough, or pressures are low enough, that the gas molecules collide primarily with the pore walls rather than with other molecules. From eqn (6) it can be seen that Knudsen diffusion becomes important when DKigis much smaller than Dijg. In this case the first term in eqn (6) becomes negligible and the diffusive flux is controlled by the second term. The Stefan–Maxwell equations are a spe-cial case of eqn (6) produced by neglecting the pressure gradient term, =pg, and the Knudsen diffusion term,
Nig=DKig. Thorstenson and Pollock21 discuss a number of simplifications of eqn (6) such as the pure molecular diffu-sion regimes, the pure Knudsen regime, and the simplified equations for binary systems.
In the present model gas-phase diffusion coefficients are assumed to be a function of pressure and temperature according to the Fuller, Schettler and Giddings method19:
DAB¼
where T is the temperature (K), P is the pressure (atm), MA
and MBare the molecular weights of A and B, and theon
terms are the diffusion volumes determined from the struc-ture of the molecules19.
The Knudsen coefficient is related to the gas molecular weight and the pore size of the soil. Geankopolis9 recom-mended the relationship
where r is the pore radius (cm), Miis the molecular weight (g/gmol), and DKig in cm
2
s¹1.
Thorstenson and Pollock21 discussed the relationship between the Knudsen diffusion coefficient and the Klinkenberg parameter. They derived the relationship
DKig¼ kbi
mig
(9)
where biis the Klinkenberg parameter that is a character-istic of the soil. For air, Thorstenson and Pollock21 pre-sented the relationship of Heid et al.12:
bair¼0:11k
¹0:39
(10)
with bairin Pa and k in m2. The b values for other
compo-nents can be determined from
bi¼bair
It should be noted that in eqn (6) it has been assumed that the same tortuosity factor can be used for molecular diffu-sion and Knudsen diffudiffu-sion. This may not be appropriate since the two processes are different phenomena. In
particular, the effect of increasing water saturation on tor-tuosity factors for Knudsen diffusion is unknown. For the present study the Millington and Quirk18 relationship has been used to calculate tortuosities.
The discretized form of eqn (6) may be written as
Xnp
where m is the maximum number of neighboring blocks for block k, and the subscript l, k on the flux terms represents the fluxes between blocks l and k, evaluated at the interface between l and k. The calculation of advective, dispersive and Fickian diffusive fluxes in the simulator is outlined in Sleep and Sykes20.
To apply the dusty gas model, eqn (6) is used to calculate the diffusive gas-phase fluxes between neighboring blocks,
(Na b)l , k. For a system of nc components eqn (6) may be
written as a matrix equation16:
AF¼B (13)
where A is a matrix with the terms
Aii¼ ¹
The xigterms in eqns (14) and (15) represent the gas-phase mole fractions of species i at the interface between blocks. Any standard spatial weighting technique such as midpoint weighting or upstream weighting may be used to calculate the interface value of xjg(see Sleep and Sykes20for a dis-cussion of various weighting techniques). In the present model formulation, midpoint weighting is used. The F matrix is the vector of molar fluxes between neighboring blocks:
Fi¼(Nig)l,k (16)
The B matrix is derived from the right-hand side of eqn (6):
Bi¼ 1
RT(pg=xigþxig=pgþxigrgg=z) (17) The gradient terms in eqn (17) are calculated using central differences. For example, for neighboring blocks k and l the pressure gradient in the x-direction (xl. xk) is calculated from
determine the derivatives of each of the fluxes with respect to mole fractions and pressures. Once eqn (14) is solved for the fluxes, using the mole fractions and pressures from the previous Newton iteration, the derivatives may be calcu-lated from the formula
where y is a mole fraction, a pressure, or other state vari-able that mole fractions, densities, diffusion coefficients or tortuosities may depend upon. If an LU decomposition is used to solve eqn (14), then ]]Fy may be found by calculat-ing the right-hand side of eqn (19) and then solvcalculat-ing uscalculat-ing forward and backward substitution. Eqn (19) must be solved for each primary variable to generate all the required derivatives.
3 MODEL APPLICATIONS
3.1 One-dimensional simulations
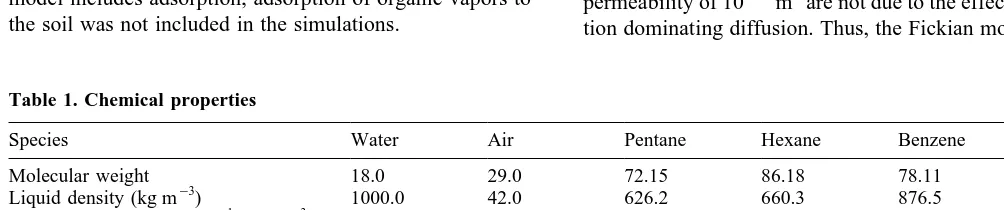
A series of simulations of volatilization and subsequent movement of pentane and hexane in a one-dimensional col-umn of dry soil was performed in order to examine the differences between predictions of the dusty gas model and predictions based on Fick’s law. The properties of pen-tane and hexane are summarized in Table 1. Soil permeabil-ities of 10¹11, 10¹13 and 10¹16m2 were used in the simulations. The temperature for simulations was 238C and air was considered as a single species with the proper-ties of nitrogen gas. The corresponding Knudsen coeffi-cients for air, calculated from eqn (10), are 1.053 10¹2, 6.35310¹4and 9.39310¹6m2s¹1, respectively. In each simulation a horizontal domain of length 30 m (150 finite difference blocks of 0.2 m) was used. A liquid mixture of 50 mol% pentane and 50 mol% hexane was injected into the end of the column for a period of 3 min at a rate of 1.03 10¹4m3(m2s)¹1. The organic liquid saturation reached a maximum of 0.21, just slightly greater than the residual saturation, so the NAPL phase did not move more than 0.4 m (two model cells) in the simulation. Although the model includes adsorption, adsorption of organic vapors to the soil was not included in the simulations.
The results of the one-dimensional simulations in dry soil are shown in Figs 1 and 2. The organic was injected at the end of the column, corresponding to a horizontal distance of 30 m on Figs 1 and 2. The changes in gas-phase concentra-tions at this end of the column, where the organic is present at residual saturation, are reflective of changes in the com-position of the organic phase. It may be noted that, as time proceeds, the concentrations of pentane at the end of the column decrease while the concentrations of hexane increase. This is due to the preferential volatilization of pentane from the residual organic phase, leading to enrich-ment of the organic phase in hexane.
The differences between the predictions using Fick’s law for diffusion and predictions using the dusty gas model are small for a permeability of 10¹11m2. The difference at 710 days is slightly greater for hexane than it is for pentane since the binary diffusion coefficients used for each organic com-pound are the organic–air coefficients. The concentrations of pentane in air are high due to the higher vapor pressure of pentane. The binary diffusion coefficient for a hexane–pen-tane mixture, calculated from eqn (7), is 3.5310¹6
m2s¹1, which is lower than the value of 7.6 3 10¹6m2s¹1for a hexane–air mixture. In other words, the high pentane con-centrations hinder the diffusion of hexane, a phenomenon not accounted for when Fick’s law is applied as though each of the gases were diffusing independently through the air.
With a soil permeability of 10¹11m2, the ratios of diffu-sive fluxes to advective fluxes varied with time and location in the column, but the diffusive fluxes always exceeded the advective fluxes, except at an early time near the point of injection of the liquid organic. Typically, at the midpoint of the column the diffusive fluxes exceeded the advective fluxes by a factor of four or more. To determine whether the small difference between the dusty gas model and Fickian model predictions was due to advective effects, additional simulations (not shown) were conducted in which air was allowed to move by advection, but advection of hexane and pentane was set to zero. The results of these simulations were very similar to those plotted in Fig. 1a and 2a, in which advection of hexane and pentane was included. This indicates that the similarities between predic-tions of the dusty gas and Fickian models for the soil with a permeability of 10¹11m2are not due to the effects of advec-tion dominating diffusion. Thus, the Fickian model may be
Table 1. Chemical properties
Species Water Air Pentane Hexane Benzene Toluene
Molecular weight 18.0 29.0 72.15 86.18 78.11 92.14
Liquid density (kg m¹3) 1000.0 42.0 626.2 660.3 876.5 866.9
Liquid viscosity (kg (m s)¹1)310¹3) 1.0 0.240 0.326 0.652 0.590
Aqueous solubility (mg l¹1) 40 10 1780 515
Vapor viscosity (kg (m s)¹1310¹6) 18.50 6.0 6.5 7.4 6.7
Diffusion volume 12.7 20.1 106.5 127.0 91.0 111.5
Vapor pressure (Pa) 58 250 16 020 10 130 2930
Air-liquid interfacial tension (dyne cm¹1) 72.75 18.43 18.43 28.85 28.5
Fig. 1. Pentane diffusion in a one-dimensional system. Pentane concentration profiles for permeabilities of (a) 10¹11m2, (b)
10¹13m2, (c) 10¹16m2.
Fig. 2. Hexane diffusion in a one-dimensional system. Hexane concentration profiles for or permeabilities of (a) 10¹11m2, (b)
adequate for simulating transport of organic vapors such as pentane and hexane in dry soils with intrinsic permeabilities of 10¹11m2or greater.
For a permeability of 10¹13m2, the differences between Fick’s law and dusty gas model predictions are significant, as shown in Fig. 1b and 2b. In particular, the concentration of pentane at the end of the column containing the organic liquid is lower for the Fick’s law case, due to the greater
rates of pentane diffusion predicted by Fick’s law. Thus, at a permeability of 10¹13m2, the effects of collisions with pore walls have an impact on diffusion rates. The Knudsen diffu-sion coefficients for pentane and hexane for a permeability of 10¹13m2predicted by eqns (9)–(11) are 1.31310¹4and
1.29 3 10¹4
m2s¹1 respectively, whereas the respective air–organic diffusion coefficients are 8.21 3 10¹5
and 7.43 3 10¹5m2s¹1. Thus, since the molecular diffusion coefficients and the Knudsen diffusion coefficients are of the same order of magnitude, this permeability is in the transition regime between molecular diffusion and Knudsen diffusion.
At a permeability of 10¹16m2there is a great difference between the predictions of the dusty gas model and Fick’s law, as shown in Fig. 1c and 2c. The concentration of pen-tane left in the organic liquid source is 50% lower at 710 days for Fick’s law than for the dusty gas model prediction. Hexane concentrations are much greater for the Fick’s law case caused by the greater preferential stripping of the more volatile pentane caused by the higher Fick’s law flux rates. The concentrations are also plotted for a case in which the Knudsen diffusion coefficients were set to very large values to make the Knudsen effect insignificant. In this case, the predicted flux rates are only slightly lower than those pre-dicted by Fick’s law, because of multicomponent effects. Thus, the major impact of the reduced soil permeability on vapor diffusion is the result of Knudsen diffusion and not multicomponent effects. It may be possible, in cases where pressure gradient effects are not important, to model vapor diffusion with a Fickian model that incorporates Knudsen diffusion. This model could be developed from eqn (6).
An additional simulation of pentane and hexane volatili-zation and transport was conducted in the 10¹11m2 perme-ability soil at a water saturation of 0.9. In the absence of information about the effect of water saturation on Knudsen coefficients, it was assumed that the Knudsen coefficients were related to the soil’s intrinsic permeability through eqn (9), and that the effect of increased water saturation was accounted for by the reduced tortuosity calculated from
Fig. 3. Pentane diffusion in soil with permeability of 10¹11m2, water saturation of 0.9.
Fig. 4. System configuration for two-dimensional examples.
the Millington–Quirk expression18. The same tortuosity factor was used for both molecular and Knudsen diffusion, so that the effective Knudsen coefficient was smaller than the effective molecular diffusion coefficient by the same factor as in the dry 10¹11m2permeability soil. Thus, with these assumptions, on the basis of the results of the dry 10¹11m2 permeability soil, Knudsen diffusion would not were significant relative to molecular diffusion in this high water saturation simulation.
The results of the simulations for the dusty gas and Fick-ian models at 140 and 7100 days are shown in Fig. 3. The diffusion rates predicted by both Fick’s law and the dusty gas model are much lower than for the dry soil case caused by the small tortuosity factor associated with a water satura-tion of 0.9. In this case of high water saturasatura-tion, where Knudsen diffusion is assumed to be insignificant, the dusty gas model predicts greater flux rates than the Fickian model, as indicated by the lower pentane concentrations at 7100 days at the end of the column where the liquid organic mixture was injected. The injection of the liquid mixture and its subsequent volatilization gives rise to a pressure gradient in the column. As seen from eqn (6), diffusion fluxes are proportional to pressure gradients as well as con-centration gradients in the dusty gas model, whereas they are assumed to be proportional only to concentration gradi-ents in Fick’s law (see Thorstenson and Pollock21 for a variety of different simplified forms of the dusty gas model that illustrate clearly the dependencies on pressure gradients and the differences between Fick’s law and the dusty gas model).
If the tortuosities for Knudsen diffusion (and thus the
effective Knudsen diffusion coefficients) decreased more quickly than tortuosities for molecular diffusion as water saturation increased, then at high water saturations the Knudsen effect might become more important. In this case the dusty gas model fluxes could be less than those predicted by Fick’s law. However, as water saturation increases the smaller pores are filled first and the average pore radius of gas-filled pores, calculated on a volume basis, increases, so that the Knudsen coefficient might be expected to increase with increasing water saturation unless there are also sub-stantial increases in the thickness of the water film adsorbed to the walls of the unsaturated pores. This is clearly an area that needs some careful experimental study.
3.2 Two-dimensional soil venting
In low permeability soils pressure diffusion and Knudsen diffusion may be significant even under forced advection conditions such as soil vacuum extraction (SVE). Simula-tions of the fate of a mixture of benzene and toluene in a
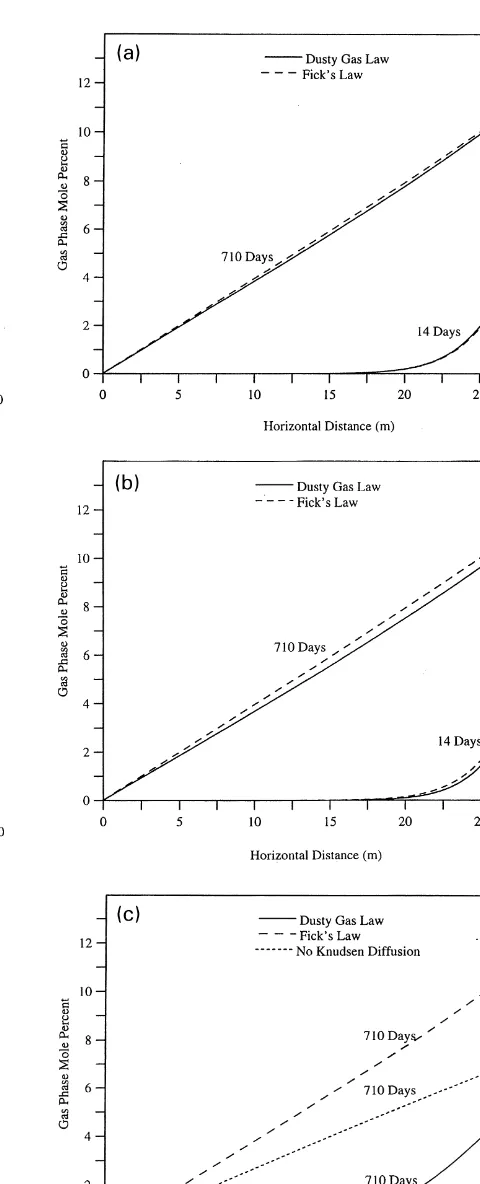
Fig. 6. Benzene distribution (mol%) in gas phase for passive conditions after 250 days, predicted by Fick’s law.
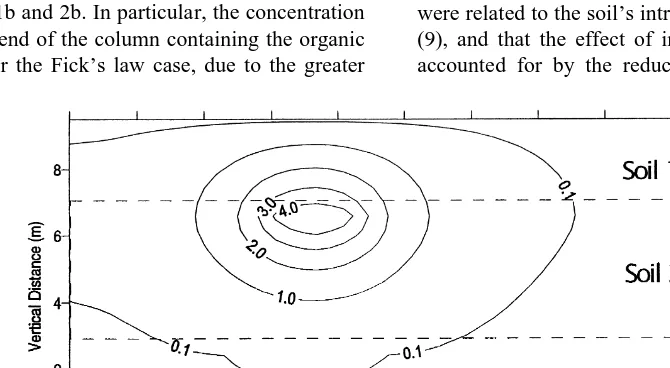
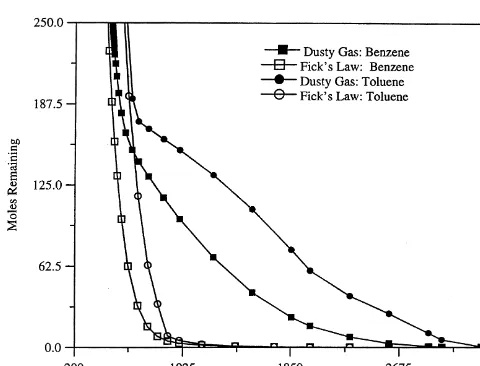
Fig. 7. Mass removal of benzene and toluene predicted by the dusty gas model and by Fick’s law for passive conditions. Table 2. Soil properties
Soil type Permeabi-lity(m2)
Porosity Brooks Coreyl
Brooks Coreypd (m water)
Swr Sor
1 10¹13 0.4 3.0 0.1 0.1 0.2
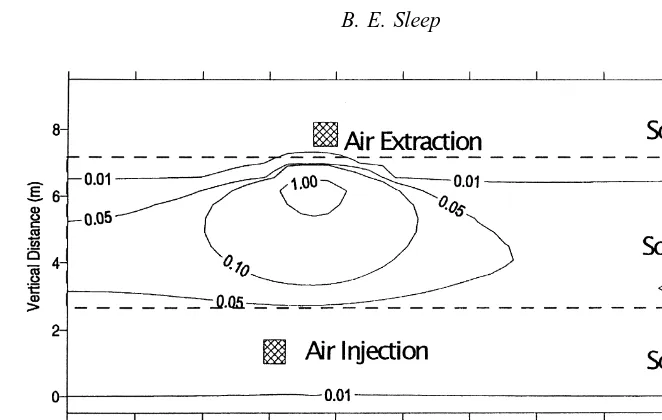
layered soil system under passive conditions and under vacuum extraction were performed to examine the differ-ences in predictions of the Fick-based model and the dusty gas model. The system configuration is shown in Fig. 4, and soil properties are given in Table 2. The top and bottom layers of soil have properties typical of a fine sand, while the middle layer has properties typical of a silty clay. 172.8 l of an organic mixture of 0.5 mole fraction benzene and 0.5 mole fraction toluene were injected into the two-dimen-sional system (1 m thick in the third dimension) at a point 2 m below the ground surface over a period of 10 days. The organic phase subsequently was allowed to redistribute, dis-solve and volatilize. Due to the small volume of organic mixture released, the organic phase did not imbibe into the silty clay layer. At 300 days in the SVE case, gas was injected at the air injection point and extracted from the air extraction point (see Fig. 4) by prescribing constant node gas-phase pressures of 4.0 and ¹4.0 m water, respectively. The predicted vapor-phase benzene concentration pro-files after 250 days for the dusty gas model and for the Fickian model are shown in Figs 5 and 6, respectively.
The extent of movement of benzene into the low permeabil-ity layer is very small for the dusty gas model compared to the Fickian model. In Fig. 7 the predicted rates of dissipa-tion of benzene and toluene concentradissipa-tions in the system due to diffusion to the atmosphere under passive conditions are plotted for the two models. The dusty gas model predicts that more than 5000 days are required for the amount of benzene remaining to reach less than 1 mole, while the Fickian model predicts that this level will be reached after about 3000 days. The predicted rate of dissipation of toluene is also greater for the Fickian model than for the dusty gas model.
The vapor-phase benzene concentration profiles with soil vacuum extraction after 1000 days (700 days of soil vacuum extraction) for the dusty gas model and for the Fickian model are shown in Figs 8 and 9 respectively. There is still considerable benzene left in the low permeability layer in the dusty gas model simulation, compared to the Fickian simulation. In Fig. 10 the predicted rates of dissipation of benzene and toluene con-centrations in the system with soil vacuum extraction are
Fig. 8. Benzene distribution (mol%) in gas phase for soil venting conditions after 1000 days, predicted by dusty gas model.
plotted for the two models. The Fickian model predicts much more rapid removal of benzene and toluene than does the dusty gas model. In the dusty gas model there is an initial phase of rapid decline in the amount of benzene and toluene in the system as these compounds are removed from the high permeability layer. There is an inflection point in the toluene removal rates at about 950 days as the organic in the more permeable zone is depleted. Beyond this point the removal of organic from the low permeability layer is much slower due to the Knudsen limitation of diffusion. In the Fickian model the rate of diffusion from the low perme-ability layer to the high permeperme-ability layer is much higher and therefore the removal of organic from the system by the extraction well is much faster.
4 SUMMARY
The dusty gas model for gas-phase diffusion was incorpo-rated into a multiphase, multicomponent compositional simulator. Simulations in one-dimensional systems showed that predictions of the dusty gas model were very similar to those of the Fickian model at permeabilities of 10¹11m2. As permeabilities were reduced the differences between the models increased. At a permeability of 10¹16m2the differ-ences in the two models were very large, with much lower fluxes predicted by the dusty gas model. The major reason for this was the reduced diffusion rates caused by the Knud-sen effect in the finer grained soils. The possibility of incor-porating Knudsen diffusion into a Fickian model for modeling vapor transport in fine grained soils should be further investigated.
At high moisture contents the dusty gas model predicted higher flux rates than did the Fickian model, caused by the dependence of diffusion rates on pressure gradients in the dusty gas model. The pressure gradients were produced by the injection of liquid organic and its subsequent
volatiliza-tion. However, the effect of moisture content on Knudsen coefficients requires more study.
The results of two-dimensional simulations of passive movement and soil vacuum extraction of benzene and toluene demonstrated that removal rates predicted by the Fickian model are much greater than those predicted by the dusty gas model. Removal rates were limited by the slow rates of diffusion of the organic vapors through the low permeability soil layer.
REFERENCES
1. Abriola, L.M. and Pinder, G.F. A multiphase approach to the modeling of porous media contamination by organic com-pounds: numerical simulation. Water Resour. Res., 1985, 21(1), 19–26.
2. Abriola, L.M., Fen, C.-S. and Reeves, H.W., Numerical simulation of unsteady organic vapor transport in porous media using the dusty gas model. In Proceedings of the IAH Conference on Subsurface Contamination by Immiscible Fluids, Calgary, Alberta, 18–20 April 1990, ed. K.U. Weyer, A.A. Balkema, publ., Rotterdam, 1992, pp. 195–202. 4. Abu-El-Sha’r, W. and Abriola, L.M. Experimental
assess-ment of gas transport mechanisms in natural porous media: parameter evaluation. Water Resour. Res., 1997, 33(4), 505– 516.
5. Arnost, D. and Schneider, P. Dynamic transport of multi-component mixtures of gases in porous solids. Chem. Eng. J., 1995, 57, 91–99.
6. Baehr, A.L. and Bruell, C.J. Application of the Stefan– Maxwell equations to determine limitations of Fick’s law when modeling vapor transport in sand columns. Water Resour. Res., 1990, 26(6), 1155–1163.
7. Cunningham, R.E. and Williams, R.J., Diffusion in Gases and Porous Media. Plenum, New York, 1980.
8. Falta, R.W., Javandel, I., Pruess, K. and Witherspoon, P.A., Density-driven flow of gas in the unsaturated zone due to evaporation of volatile organic compounds. Water Resour. Res., 1989, 25(10).
9. Geankopolis, C.J., Mass transport phenomena. Holt, Rinehart and Winston, New York, 1972.
10. Kaluarachchi, J.J. and Parker, J.C. Modeling multicomponent organic chemical transport in three-fluid-phase porous media. J. Cont. Hydrol., 1990, 5, 349–374.
11. Gierke, J.S., Hutzler, N.J. and Crittenden, J.C. Modeling the movement of organic chemicals in columns of unsaturated soil. Water Resour. Res., 1990, 26(7), 1529–1547.
12. Heid, J.G., MacMahon, J.J., Nielsen, R.F. and Yuster, S.T., Study of the permeability of rocks to homogeneous fluids. In Drilling and Production Practice. American Petroleum Institute, New York, 1950, pp. 230–244.
13. Lide, D.R., CRC Handbook of Chemistry and Physics, 74th edn. 1993.
14. Mackay, D., Shiu, W.Y. and Ma, K.C., Illustrated Handbook of Physical–Chemical Properties and Environmental Fate for Organic Chemicals, Vol. III, Volatile Organic Chemicals. Lewis Publishers, 1993, pp. 522–526.
15. Mason, E.A. and Malinauskas, A.P., Gas transport in porous media: the dusty gas model. Chem. Eng. Monogr., 17. Elsevier, New York, 1983.
16. Massmann, J. and Farrier, D.F. Effects of atmospheric pressures on gas transport in the vadose zone. Water Resour. Res., 1992, 28(3), 777–792.
17. Mendoza, C.A. and Frind, E.O. Advective–dispersive Fig. 10. Mass removal of benzene and toluene predicted by the
transport of dense organic vapors in the unsaturated zone, 1: Model development. Water Resour. Res., 1990, 26(3), 379– 387.
18. Millington, R.J. and Quirk, J.M. Permeability of porous solids. Trans. Faraday Soc., 1961, 57, 1200–1207.
19. Reid, R.C., Prausnitz, J.M. and Sherwood, T.K., The Proper-ties of Gases and Liquids, 3rd edn. McGraw-Hill, New York, 1977, 688 pp.
20. Sleep, B.E. and Sykes, J.F. Compositional simulation of
groundwater contamination by organic compounds, 1: Model development and verification. Water Resour. Res., 1993, 29(6), 1697–1708.
21. Thorstenson, D.C. and Pollock, D.W. Gas transport in unsa-turated porous media: Multicomponent systems and the ade-quacy of Fick’s law. Water Resour. Res., 1989, 25(3), 477– 507.