L
Journal of Experimental Marine Biology and Ecology 246 (2000) 163–178
www.elsevier.nl / locate / jembe
Gill Na,K-ATPase in the spiny lobster Palinurus elephas and
other marine osmoconformers
Adaptiveness of enzymes from osmoconformity to
hyperregulation
a , a b b
ˇ * ˇ ˇ
Cedomil Lucu , Massimo Devescovi , Bosko Skaramuca , Valter Kozul
a
ˇ ´ Center for Marine Research Rovinj, 52210 Rovinj, Institute Ruder Boskovic, Croatia
b
Institute of Oceanography and Fisheries, 20000 Dubrovnik, Croatia
Received 18 July 1999; received in revised form 1 November 1999; accepted 29 November 1999
Abstract
Haemolymph inorganic osmolyte changes and Na,K-ATPase activities in trichobranchiate and epipodite tissues were examined in the spiny lobster Palinurus elephas gradually acclimated from seawater (SW; 38 ppt, salinity; 1291 mOsmol / l) down to dilute seawater (DSW; 20 ppt, salinity; 679 mOsmol / l). During acclimation to DSW haemolymph was only transiently hypoosmotic, becoming isosmotic to the medium over a 24-h period of acclimation. Na,K-ATPase specific activities in homogenates of the trichobranchiate gills from SW- and DSW-acclimated spiny lobsters were in the range of 2–3mmol Pi / h / mg protein and were not significantly different. It has also been confirmed for the marine stenohaline crustaceans Maja crispata and Dromia personata that gill Na,K-ATPase maintains the same level of specific activity in SW- and DSW-acclimated crabs. The saponin-treated fraction of Na,K-ATPase activity in trichobranchiate gills was 67–89% and epipodites 63–64% over the native homogenates’ activity and no differences in enzyme activities upon saponin treatment between SW- and DSW-acclimated spiny lobsters were found. Recovery of 6% and enrichment factor (1.6) of Na,K-ATPase in partially purified plasma membrane fractions of epipodites was relatively low and not different in SW- and DSW-acclimated spiny lobsters. In the hemiepipodite, negative short-circuit current was in the
22
range from 216.7 to 222.7mA cm and conductance varied in the range of 205–290 mS
22
cm , values which were not significantly different in spiny lobsters residing in SW or DSW. Very high conductance suggests leakiness of the hemiepipodite epithelium–cuticular complex. In contrast to the group of euryhaline hyperosmoregulating Crustacea in which activation of the specific activity of Na,K-ATPase upon acclimation to dilute seawater occurs, in marine osmoconformers there is no activation of the enzyme in dilute seawater. Based on the literature data and our own results, we have reported a correlation coefficient of 0.65 between specific activity of Na,K-ATPase and the sodium gradient (mmol Na / l; haemolymph–seawater) between
*Corresponding author.
12 species of osmoconforming and osmoregulating Crustacea. During evolution, hyperos-moregulating Crustacea have achieved internal osmolyte gradients generated by Na,K-ATPase and lowering the gill surface permeability. However these adaptive characteristics are not present in marine osmoconforming Crustacea, restraining them to migrate in the brackish water habitats.
2000 Elsevier Science B.V. All rights reserved.
Keywords: Isosmotic haemolymph; Na; K-ATPase activity; Trichobranchiate gills; Epipodite; Voltage clamp
1. Introduction
In spite of the fact that representatives of the superfamily Palinuroidea (Latreille 1802) has great economic value on the market, there is a dearth of publications on their physiology and biochemistry (Dall, 1974). No substantial progress, except in the field of the nervous system and its physiology (Schmidt and Ache, 1994), has been attained up to this point. The Western rock lobster Panulirus longipes is an isosmotic crustacean tolerating seawater salinity in the range 25–40 ppt (Dall, 1974). The osmotic adjustment of principal monovalent ions is made by the gills and Ca and Mg regulation by the gut (Dall, 1974; 1977).
Marine osmoconforming Crustacea have haemolymph in osmotic equilibrium with seawater and all show very limited ionic regulation. Differences between plasma and seawater in these species are mostly due to the indiffusibility of haemolymph proteins and formation of complexes between proteins and inorganic osmolytes. In isolated perfused gills of the osmoconforming decapod Maja squinado, transepithelial potential was close to 0 mV and no net sodium fluxes were found over the concentration range 20–100% seawater (King and Schoffeniels, 1969). In these osmoconforming species which allow their blood osmolarity to parallel their environment over a narrow salinity range, intracellular volume regulation occurs controlling organic osmolyte content in the cells (Gilles, 1987).
In the hyperosmoregulating Crustacea, gill Na,K-ATPase activity increases pro-portionally with the increase in the number of chloride cells (Neufeld et al., 1980) and as a key enzyme for Na / K exchange indirectly regulates activities of the Na / H exchanger (Shetlar and Towle, 1989; Towle et al., 1997) and Na / K / 2Cl cotransporter (Riestenpatt et al., 1996) on the apical membrane side, and the Na / Ca exchanger on the basolateral membrane side (Flik et al., 1994). In the marine osmoconforming Crustacea Calappa hepatica (Spencer et al., 1979), Cancer and Nephrops (Harris and Bayliss, 1988), haemolymph is isosmotic with surrounding seawater and Na,K-ATPase specific ac-tivities were reported to be at the detection limits. Accordingly, specific activity of the gill Na,K-ATPase in marine Crustacea is lower than in the brackish water and freshwater Crustacea and sodium gradients between haemolymph and medium are in positive correlation with Na,K-ATPase activity (Harris and Bayliss, 1988).
Na,K-ATPase specific activity of the marine osmoconformers Maja crispata and Dromia personata were examined to find out any adaptive distinction between the Na,K-ATPase activities of stenohaline-osmoconforming and euryhaline-hyperosmoregulating Crus-tacea.
2. Materials and methods
2.1. Materials
Spiny lobsters Palinurus elephas (Fabricius, 1787) were caught by local fishermen in regions of small salinity fluctuations (3861 ppt) in the springtime and autumn of 1998 in the South Adriatic near Dubrovnik (Croatia). Animals were kept alive in plastic tanks where seawater was renewed three times weekly (T518628C; aeration). Weight of the intermoult animals was 156679 g; length from medial frontal spine to telson was 1763 cm. Stenohaline osmoconforming spider crab Maja crispata (Risso, 1827; length of carapace 9.161.2 cm; weight 128630 g) and sponge crab Dromia personata (Linneaus, 1587; length of carapace 7.961.6 cm; weight 233650 g) were kept in the vicinity of town of Rovinj (North Adriatic) where seawater salinity fluctuates during a year not more than in narrow range of 36.9560.83 ppt. In preliminary experiments we find that spiny lobsters can survive direct transfer from seawater (38 ppt) to 27 ppt seawater, but direct transfer to lower salinities induces mortality. For experimental purposes spiny lobsters were transferred from 38 to 30 ppt salinity and consecutively salinities were gradually decreased for 2 ppt each 2 days until 20 ppt salinity, where the spiny lobster were acclimated for 2 weeks. Brachyuran crabs Maja crispata and Dromia personata were acclimated to DSW by steadily decreasing of the seawater concentration by 2 ppt each 2 days until the salinity where decapods were acclimated for at least 2 weeks. Animals were fed with fish fillet and a strong tonus of the chaeliped was an indication of successful acclimation to the lowest salinity.
2.2. Blood sampling and ion determination
Haemolymph was withdrawn directly from the pericardial sinus. The haemolymph was allowed to clot at room temperature, the clot was broken up, and the haemolymph was centrifuged at 10 000 rpm for 10 min and frozen at 2208C until measurements were performed. For osmometric and chloride measurements, serum was diluted 5 times and for sodium, calcium and magnesium 1000 times with doubly distilled water. Chloride concentrations were determined using a CMT-10 chloride titrator (Radiometer, Copenhagen), sodium by flame photometry and osmolarity by vapour pressure os-mometry (Knauer, Germany). Total Ca and Mg concentrations in serum were measured with inductively coupled plasma atomic emission spectrophotometry (ICP-AES, Plasma IL 200 Thermo Electron, UDA).
gills and epipodites from individual animals were dissected out, blotted dry and frozen for not longer than 2 weeks at 2808C.
2.3. Protein determination
Protein concentration of gill and epipodite homogenates was measured by the Coomassie Brilliant Blue dye binding technique (Bio-Rad protein assay) with bovine serum albumin as a standard.
2.4. Na,K-ATPase determination
Preparation of the tissue and enzyme determination were performed as previously described in detail (Lucu and Devescovi, 1999). Briefly, tissues were homogenized by 40 strokes in a Dounce homogenizer with a loosely fitting pestle in 10 ml hypotonic solution / g fresh weight of tissue. Hypotonic saline contained 12.5 mmol / l NaCl; 1 mmol / l dithiothreitol; 0.5 mmol / l EDTA with the serine protease inhibitor aprotinin (300 I.U. / l). Homogenate was filtered on plastic mesh (200mm) to remove cuticle and branchial septa. The homogenate was kept on ice (08C). Partially purified membrane vesicles were prepared by first centrifuging the homogenate at 500 g for 5 min. The resulting supernatant was centrifuged for 30 min at 10 000 g to remove mitochondria. The second supernatant was centrifuged for 1 h at 50 000 g and the pellets were resuspended in saline solution (300 mmol / l sucrose; 20 mmol / l Hepes; 0.5 mmol / l EDTA; 2.5 mmol / l DTT and pH was adjusted at 7.5 by Tris base) and frozen at 2808C before use for not longer than 1 week before enzyme analyses. We used 0.2 mg of saponin per mg of proteins to unmask enzyme activity in native homogenates of gills and epipodites (Flik et al., 1994; Lucu and Devescovi, 1999). Total ATPase activity and Na,K-ATPase activity were determined as described in details previously (Flik et al., 1994; Lucu and Devescovi, 1999). The assay procedure was based on determination of the P (inorganic phosphate) released from the substrate ATP in medium containing 100i
mmol / l NaCl, 2.5 mmol / l KCl, 30 mmol / l imidazole, 3 mmol / l ATP, pH 7.5 and the amount of P released in the same medium but without KCl and ouabain to 1 mmol / li
was added. The difference between the mean values for total and ouabain-insensitive ATPase was noted as Na,K-ATPase specific activity expressed in mmol P / h per mgi
protein. Each experiment was performed in triplicate tubes chilled to 08C where 20 ml homogenate or 5ml partially purified membranes and 250ml assay medium was added and the mixture incubated at 378C for 15 min. Reaction was stopped by 1 ml of trichloracetic acid–ammonium heptamolybdate. Absorption was measured at 700 nm with a Unicam 8620 UV–Vis spectrophotometer.
2.5. Electrophysiological studies
After isolation the epipodite edges were cut off, lifted and separated into two halves of which one hemiepipodite with supporting cuticle and epithelium layer was mounted in a
2
potential difference and current pulses were measured by calomel reference electrodes (Ingold C., Germany) connected by 3 M KCl agar bridge in a micro-Ussing chamber. The open-circuit potential and short-circuit current were measured using an automatic voltage clamp device (Bioengineerenig, The University of Iowa, USA). Tissue conduct-ance (G ) was calculated from current resulting from a single voltage pulse of 1 mVt
every 500 s. Cuticular and basolateral sides (haemolymph oriented side) were continu-ously superfused (flow-rate was 0.25 ml / min) with the following saline identical on both sides (in mmol / l): NaCl, 300; KCl, 5; MgCl , 2; CaCl , 4; glucose, 6 and Hepes, 6 and2 2
adjusted to pH 7.6 with Trizma-base buffer.
3. Results
3.1. Haemolymph osmoconcentration and ionic changes
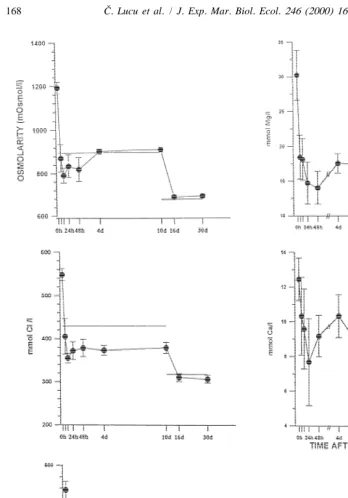
The osmoconforming spiny lobster Palinurus elephas survives sudden transfer from seawater (SW; 38 ppt, salinity; 1291 mOsmol / l) to intermediate 27 ppt, salinity and by gradual acclimation shows increased tolerance to a more extreme salinity dilution at 20 ppt (DSW; 679 mOsmol / l). After transfer of spiny lobsters from 38 ppt to 27 ppt, the new steady state in haemolymph osmolarity and sodium and chloride was reached within 24 h. Gradual acclimation from 27 to 20 ppt followed the same pattern, i.e. osmolarity and sodium concentration of the haemolymph remain in equilibrium with DSW (Fig. 1). Haemolymph chloride concentration in SW and DSW was slightly hyporegulated.
In P. elephas, serum calcium and magnesium (12.5 mmol Ca / l; 34.2 mmol Mg / l) decreased 26 and 42%, respectively, 2 and 4 days after transfer from SW to DSW and slightly increased to a new steady state. A steady state of serum calcium was reached at 9.17 mmol / l, about 2.9 mmol / l above the calcium concentration in DSW (6.2 mmol / l), and magnesium concentration was 17.56 mmol / l, about 14 mmol / l below magnesium in DSW (31.2 mmol / l; Fig. 1). In the spiny lobster Panulirus longipes calcium con-centration in the blood was 53 and 17% over the respective ion concon-centration in sea water salinities of 20 and 45 ppt (Dall, 1974). Under the same conditions, blood magnesium was about 33% less than the external seawater concentration. After abrupt transfer from seawater (36–38 ppt) to lower or higher salinities, a new steady state was reached in 10 h.
3.2. Na,K-ATPase and seawater osmoconcentration changes
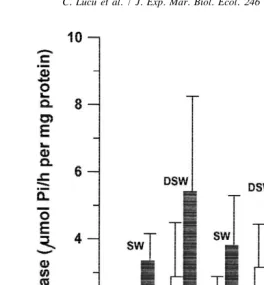
In trichobranchiate gills, specific activity of the native homogenates ranged from 1.9 to 3.2mmol P / h per mg protein and for saponin treated homogenate ranged from 3.4 toi
5.4 mmol P / h per mg protein and exhibited no significant change from SW- andi
DSW-acclimated spiny lobsters (Fig. 2). In the osmoconforming crabs studied we have found equal distribution of the enzyme in anterior and posterior gill pairs. Saponin treatment increased Na,K-ATPase in homogenates isolated from trichobranchiate gills by 67–89% and in epipodites by 63–64% (Fig. 2; Table 1).
Fig. 1. Time course of changes in osmolarity and ion concentration in the haemolymph of spiny lobster
Palinurus elephas acclimated to seawater (0 h; 38 ppt, salinity; 1291 mOsmo / l) and transferred to dilute
Fig. 2. Na,K-ATPase specific activity of the native homogenates (white bars) and of saponin-treated homogenates (black bars) in pleurobranchia (pleuro), arthrobranchia (arthro), and podobranchia (podo) from spiny lobsters Palinurus elephas acclimated to seawater (38 ppt salinity; 1291 mOsmol / l) and dilute seawater (20 ppt salinity; 679 mOsmol / l). The mean values were calculated from 5–6 individual measurements. The differences between values for SW- and DSW-acclimated animals (native homogenates and saponin treated homogenates) were not significant at the level P,0.05 using Student’s t-test.
membranes from the epipodite epithelium of SW- and DSW-acclimated spiny lobsters were not significantly different (Table 1). Similar results were obtained with partially purified membrane vesicles from trichobranchiate gills (results not shown). The percentage of recovery (Vtotal-M/Vtotal H) for gills was 2.7–3.1% (results not shown) and for epipodites isolated from SW- and DSW-acclimated osmoconforming spiny lobsters about 6% (Table 1), much less than for gills from the osmoregulating shore crab Carcinus (13–15%; Lucu and Flik, 1999). The enrichment factor (Vsp membranes /Vsp
homogenate) was 1.6 in both SW- and DSW-acclimated animals (Table 1).
3.3. Short-circuit current and conductance of hemiepipodite
Table 1
Specific Na,K-ATPase activity of native and saponin-treated homogenates and membrane vesicles from the epipodites isolated from spiny lobster Palinurus elephas acclimated to seawater (SW) and dilute seawater
a
Vspecspecific activity of Na,K-ATPase (mmol P / h per mg protein); recovery (per cent) is the ratio of thei total Na,K-ATPase activity of the vesicles (Vspec3total protein content; mg) and the total Na,K-ATPase activity of homogenate (Vspec3total protein content; mg)3100; enrichment factor is the Vspec(vesicles) /Vspec (homogenate); values are means6S.D. calculated from 4–7 individual experiments; ppt5salinity of the seawater; no significant differences between SW and DSW were detected at P,0.05 using the paired Student’s
t-test.
22 22
(222.7 mA cm ) and dilute seawater (216.7 mA cm ) acclimated spiny lobsters, short-circuit currents were not significantly different from each other. The corresponding
22
transepithelial conductance ranged from 205.0 (DSW) to 289.8 mS cm (SW) and were not statistically significantly different when measured in SW- and DSW-acclimated epipodites.
4. Discussion
Besides aquatic Crustacea, there are few systematic categories of invertebrates with such a variety of osmotic behaviour. Marine osmoconforming Crustacea have no ability of osmoregulation, surviving limited dilutions in the osmoconcentration of seawater. Their haemolymph ion composition and osmoconcentration vary with external seawater concentration. According to this study, after acclimation of the spiny lobster to SW and DSW, haemolymph osmolarity was, respectively, 17.3 and 19.4% higher than the
1 2
predicted osmolarities calculated from the sum of respective Na and Cl
con-Table 2
Short-circuit current (I ) and conductance (G) across single split epipodite isolated from spiny lobstersc a
Palinurus elephas acclimated to seawater (38 ppt, salinity) and to dilute seawater (20 ppt, salinity)
Seawater Dilute seawater
centrations alone; the remainder of the osmolarity may be related to an accumulation of organic osmolytes (Pequeux, 1995). The Western rock lobster Panulirus longipes acclimated in this range showed slight hyperionic regulation of sodium and slight hyporegulation of chloride in haemolymph (Dall, 1974; Malley, 1977). Sensillar lymph of the spiny lobster Panulirus argus has a composition of Na, K, Ca and Mg similar to that of seawater and haemolymph, however Cl is present at a reduced level (Gleeson et al., 1993).
Hyperosmoregulating Crustacea distributed within estuaries show regulation of body fluids osmoconcentration that protect the intracellular space from drastic changes in cell homeostasis. The moderate hyperosmoregulator Carcinus can withstand external salinities close to 10 ppt, and the weak osmoregulator Homarus can tolerate 20 ppt, keeping blood osmolarities, respectively, 300 and 150 mOsmol hyperosmostic to their medium (Zanders, 1980; Lucu and Devescovi, 1999). Crustacean gills in hyperosmo-regulating crabs act as a selective interface actively absorbing Na and Cl from diluted external medium. The gills of osmoregulating Crustacea are a convenient model in which to study Na,K-ATPase activation (Lucu and Flik, 1999).
In the next sections we will discuss the differences in responses of Na,K-ATPase of the osmoregulatory tissues located in branchial cavity from spiny lobster and a few brachyuran osmoconformers with osmoregulating Crustacea.
4.1. Na,K-ATPase activity and short-circuit current (SCC) of epipodite in osmoconforming and weakly regulating lobsters
Fig. 3. Comparison of specific activity of the enzyme Na,K-ATPase in epipodite homogenates and of partially purified membrane fraction from lobster Homarus gammarus (Lucu and Devescovi, 1999) and of spiny lobster
Palinurus elephas (this study). Na,K-ATPase specific activities from native homogenates (H) and partially
purified membranes (M) from animals acclimated to seawater (white bars) and to dilute seawater (black striped bars) is presented. Statistical significance of differences between seawater (38 ppt) and dilute seawater (20 ppt, salinity). Na,K-ATPase activities were tested by paired Student’s t-test. Homarus: H, P,0.001; M, P,0.01 (n55–6). Palinurus: In H and M seawater enzyme activities were not statistically significantly different at the level P,0.05 using Student’s t-test from those in dilute seawater (n56).
from the spiny lobster (1.6). (c) In the hemiepipodite epithelium isolated from the spiny lobster the SCC was used as a good alternative estimation of the active transport (negative charge flow driven from apical to basolateral side of preparation). The SCC of hemiepipodite from SW and DSW acclimated spiny lobsters are very low and do not
22
differ, ranged from 216.7 to 222.7 mA cm (cuticle1epithelium layer; Table 2) 10–14 times less than in hemiepipodite isolated from the weakly hyperosmoregulating lobster Homarus gammarus. Moreover, conductance of spiny lobster hemiepipodite
22
(205–290 mS cm ; Table 2) was 3–4.5 fold higher than the Homarus hemiepipodite 22
that in epipodites of the spiny lobsters acclimated to DSW there are no substantial changes in turnover rate or densities of sodium pump.
4.2. Gill Na,K-ATPase activity in Crustacea — adaptivness from osmoconformity to regulation
Upon acclimation of the spiny lobsters to dilute seawater, Na,K-ATPase specific activity of native and saponin-treated trichobranchiate homogenates was not changed compared to gill enzyme activity from the seawater acclimated animals (Fig. 2).
Table 3 depicts the relationship between Na,K-ATPase specific activity in gill homogenates and the haemolymph osmoconcentration gradient (mOsmol / l; haemolymph-medium) during acclimation to the changed osmoconcentration of their aquatic environment in the osmoconforming and regulating Crustacea. Because of the lack of evidence regarding Na,K-ATPase activity in other taxonomic groups of marine osmoconforming Crustacea upon modest dilution of the seawater, besides the present study on spiny lobsters, we have examined gill Na,K-ATPase activity in the other osmoconformers, i.e. marine stenohaline sponge crab Dromia personata and spider crab Maja crispata gradually acclimated for 2 weeks in SW and DSW (20 ppt salinity; Table 3). Haemolymph osmolarity was like for the spiny lobster, only slightly below SW, and no changes in Na,K-ATPase in gill homogenates were found between SW- and DSW-acclimated groups (Table 3). The lack of osmoregulatory ability of the haemolymph confines life of the osmoconformers to limited sea water osmodilution, preventing their migration into extremely dilute waters. We predict similar responses for a large group of stenohaline marine osmoconforming Crustacea not yet studied.
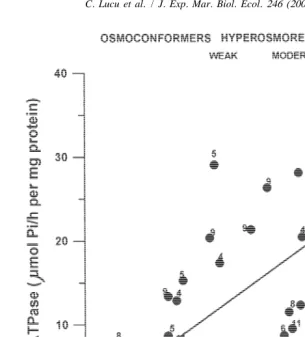
In the hyperosmoregulating Crustacea, Na,K-ATPase activity is increased during acclimation to dilute seawater (more expressed in posterior than anterior gills), likely leading to the generation of a hyperosmotic haemolymph osmoconcentration gradient in low salinities (Table 3). Studies on hyperosmoregulators have shown that increased gill Na,K-ATPase activity is related to the increase in the haemolymph osmoconcentration gradient (mOsmol / l, haemolymph-medium; Table 3). There are numerous data sug-gesting that the pattern of osmoconcentration is quite similar to the pattern of sodium regulation for aquatic Crustacea. Analysis of the relationship between gill Na,K-ATPase activity (see Table 3) and the sodium gradient (mmol / l Na; haemolymph-medium) of osmoconformers and regulators during a course of acclimation to different osmoconcen-trations of medium has shown correlation of these parameters (regression coefficient of 0.65; P,0.01; Fig. 4).
Table 3
Gills Na,K-ATPase in aquatic Crustacea in relation to osmoconcentration differences between haemolymph a
Trichobranchiate 38 2.1–2.9 38 14 days This study 20 2.1–3.2 16
Epipodites 38 3.660.4 38 20 5.361.3 16
2
Maja crispata 38 2.161.7 221 SW→DSW This study 20 1.260.2 3 14 days
3
Dromia personata 38 1.561.0 214 SW→DSW This study 20 0.960.5 5 14 days
HYPEROSMOTIC
4
Callinectes sapidus 30–35 12.960.6 42 SW→DSW Neufeld et al. 1980 24 17.4 150 2–3 weeks
15 20.560.9 360
6 24.11 562
5
Callinectes similis 30 8.762.4 20 SW→DSW Piller et al., 1995 20 15.863.5 94 2 weeks
10 29.862.4 235
6
Carcinus maenas 30 7.062.4 50 SW→DSW Siebers et al., 1987 20 8.860.6 130 4 weeks
10 12.461.4 220
7
Homarus gammarus SW→DSW Lucu and Devescovi, 1999 Trichobranchiate 38 1.4 to 1.8 226 10 days
20 3.1 to 3.8 154 Epipodites 38 4.561.1 226 20 12.663.4 154
8
Macrobrachium olfersii 28 7.9 40 FW→SW Lima et al., 1997 21 7.3 128 10 days
Freshwater 11.6 352
Eriocheir sinensis 34–36 1.460.3 FW→SW Pequeux and Gilles, 1984 2 weeks
Freshwater 4.560.5
36 2.060.1 FW→SW Bigalke, 1986 Freshwater 2.060.1 Few weeks
9
Hemigrapsus nudus 30 13.461.9 20 SW→DSW Corotto and Holliday, 1996 22.7 20.462.2 161
15.1 26.461.9 306 7.6 21.461.9 287
HYPER-HYPOSMOTIC
10
Uca minax 17–18 5.760.4 156 Few weeks Wanson et al., 1984 4–5 5.760.4 444
11
Ucides cordatus 26 7.261.7 3.4 SW→DSW Harris and Santos, 1993 9 9.661.2 336 Few weeks
12
Uca pugnax 15.2 28.2 296 SW→DSW Holliday, 1985 3.0 40.0 622 3 weeks
a
Fig. 4. Summarized literature sources and present results on dependence of Na,K-ATPase specific activity in gill homogenates on the sodium haemolymph gradient (mmol Na / l; haemolymph-medium) of regulating and osmoconforming Crustacea during acclimation to dilute seawater. The regression line fitted by the equation
y50.071x16.90 (regression coefficient R50.65; P,0.01, n531). Individual specific activities of Na,K-ATPase indicated above full circles are related to appropriate specimens from Table 3 (indexes above
11
descriptions of species). Additional data for sodium gradients for Ucides cordatus (Martelo and Zanders,
6 5
1984); Carcinus maenas (Zanders, 1980) and Callinectes similis (Piller et al., 1995) and Callinectes 4
sapidus (Colvocoresses et al., 1974) were used.
(Pequeux and Gilles, 1984; Table 3). Moreover, the gill epithelium of Eriocheir is 30 times less permeable (Onken et al., 1991) when compared to the regulating Uca (Schwarz, 1990), providing an additional adaptive mechanism that makes these crabs functionally divergent. These controversies should be cleared up in the future and therefore we have not introduced these results in Fig. 4. Thus sodium losses through leaky permeable surfaces in dilute medium are replaced by active sodium pumping across branchial cavity cells into the haemolymph space (Lucu and Siebers, 1987). The Na,K-ATPase located basolaterally (Towle and Kays, 1986) is responsible for active transport of sodium and for generation of the sodium gradients that energize secondary active transporters important for maintaining other ion gradient in euryhaline hy-perosmotic Crustacea (Riestenpatt et al., 1996).
Besides Na,K-ATPase activation, one additional physiological adaptive peculiarity for the invasion of the brackish water and freshwater habitats is a reduction of body surface permeabilities in order to restrict inward water flux and diffusive losses of electrolytes. The cuticle, which covers the gill and epipodite epithelium, has to be considered as a selective barrier for cation and anion movement and this selectivity is more expressed in hyperosmoregulating Crustacea such as Eriocheir and Carcinus and less than for osmoconforming Maja squinado and Nephrops norvegicus (Pequeux and Lignon, 1991). In the spiny lobster acclimated to DSW, the isolated gill cuticle conductance was 1961
22
mS cm , about 2.6–4 times more than reported for cuticle of the euryhaline shore crab 22
Carcinus acclimated to dilute sea water (500–750 mS cm , Onken and Riestenpatt, 1998). There is little doubt that the branchial structures are more leaky in osmoconfor-mers than in osmoregulating euryhaline Crustacea. Accordingly,the conductances for gill 22
epithelium (cuticle1epithelium layer) of osmoconforming P. elephas (289 mS cm ; 22
this study) are larger than for moderate hyperosmoregulator Carcinus (40 mS cm ; 22
Onken and Siebers, 1992) and Uca (90 mS cm ; Schwarz, 1990) and much larger than for the tight branchial epithelium of the extremely euryhaline Chinese crab Eriocheir
22
sinensis (3 mS cm ; Onken et al., 1991).
One of the major steps in the evolution of homeostatic control in osmoregulating Crustacea is the development of ionic and osmotic composition of the extracellular fluids different from seawater. In the most primitive osmotic conformers, the internal environment is almost totally dependent upon the external environment. Under these conditions the Na,K-ATPase is not involved in anisosmotic regulation of the haemolymph during acclimation in the narrow salinity ranges but rather plays a role in maintaining intracellular homeostasis.
an osmoconcentration gradient between haemolymph and aquatic environment, a prerequisite for invasion of Crustacea into brackish and freshwater habitats.
Acknowledgements
This research was supported by the Ministry of Science and Technology of the Republic of Croatia. Thanks the Research School M&T, Wageningen, The Netherlands for financial support. We would like to thank David W. Towle for helpful comments and
´ ´
to B. Jagio and I. Korenic for technical help. [SS]
References
1 1 1 1
Bigalke, T. 1986. Characterisierung der (Na ,K )-ATPase und des Na / H -austauschers aus dem ionentran-sportierenden kiemenepithel der Wollhandkrabbe Eriocheir sinensis. Dissertation, Freie Universitat Berlin, p. 139.
Colvocoresses, J.A., Lynch, M.P., Webb, K.L., 1974. Variation in serum constituents of the blue crab
Callinectes sapidus: major cations. Comp. Biochem. Physiol. 49A, 787–803.
Corotto, F.S., Holliday, C.W., 1996. Branchial Na,K-ATPase and osmoregulation in the purple shore crab
Hemigrapsus nudus (Dana). Comp. Biochem. Physiol. 113A, 361–368.
Dall, W., 1974. Osmotic and ionic regulation in the Western rock lobster Panulirus longipes (Milne-Edwards). J. Exp. Mar. Biol. Ecol. 15, 97–125.
Dall, W., 1977. Indices of nutritional state in the western rock lobster Panulirus longipes (Milne Edwards) I. Blood and tissue constituents and water content. J. Exp. Mar. Biol. Ecol. 16, 167–180.
ˇ
Flik, G., Verbost, P.M., Atsma, W., Lucu, C., 1994. Calcium transport in gill plasma membranes of the crab
Carcinus maenas: evidence for carriers driven by ATP and a Na gradient. J. Exp. Biol. 195, 109–122.
Gilles, R., 1987. Volume regulation in cells of euryhaline invertebrates. Curr. Top. Memb. Trans. 20, 205–247. Gleeson, R.A., Aldrich, H.C., White, J.F., Trapido-Rosenthal, H.G., Carr, W.E.S., 1993. Ionic and elemental analyses of olfactory sensillar lymph in the spiny lobster Panulirus argus. Comp. Biochem. Physiol. A 105, 29–34.
Haond, C., Flik, G., Charmantier, G., 1998. Confocal laser scanning and electron microscopical studies on osmoregulatory epithelia in the branchial cavity of the lobster Homarus gammarus. J. Exp. Biol. 201, 1817–1833.
1 1
Harris, R.R., Bayliss, D., 1988. Gill (Na 1K )-ATPase in decapod crustaceans: distribution and
characteris-1
tics in relation to Na regulation. Comp. Biochem. Physiol. 90A, 303–308.
1 1 1
Harris, R.R., Santos, C.M.F., 1993. Sodium uptake and transport (Na 1K ) ATPase changes following Na depletion and low salinity acclimation in the mangrove crab Ucides cordatus (L.) Comp. Biochem. Physiol. 105A, 35–42.
Holliday, C.W., 1985. Salinity-induced changes in gill Na,K-ATPase activity in the mud fiddler crab Uca
pugnax. J. Exp. Zool. 233, 199–208.
King, E.N., Schoffeniels, R., 1969. In vitro preparation of crab gill for use in ion transport studies. Arch. Int. Physiol. Biochim. 77, 105–111.
Lima, A.G., McNamara, J.C., Terra, W.R., 1997. Regulation of hemolymph osmolytes and gill Na / K-ATPase activities during acclimation to saline media in the freshwater shrimp Macrobrachium olfersii (Wiegmann,1836; Decapoda Palaemonidae). J. Exp. Mar. Ecol. 215, 81–89.
ˇ
Lucu, C., Siebers, D., 1987. Linkage of Cl fluxes with ouabain sensitive Na / K exchange through Carcinus gill epithelia. Comp. Biochem. Physiol. 87A, 807–811.
ˇ
Lucu, C., Devescovi, M., 1999. Osmoregulation and branchial Na,K-ATPase in the lobster Homarus
1 21
ˇ
Lucu, C., Flik, G., 1999. Na,K-ATPase and Na / Ca -exchange activities in gills of hyperregulating Carcinus
maenas. Am. J. Physiol. 276, R490–R499.
Malley, D.F., 1977. Salt and water balance of the spiny lobster Panulirus argus: the role of the antennal gland. J. Exp. Biol. 70, 221–230.
Martelo, M.J., Zanders, I.P., 1984. Influence of temperature on ionic regulation in blood and urine of the mangrove crab Ucides cordatus (L.) Comp. Biochem. Physiol. 78A, 255–258.
Moore, R.C. 1969. Treatise on invertebrate paleontology. Part R Arthropoda, The Geological Society of America and The University of Kansas, R437–R446.
Neufeld, G.J., Holliday, C.W., Pritchard, J.B., 1980. Salinity adaptation of gill Na,K-ATPase in the blue crab
Callinectes sapidus. J. Exp. Zool. 211, 215–224.
Onken, H., Siebers, D., 1992. Voltage-clamp measurements of single split lamelae of posterior gills of the shore crab Carcinus maenas. Mar. Biol. 114, 385–390.
Onken, H., Riestenpatt, S., 1998. NaCl absorption across split lamellae of hyperregulating crabs: Transport mechanisms and their regulation. Comp. Biochem. Physiol. 119A, 883–893.
Onken, H., Graszynski, K., Zeiske, W., 1991. Na independent electrogenic Cl uptake across the posterior gills of Chinese crab (Eriocheir sinensis): voltage-clamp microelectrode studies. J. Comp. Physiol. 161, 293–301.
Pequeux, A., Gilles, R., 1984. Control of the extracellular fluid osmolarity in crustaceans. In: Pequeux, A., Gilles, R., Bolis, L. (Eds.), Osmoregulation in Estuarine and Marine Animals. Lecture Notes On Coastal and Estuarine Studies, Vol. 9, Springer Verlag, Berlin, pp. 17–34.
Pequeux, A., Lignon, J., 1991. Permearabilite cuticulaire et ionoregulation chez les Crustaces Decapodes. Cah. Biol. Mar. 32, 203–211.
Pequeux, A., 1995. Osmotic regulation in Crustacea. J. Crustac. Biol. 15, 1–60.
Piller, S.C., Henry, R., Doeller, J.E., Kraus, D.W., 1995. A comparison of the gill physiology of two euryhaline crab species Callinectes sapidus and Callinectes similis: energy production, transport-related enzymes and osmoregulation as a function of acclimation salinity. J. Exp. Biol. 38, 349–358.
1 2
Riestenpatt, S., Onken, H., Siebers, D., 1996. Active reabsorption of Na and Cl across the gill epithelium of the shore crab Carcinus maenas: voltage-clamp and ion-flux studies. J. Exp. Biol. 199, 1545–1554. Schmidt, M., Ache, B.W., 1994. Descending neurons with dopamine-like or with substance P/ FMR Famid-like
immunoreactivity target the somata of olfactory interneurons in the brain of the spiny lobster Panulirus
argus. Cell Tissue Res. 278, 337–352.
Schwarz, H.J. 1990. Electrophysiologische untersuchungen des transportepithelialen Natrium-Transportes ¨
isolierter almkiemenplattchen der posterior Kiemen deer Wollhandkrabben Eriocheir sinensis und der Winkerlkrabbe Uca tangeri. PhD thesis, Frei Universitat Berlin, p. 256.
Siebers, D., Lucu, C., Winkler, A., 1987. Active influx of ions across the gill of osmoregulatory shore crabs
Carcinus maenas. Zool. Beitr. N.F. 30, 315–328.
Shetlar, R.E., Towle, D.W., 1989. Electrogenic sodium-proton exchange in membrane vesicles from crab (Carcinus maenas) gill. Am. J. Physiol. 257, R924–R931.
Spencer, A.M., Fielding, A.H., Kamemoto, F.I., 1979. The relationship between gill Na,K-ATPase activity and osmoregulation capacity in various crabs. Physiol. Zool. 52, 1–10.
Towle, W.D., Kays, W.T., 1986. Basolateral localization of Na,K-ATPase in gill epithelium of two osmoregulating crabs, Callinectes sapidus and Carcinus maenas. J. Exp. Zool. 239, 311–318.
Towle, D.W., Rushton, M.E., Heidysch, D., Magnani, J.J., Rose, M.J., Amstuz, A., Jordan, M.K., Shearer, D.W., Wu, W.S., 1997. Sodium / proton antiporter in the euryhaline crab Carcinus maenas: molecular cloning, expression and tissue distribution. J. Exp. Biol. 200, 1003–1014.
1 1
Vasilets, L.A., Schwarz, W., 1993. Structure–function relationships of cation-binding in the Na / K -ATPase. Biochim. Biophys. Acta 1154, 201–222.
1 1 1
Wanson, S.A., Pequeux, A.J.R., Roer, R.D., 1984. Na regulation and (Na ,K ) ATPase activity in the euryhaline fiddler crab Uca monax (La Conte). Comp. Biochem. Physiol. 79A, 673–678.