Soil biochemical indicators as a tool to assess the short-term
impact of agricultural management on changes in organic C
in a Mediterranean environment
A. Lagomarsino
a,*, M.C. Moscatelli
a, A. Di Tizio
a, R. Mancinelli
b, S. Grego
a, S. Marinari
a aDepartment of Agrobiology and Agrochemistry, University of Tuscia, Via S.Camillo de Lellis s.n.c., 01100 Viterbo, ItalybDepartment of Crop Production, Via S.Camillo de Lellis s.n.c., 01100 Viterbo, Italy
1.
Introduction
Total organic carbon (TOC) in soil is usually considered as one of the most important properties of soils because of its impact on ecosystem sustainability, affecting other physical, chemi-cal and biologichemi-cal characteristics of soil (Reeves, 1997). Nevertheless, only drastic changes can modify this parameter
in the short-term. Differently, when comparing soils with similar physico-chemical properties subjected to different managements, biochemical properties can be used as indica-tors because of their great sensitivity to even slight modifica-tions in the short-term (Gil-Sotres et al., 2005). In this study we selected some biochemical indicators (microbial biomass, enzyme activities and microbial indices) on the basis of their
a r t i c l e
i n f o
Article history: Received 23 April 2008 Received in revised form 2 July 2008
Accepted 4 July 2008
Keywords:
Organic management Soil indicators Hydrolytic enzymes Dehydrogenase C accumulation Microbial biomass
a b s t r a c t
Two management systems (conventional vs. organic) in a 3-years crop rotation (pea–durum wheat–tomato) were compared after 4 years in order to assess soil carbon (C) changes in a short-term period. Biochemical properties of soil, such as microbial biomass C and N (MBC and MBN), microbial respiration, N mineralization, dehydrogenase, chitinase, acid–phos-phatase, arylsulfatase andb-glucosidase activities, were chosen as indicators of soil organic matter biochemical alteration. The main questions addressed in this study were (1) do soil biochemical properties discriminate between organic and conventional management sys-tems in a short-term period? (2) Which biochemical indicator is more effective in predicting soil organic C accumulation in organically managed agricultural soils?
A general increase of hydrolytic enzymes activities has been observed in soil under organic management. MBC, MBN and the MBC/TOC ratio (qmic) increased in organic soil under pea (100%, 50% and 100%, respectively) and durum wheat (55%, 28% and 42%, respectively), while the basal respiration per unit of microbial biomass (qCO2) decreased
(48% and 40% under pea and durum wheat, respectively). Moreover, the specific activity ofb -glucosidase was significantly lower under organic management of pea and durum wheat and was positively correlated withqCO2, suggesting a lower maintenance energy
require-ment of the microbial community.
Soil microbial biomass and enzymatic activities were successfully used to detect short-term changes in soil and, taking into account its role in soil functioning,b-glucosidase resulted the most suitable indicator to predict organic C accumulation in soil under organic management in a Mediterranean environment.
#2008 Elsevier Ltd. All rights reserved.
*Corresponding author. Tel.: +39 0761 357248; fax: +39 0761 357242. E-mail address:[email protected](A. Lagomarsino).
a v a i l a b l e a t w w w . s c i e n c e d i r e c t . c o m
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / e c o l i n d
role in soil C and N cycling, which represent the key elements to evaluate agricultural management sustainability.
In particular, microbial biomass has been suggested as an integrative signal of the microbial significance in soils because it is one of the few fractions of soil organic matter (SOM) that is biologically meaningful, easily measurable, and sensitive to management or pollution (Powlson, 1994). It is both a source and sink for nutrients, it participates in the main biogeo-chemical transformation of C, N, P, S and it contributes to soil structure and stabilization. For these reasons, microbial biomass is widely used as indicator in many soil-monitoring programs (Winding et al., 2005). At the same time, microbially mediated nitrogen (N) mineralization in soil is important in order to sustain plant productivity without massive use of mineral fertilizers.
Moreover enzyme activities can provide indication for quantitative changes in SOM. It is known that the activities of most enzymes increase as native SOM content increases, reflecting larger microbial communities and stabilization of enzymes on humic materials (Burns, 1982; Bending et al., 2002). Enzymes allow microbes to access energy and nutrients present in complex substrates and catalyze decomposition and nutrient mineralization as well as humification processes (Sinsabaugh et al., 1993; Asmar et al., 1994; Masciandaro and Ceccanti, 1999; Allison and Vitousek, 2005). Among hydrolytic enzymes, acid phosphatase andb-glucosidase activities have been frequently used as indicators of changes in quantity and quality of SOM (Gil-Sotres et al., 2005). N-Acetyl-glucosamini-dase activity is considered to be important in C and N cycling because it participates in the processes whereby chitin is converted to amino sugars, which is one of the major sources of mineralizable N in soils (Ekenler and Tabatabai, 2002). N-Acetyl-glucosaminidase and arylsulphatase activities are also considered as indirect indicators of the presence of fungal biomass because sulphate esters (substrates of arylsulpha-tase) are only present in fungal cells (Bandick and Dick, 1999) and chitin is the main constituent of fungal cell wall tissue (Miller et al., 1998). Dehydrogenase activity typically occurs in all intact, viable microbial cells. Thus, its measurement is usually related to the presence of viable microorganisms and their oxidative capability (Trevors, 1984).
Representing single biochemical properties as an index can be a valid approach to evaluate the significance of microbial populations and microbial activity in the cycling of elements in soils under different management (Nannipieri, 1994; Anderson, 2003). The use of quotients avoids the problems of comparing trends in soils with different organic matter or microbial biomass content (Sparling, 1997) and appears to provide more sensitive indications of soil changes than either activity or population measurements alone (Brookes, 1995; Dilly and Munch, 1998). The metabolic quotient (basal respiration per unit of microbial biomass,qCO2) reflects the maintenance energy requirement of soil microbes (Anderson, 2003) and can be a relative measure of how efficiently the soil microbial biomass is utilizing C resources, as well as the degree of substrate limitation for soil microbes (Wardle and Ghani, 1995; Dilly and Munch, 1998).Anderson (2003)indicated above 2 g C–CO2h 1kg MBC 1as a critical threshold for the ‘‘baseline performance’’ of microbial communities. The percentage of MBC to TOC (qmic) could be used as a stability
indicator for quick recognition of an environmental change (Anderson, 2003), it reflects the contribution of microbial biomass to soil organic carbon (Anderson and Domsch, 1989) and it indicates the substrate availability to the soil microbes, being values below 2% a signal of SOM depletion (Anderson, 2003). The enzyme activities can also be expressed per unit of MBC (specific activities), which represent a measure of microbial physiological capacity, and have been found to be more closely related to community composition than total enzyme activity (Waldrop et al., 2000).
It has been shown that in the long-term organic manage-ment of agricultural soils positively influences soil properties (Schjonning et al., 2002; Tu et al., 2006a) since the addition of organic amendments can improve TOC accumulation in soil by increasing C pools with a slow turnover time (Lal and Kimble, 1997). Moreover, green manuring is considered a good agricultural practice because of its positive effect on soil fertility, quality and biodiversity (Stark et al., 2007). In the short-term, several studies supported the evidence of an increase of microbial biomass and activity under organic management, leading to high nutrients availability for plants (Zaman et al., 1999; Tu et al., 2003; Wang et al., 2004; Marinari
et al., 2006). Tu et al. (2003) showed that enhanced soil
microbial biomass and activity were associated with high net N mineralization rates, which resulted in larger N availability. In separate studies,Monokrousos et al. (2006)andMelero et al. (2006)found the activities of enzymes involved in the cycles of N and phosphorus (P) enhanced through the organic manage-ment, with beneficial effects for soil nutrients supply.
Stating that (i) the management systems react differently in diverse climatic regimes with respect to TOC accumulation in soil and (ii) short-term TOC changes are usually not detectable due to the high background of soil C level, the main questions addressed in this study were: (1) do soil biochemical properties discriminate the management sys-tems in a short-term period? (2) Which biochemical indicator is more effective in predicting TOC changes in soil under Mediterranean environment?
2.
Materials and methods
2.1. Site description
At the University of Tuscia experimental farm (Viterbo) a long-term field study was established in 2001, in order to compare organically (ORG) and conventionally (CONV) managed soil in a randomized block design with three replicated plots of 108 m2. The soil is clay loam and classified asTypic Xerofluvent (Soil Taxonomy, Biondi, personal communication). For further details seeMancinelli et al. (2007). In the conventional system the traditional agricultural practices (e.g. chemical fertilizers and pesticides, etc.) are adopted, whereas the organic system is managed following the Regulation 2092/91/EEC. Both systems have a 3 years crop rotation (pea,Pisum sativumL.; durum wheat, Triticum durum Desf.; tomato, Licopersicum esculentumMill.). Tomato was irrigated during the vegetative period, providing 3500–4000 m3ha 1 following potential evapo-transpiration. In the organic management, the rotation is implemented with common vetch (Vicia sativa L.) and
sorghum (Sorgum vulgareL.) cover crops used as green manure before tomato transplanting and pea planting, respectively. Organic fertilizers (Guanito, DIX10; green manure), and mineral nitrogen fertilizer (NH4NO3) were applied to organic and conventional fields, respectively, using the equivalent dose of 120 and 200 kg N ha 1for durum wheat and tomato, respectively. Moreover, the crops in both management systems were sown after minimum tillage (harrowing 15 cm depth). The three crops are simultaneously cropped in the experimental field that includes thus 18 plots: 2 systems3 crops3 replicates.
2.2. Soil sampling
Soil sampling was performed in February 2006 and at the end of the vegetative cycle for each crop (June for durum wheat and pea, August for tomato), in order to take into account seasonal fluctuations of biochemical properties (Wick et al., 2002). After removal of litter layer two soil cores were taken inside each plot at 20 cm depth and then pooled together. Soil samples were sieved (<2 mm) and kept at 48C prior to analyses. Soil moisture was adjusted to 60% of water holding capacity (WHC); then soil samples were left to equilibrate at room temperature in the dark for 1 day before biochemical assays.
2.3. Chemical and biochemical analyses
Microbial biomass carbon (MBC) was determined following the Fumigation Extraction (FE) method (Vance et al., 1987). Microbial biomass was calculated as follows: Biomass C =EC: kEC where EC is the difference between organic C extracted from fumigated soils and organic C extracted from non-fumigated soils andkEC= 0.38 (Joergensen, 1996). Micro-bial biomass nitrogen (MBN) was estimated using ninhydrin reagent solution (Sigma) on fumigated and non-fumigated samples following the method reported byJoergensen and Brookes (1990).
Microbial respiration was measured at 288C. The CO2 evolved was trapped after 1, 3, 7, 10, 14, 21, 28 days of incubation, in 2 ml 1 M NaOH and determined by titration of the excess NaOH with 0.1 M HCl (Badalucco et al., 1992). The CO2evolved during the 28th day of incubation was used as the basal respiration value (MRbasal). The metabolic quotient (qCO
2) was calculated as MRbasalper unit of MBC (Dilly and Munch, 1998) and expressed as (g C–CO2h 1kg MBC 1).
N mineralization activity was assessed for samples collected in winter following the method of Stanford and
Smith (1972)with a 30 weeks aerobic incubation. Ammonium
and nitrate contents produced during the incubation time were determined colorimetrically following Anderson and Ingram (1993)andCataldo et al. (1975), respectively after 2, 4, 8, 12, 22 and 30 weeks at 288C.
Total organic carbon (TOC) and total nitrogen (TN) were determined using an elemental analyzer (Thermo Soil NC— Flash EA1112).qmicwas calculated as the ratio of MBC to TOC and expressed asmg MBCmg TOC 1(Anderson and Domsch, 1989).
The enzymatic activities were detected colorimetrically using a SHIMADZU UV–VIS 1240 spectrophotometer to
measure the pNP (p-nitrophenol) released after enzymatic reaction at 400 nm wavelength. Acid phosphatase activity (Ac. P) was determined after incubation at 378C with 0.115 M p-nitrophenylphosphate at pH 5.0 as substrate (Garzillo et al., 1996).b-Glucosidase (bG) activity was assayed using 25 mM p-nitrophenylglucoside at pH 6.0 as substrate (Alef and Nannipieri, 1995). Arylsulphatase activity (Aryl) was detected according toElsgaard et al. (2002)using 25 mMp-nitrophenyl sulphate at pH 5.8 as substrate. 5 mM p-nitrophenyl-N-acetyl-b-D-glucosaminide at pH 5.8 was used as substrate to test N-acetyl-b-D-glucosaminidase activity (NAG) (Parham and Deng, 2000). Dehydrogenase activity (DH) was determined by the reduction of 2-(p-iodophenyl)-3-( p-nitrophenyl)-5-phenyl-tetrazolium chloride (INT) to iodo-nitrophenyl formazan (INTF) measured at 490 nm (Garcı`a et al., 1997). The enzyme activities per unit of microbial biomass C (specific activity) have been calculated in order to normalize activity to the size of the microbial biomass and are indicated by an asterisk (*) following the acronym of the enzyme (Waldrop et al., 2000).
2.4. Statistical analysis
Analysis of variance (ANOVA) was performed using Statistica 6.0 software package (Statsoft, Tulsa, USA). The main effects of management system (organic vs. conventional), crop, time and their interactions on soil properties were evaluated. Data were tested for normality with the Shapiro–Wilk statistics. Data of soil chemical properties are reported as average values of 3 replicates in the two sampling dates, since no significant variations in the two periods were observed. Data of soil biochemical properties are reported separately for the two sampling dates to show the seasonal fluctuation. The correlation matrix was performed using the average of the two sampling dates in 2006 in order to avoid the seasonal fluctuation of soil biochemical properties and to improve the correlation with chemical properties.
3.
Results
3.1. Chemical and biochemical properties of soil
In the studied area the chemical properties of soil did not greatly change in the two management systems (Table 1); after 4 years of organic management the amount of TOC in soil slightly increased (8%,p<0.1), while pH, TN and C/N ratio did not change significantly (Table 1).
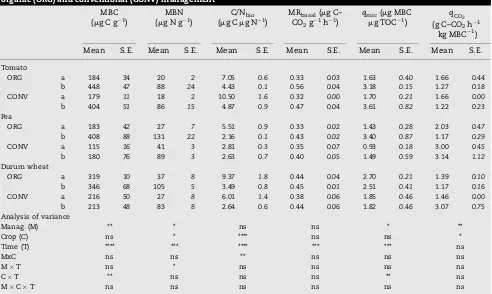
On the contrary, the biochemical properties of soil resulted much more sensitive to the management system. MBC, MBN andqmicsignificantly increased in ORG soil under pea (100%, 50% and 100%, respectively, as average of the two periods) and durum wheat (55%, 28% and 42%, respectively, as average of the two periods). MRbasaldid not show significant differences between the management systems, while the basal respira-tion per unit of microbial biomass (qCO
2) significantly decreased in ORG soil under pea and durum wheat (48% and 40%, respectively, as average of the two periods) (Table 2). Greater values ofqCO
February (Table 2). MBN showed the greatest increase in ORG soil at the end of the cycle, as showed by the significant interaction managementtime (Table 2). C/Nbio showed greater values in February than at the end of the vegetative cycle (Table 2). The greatest values were observed under tomato, while the increase in ORG soil was significant only under durum wheat, as showed by the interaction
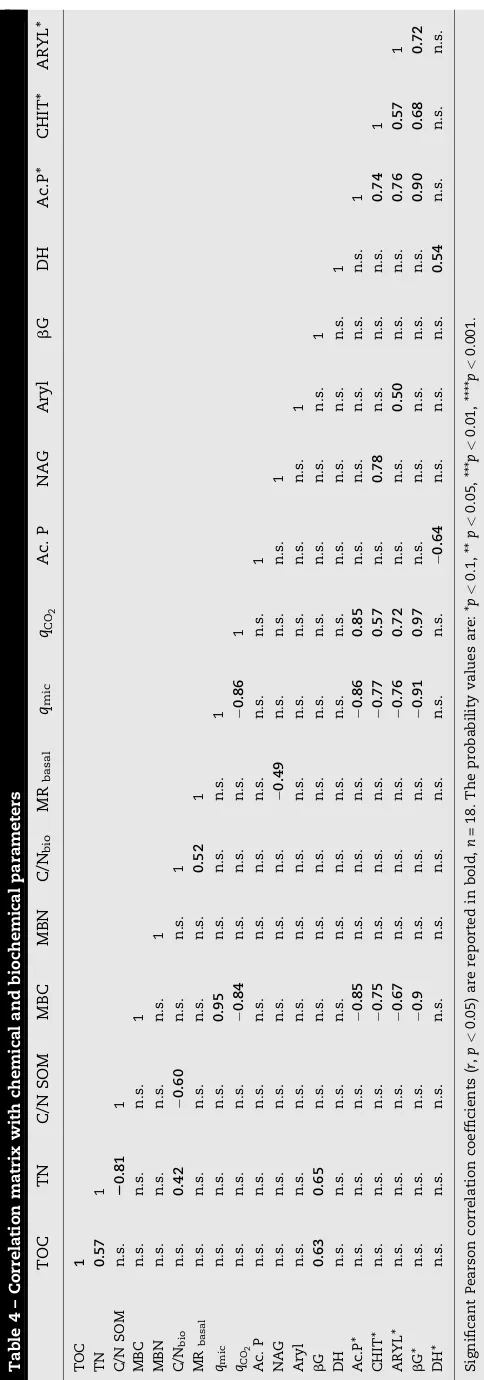
manage-mentcrop. C/Nbio significantly correlated with TN and MRbasal(Table 4).
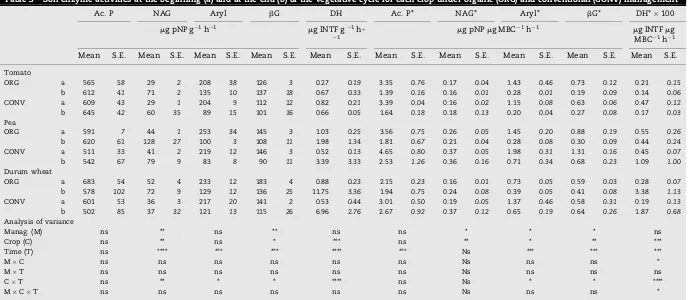
NAG and bG were significantly higher in ORG soil; this effect was greater under pea and durum wheat (Table 3). The three crops differently affected soil enzyme activities: NAG showed the greatest values under pea, bG and DH under durum wheat. Moreover, a different temporal pattern was Table 1 – Chemical properties of organic (ORG) and conventional (CONV) soils for each crop as average of the two sampling dates in 2006
pH (H2O) pH (KCl) TOC (%) TN (%) C/N ratio
Mean S.E. Mean S.E. Mean S.E. Mean S.E. Mean S.E.
Tomato
ORG 6.73 0.04 5.29 0.10 1.28 0.07 0.16 0.03 9.22 0.59
CONV 6.87 0.15 5.56 0.13 1.13 0.10 0.14 0.01 8.74 0.18
Pea
ORG 6.80 0.10 5.60 0.19 1.25 0.01 0.13 0.01 9.80 0.62
CONV 6.94 0.08 5.68 0.14 1.22 0.02 0.13 0.01 9.78 0.60
Durum wheat
ORG 6.91 0.00 5.57 0.01 1.28 0.04 0.17 0.02 8.28 1.01
CONV 6.92 0.04 5.60 0.02 1.19 0.02 0.14 0.02 9.32 0.81
Analysis of variance
Management (M) ns ns * ns ns
Crop (C) ns ns ns ns ns
MC ns ns ns ns ns
Standard errors (S.E.) are reported in Italic. Analysis of variance: *p<0.1, **p<0.05, ***p<0.01, ****p<0.001,n= 3.
Table 2 – Soil biochemical properties at the beginning (a) and at the end (b) of the vegetative cycle for each crop under organic (ORG) and conventional (CONV) management
MBC
(mg C g 1) (mg N gMBN1) (mg CC/Nmg Nbio 1) MRCObasal(mg C– 2g 1h 1)
qmic(mg MBC
mg TOC 1) qCO2 (g C–CO2h 1
kg MBC 1)
Mean S.E. Mean S.E. Mean S.E. Mean S.E. Mean S.E. Mean S.E.
Tomato
ORG a 184 34 20 2 7.05 0.6 0.33 0.03 1.63 0.40 1.66 0.44
b 448 47 88 24 4.43 0.1 0.56 0.04 3.18 0.15 1.27 0.18
CONV a 179 11 18 2 10.50 1.6 0.32 0.00 1.70 0.21 1.66 0.00
b 404 51 86 15 4.87 0.9 0.47 0.04 3.61 0.82 1.22 0.23
Pea
ORG a 183 42 27 7 5.51 0.9 0.33 0.02 1.43 0.28 2.03 0.47
b 408 88 131 22 2.16 0.1 0.43 0.02 3.40 0.87 1.17 0.29
CONV a 115 16 41 3 2.81 0.3 0.35 0.07 0.93 0.18 3.00 0.45
b 180 76 89 3 2.63 0.7 0.40 0.05 1.49 0.59 3.14 1.12
Durum wheat
ORG a 319 10 37 8 9.37 1.8 0.44 0.04 2.70 0.21 1.39 0.10
b 346 68 105 5 3.49 0.8 0.45 0.01 2.51 0.41 1.17 0.16
CONV a 216 50 27 8 6.01 1.4 0.38 0.06 1.85 0.46 1.46 0.00
b 213 48 83 8 2.64 0.6 0.44 0.06 1.82 0.46 3.07 0.75
Analysis of variance
Manag. (M) ** * ns ns * **
Crop (C) ns * **** ns ns *
Time (T) **** *** **** *** *** ns
MxC ns ns ** ns ns ns
MT ns * ns ns ns ns
CT ** ns ns ns ** ns
MCT ns ns ns ns ns ns
Microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), microbial C/N ratio (C/Nbio), microbial basal respiration (MRbasal), microbial
quotient (qmic), metabolic quotient (qCO2). Standard errors (S.E.) are reported inItalic. Analysis of variance: *p<0.1, **p<0.05, ***p<0.01,
****p<0.001,n= 3.
Table 3 – Soil enzyme activities at the beginning (a) and at the end (b) of the vegetative cycle for each crop under organic (ORG) and conventional (CONV) management
Ac. P NAG Aryl bG DH Ac. P* NAG* Aryl* bG* DH*100
mg pNP g 1h 1 mg INTF g 1
h-1 mg pNPmg MBC
1h 1 mg INTFmg
MBC 1h 1
Mean S.E. Mean S.E. Mean S.E. Mean S.E. Mean S.E. Mean S.E. Mean S.E. Mean S.E. Mean S.E. Mean S.E.
Tomato
ORG a 565 58 29 2 208 38 126 3 0.27 0.19 3.35 0.76 0.17 0.04 1.43 0.46 0.73 0.12 0.21 0.15
b 612 41 71 2 135 10 137 18 0.67 0.33 1.39 0.16 0.16 0.01 0.28 0.01 0.19 0.09 0.14 0.06
CONV a 609 43 29 1 204 9 112 12 0.82 0.21 3.39 0.04 0.16 0.02 1.15 0.08 0.63 0.06 0.47 0.12
b 645 42 60 35 89 15 101 16 0.66 0.05 1.64 0.18 0.18 0.13 0.20 0.04 0.27 0.08 0.17 0.03
Pea
ORG a 591 7 44 1 253 34 145 3 1.03 0.25 3.56 0.75 0.26 0.05 1.45 0.20 0.88 0.19 0.55 0.26
b 620 61 128 27 100 3 108 11 1.98 1.34 1.81 0.67 0.21 0.04 0.28 0.08 0.30 0.09 0.44 0.24
CONV a 511 33 41 2 219 12 146 3 0.52 0.13 4.65 0.80 0.37 0.05 1.98 0.31 1.31 0.16 0.45 0.07
b 542 67 79 9 83 8 90 11 3.39 3.33 2.53 1.26 0.36 0.16 0.71 0.34 0.68 0.23 1.09 1.00
Durum wheat
ORG a 683 54 52 4 233 12 183 4 0.88 0.23 2.15 0.23 0.16 0.01 0.73 0.05 0.59 0.03 0.28 0.07
b 578 102 72 9 129 12 136 25 11.75 3.36 1.94 0.75 0.24 0.08 0.39 0.05 0.41 0.08 3.38 1.13
CONV a 601 53 36 3 217 20 141 2 0.53 0.44 3.01 0.50 0.19 0.05 1.37 0.46 0.58 0.31 0.19 0.13
b 502 85 37 32 121 13 115 26 6.96 2.76 2.67 0.92 0.37 0.12 0.65 0.19 0.64 0.26 1.87 0.68
Analysis of variance
Manag. (M) ns ** ns ** ns ns * * * ns
Crop (C) ns ** ns * *** ns ** * ** ***
Time (T) ns **** *** *** **** *** Ns *** *** ***
MC ns ns ns ns ns ns Ns ns ns *
MT ns ns ns ns ns ns Ns ns ns ns
CT ns ** * * **** ns Ns * * ****
MCT ns ns ns ns ns ns Ns ns ns *
Acid phosphatase (Ac. P), Chitinase (NAG), Arylsulphatase (Aryl),b-glucosidase (bG), Dehydrogenase (DH) and their specific activities (*). Standard errors (s.e.) are reported inItalic. Analysis of variance: *p<0.1, **p<0.05, ***p<0.01, ****p<0.001,n= 3.
ec
olo
g
ical
indicato
rs
9
(2
009)
observed: NAG and DH activities were significantly higher at the end of the vegetative cycle than in February; the other enzymes showed an opposite pattern with lower values at the end of the vegetative cycle (Table 3). Among several biological properties,bG activity was the only parameter significantly correlated with TOC and TN (Table 4).
Specific activities of NAG, Aryl,bG and DH per unit of MBC (indicated by an asterisk) were significantly lower in ORG soil under pea and durum wheat (Table 3). Values were greater under pea than in the other two crops (Table 3). Ac.P*, NAG*, Aryl* andbG* positively correlated withqCO
2 and negatively withqmic(Table 4).
The N mineralized after 30 weeks of incubation reached the highest values in ORG soil under durum wheat (Fig. 1). Moreover, mineralized N significantly correlated with the chitinase activity determined at the beginning of the vegetative cycle (Fig. 2).
4.
Discussion
Biochemical properties are known to be highly sensitive to seasonal fluctuations and monitoring trends and changes over time is essential for their adequate application (Wick et al., 2002). In this study significant fluctuations of almost all biochemical properties were found, revealing a high degree of sensitivity to environmental constrain and vegetative status of crops. Nevertheless, with the exception of MBN, the effect of organic management on the biochemical indicators was independent of the seasonal variation and was similar in the two periods, suggesting a consistency of the response to the management system.
Since short-term TOC changes are usually not detectable due to the high background of soil C level, microbial biomass content and its related indices might be considered sensitive early indicators for TOC accumulation predictions according to several authors (Friedel and Gabel, 2001; Fließbach and Ma¨der, 2000; Kaur et al., 2005; Tu et al., 2006b; Melero et al., 2006; Marinari et al., 2006). In the present study the considerable increase of MBC, MBN and qmic in ORG soil
Table
Fig. 1 – Cumulative mineralized N after 30 weeks of incubation. Different letters indicate significant differences among treatments: conventional (Conv) organic (Org). Bars in the figure represent standard error, n= 3.
was probably due to the easily available carbon fraction of the added organic material such as green manure and fertilizer (Marinari et al., 2006) as also reported in a previous work carried out in the same experimental field (Lagomarsino et al., in press). Moreover, the increase ofqmicin ORG soil reflects the larger organic substrates availability for microbial growth (Anderson, 2003). Nevertheless, the lack of response under tomato suggests that the interactions between crops and microbes, as well as the related agricultural practices, can differently impact soil microbial biomass. The increase of hydrolytic enzymes provided other evidences of better conditions for microbial biomass in ORG soil under pea and durum wheat, but not under tomato. We believe that tomato-specific management practices such as irrigation, more frequent soil tillage and N fertilization, could reduce the positive impact of organic management.
The N mineralization activity followed the same trend of microbial biomass and reached the highest values in ORG soil under durum wheat. Increased soil microbial pool is often associated with high net mineralization and availability of N for plants as demonstrated by several authors (Wang et al., 2004; Tu et al., 2006b).Bonde et al. (1988)estimated that the N pool of microbial biomass contributed to 55–89% of total mineralized N.
An enhancement of soil enzymatic activity is generally expected in response to: (i) increased microbial synthesis and release of extracellular enzymes and (ii) improved environ-mental conditions induced by changes of soil physico-chemical properties (Aon and Colaneri, 2001). In our study the specific enzyme activities response to the organic management suggests that the increase of hydrolytic enzymes was probably more related to a larger microbial pool than to its higher physiological capacity (Waldrop et al., 2000).
Moreover, the lower metabolic quotient andbG specific activity in ORG soil suggest a decrease of the microbial community maintenance energy requirement (Dilly, 2005). According toSchjonning et al. (2002)the decrease ofqCO2and specific enzyme activity indicates: (i) a more efficient micro-bial community and (ii) a better use of the available organic substrates. Moreover,Ma¨der et al. (2002)supposed that the decrease of the metabolic quotient was related to a significant
increase of microbial diversity in organically managed soil because a diverse microbial community is able to better transform C from organic debris into biomass.
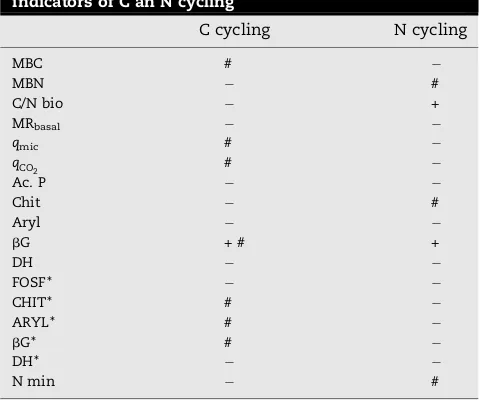
The response of selected indicators supported the hypoth-esis of TOC accumulation in the longer-term in ORG soil. Few years of contrasting soil management are not usually enough to produce a consistent difference in SOM content (Marinari et al., 2006). Therefore in our study the slight increase of TOC (+7%) after 4 years of organic management needs to be confirmed through other evidences: the biochemical indica-tors allowed us to predict changes of SOM in the longer term because of their greater sensitivity to environmental changes. According withDoran and Zeiss (2000), an indicator of soil quality should be (1) sensitive to variations in management; (2) well correlated with beneficial soil functions; (3) useful for elucidating ecosystem processes; (4) comprehensible and useful to land managers; (5) easy and inexpensive to measure. In this work, the selection of soil biochemical indicators relied on their role in reflecting short-term changes in C and N cycling. In Table 5 we summarize the effectiveness of the selected biochemical parameters as indicators of C and N cycling in soil. Two main aspects were taken into account: (1) the discriminating power between the two management systems and (2) the correlation between each indicator and soil C and N organic pools.
Among all selected parameters, MBC and MBN showed to be sensitive indicators of C and N accumulation under organic management, as already reported in other works (Friedel and Gabel, 2001; Fließbach and Ma¨der, 2000; Kaur et al., 2005; Tu et al., 2006b; Melero et al., 2006; Marinari et al., 2006). The microbial indices as well confirmed to be useful indicators of changes in C cycling, indicating both a larger availability of C substrates and a more efficient microbial community under organic management. Several previous evidences (Sparling, 1997; Anderson, 2003; Fließbach and Ma¨der, 1997;Ma¨der et al., Fig. 2 – Linear regression between chitinase activity
(mg pNP gS1hS1) and mineralized N (mg N gS130 weekS1).
The regression is significant atp< 0.001,n= 18.
Table 5 – Effectiveness of biochemical parameters as indicators of C an N cycling
C cycling N cycling
MBC #
MBN #
C/N bio +
MRbasal
qmic #
qCO
2 #
Ac. P
Chit #
Aryl
bG + # +
DH FOSF*
CHIT* #
ARYL* #
bG* #
DH*
N min #
2002; Marinari et al., 2006) supportqCO
2 andqmicas valuable soil indices. Moreover, the critical threshold indicated by
Anderson (2003) make them suitable in predicting stress
conditions for microbial biomass, suggesting in our case a better environment in ORG soil.
In the present study, not all enzymes demonstrated to be valid indicators of changes under organic management: Ac.P and Aryl activities did not respond to the different manage-ment, although other studies reported significant changes of Ac.P (Pascual et al., 1999; Monokrousos et al., 2006). Arylsulphatase did not show significant variations also in the study ofMijangos et al. (2006). DH activity appeared to be a sensitive indicator of the impact of organic management in the work ofMijangos et al. (2006)andMarinari et al. (2006). However, in this study, the extremely high variability of this parameter decreased its reliability as indicator. On the contrary, bG and NAG activities resulted to be suitable indicators of changes in C and N cycling respectively.bG indicates the SOM decomposition potential and has been proposed as a sensitive index of management effects on agricultural soils (Ndiaye et al., 2000; De la Horra et al., 2003) as well as for the development of complex indices (Puglisi et al., 2006). In our study the strict relationship betweenbG and SOM content indicates that even a slight increase of TOC can promote enzymes protection and induce significant changes of biochemical activity, demonstrating the efficacy of this enzyme to predict early changes of SOM content. In fact, bG can reflect both past biological activity and the ability of a soil to protect enzymes, since the abiontic forms ofbG contribute to a significant amount of the total activity (Knight and Dick, 2004). The results obtained in this study confirm thatbG can be used as sensitive indicator of short-term soil TOC changes, since it integrates information on microbialstatusand soil physico-chemical conditions (Aon and Colaneri, 2001). The significant relationship between chitinase and the N mineralization activity confirms the findings ofEkenler and Tabatabai (2002), who demonstrated that chitinase plays a major role in N cycling and can be used as an index of net N mineralization in soil, being its activity a rate-limiting step.
5.
Conclusions
The three crops responded differently to the treatments, suggesting that the beneficial effects of organically managed soils should be considered within the framework of the crops vegetative cycle and related to agricultural practices. In the 3 years crop rotation, considered in this study, organic fertiliza-tion supported higher rates of N mineralizafertiliza-tion and greater hydrolytic enzymatic activities, related to a larger and more efficient microbial pool under pea and durum wheat but not under tomato. Despite the contrasting effect of the single species on soil biochemical properties, the whole crop rotation under organic management seems to have a positive effect on TOC accumulation. Soil biochemical properties were effective in discriminating the two systems management in terms of C and N pools changes, revealing which crops, within the rotation, had a positive effect on C accumulation in a Mediterranean soil under organic management. In particular
b-glucosidase resulted the most suitable indicator to predict soil TOC changes in a 3 years crop rotation.
Acknowledgements
The authors wish to thank prof. Enio Campiglia and prof. Fabio Caporali for allowing the use of the experimental station; Mr. Claudio Stefanoni for technical assistance; Dr. Francesco Biondi for providing soil classification and prof. Paolo De Angelis for allowing the use of the elemental analyzer.
r e f e r e n c e s
Alef, K., Nannipieri, P., 1995.b-glucosidase activity. In: Alef, K., Nanniperi, P. (Eds.), Methods in Applied Soil Microbiology and Biochemistry. Academic Press, NY, pp. 350–351. Allison, S.D., Vitousek, P.M., 2005. Responses of extracellular
enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 37, 937–944.
Anderson, T.H., 2003. Microbial eco-physiological indicators to assess soil quality. Agric. Ecosyst. Environ. 98, 285–293. Anderson, T.H., Domsch, K.H., 1989. Ratios of microbial biomass
carbon to total organic-C in arable soils. Soil Biol. Biochem. 21, 471–479.
Anderson, J.M., Ingram, J.S.I., 1993. Colorimetric determination of ammonium. In: Anderson, J.M., Ingram, J.S.I. (Eds.), Tropical Soil Biology and Fertility: A Handbook of Methods. Second Edition. CAB International, Wallingford, U.K., Anderson, pp. 73–74.
Aon, M.A., Colaneri, A.C., 2001. Temporal and spatial evolution of enzymatic activities and physico-chemical properties in an agricultural soil. Appl. Soil Ecol. 18, 255–270.
Asmar, F., Eiland, F., Nielson, N.E., 1994. Effect of extracellular-enzyme activities on solubilization rate of soil organic nitrogen. Biol. Fertil. Soils 17, 32–38.
Badalucco, L., Grego, S., Dell’Orco, S., Nannipieri, P., 1992. Effect of liming on some chemical, biochemical and micro-biological properties of acid soil under spruce (Picea abiesL.). Biol. Fertil. Soils 14, 76–83.
Bandick, A.K., Dick, R.P., 1999. Field management effects on soil enzyme activities. Soil Biol. Biochem. 31, 1471–1479. Bending, G.D., Turner, M.K., Jones, J.E., 2002. Interactions
between crop residue and soil organic matter quality and the functional diversity of soil microbial communities. Soil Biol. Biochem. 34, 1073–1082.
Bonde, T.A., Schnu¨rer, J., Rosswall, T., 1988. Microbial biomass as a fraction of potentially mineralizable nitrogen in soils from long-term field experiments. Soil Biol. Biochem. 20, 447–452.
Brookes, P.C., 1995. The use of microbial parameters in monitoring soil pollution by heavy metals. Biol. Fertil. Soils 19, 269–279.
Burns, R.G., 1982. Enzyme activity in soil: location and a possible role in microbial ecology. Soil Biol. Biochem. 14, 423–427. Cataldo, D.A., Haroon, M., Schrader, L.E., Young, V., 1975. Rapid
colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plan. 6, 71–80. De la Horra, A.M., Conti, M.E., Palma, R.M., 2003.b-Glucosidase
and protease activities as affected by long-term
management practices in a Typic Argiudoll soil. Commun. Soil Sci. Plan. 34, 2395–2404.
Dilly, O., 2005. Microbial energetics in soils. In: Buscot, F., Varma, A. (Eds.), Microorganisms in Soils: Roles in Genesis and Functions. Springer-Verlag, Berlin, Heidelberg, pp. 123–138.
Dilly, O., Munch, J.C., 1998. Ratios between estimates of microbial biomass content and microbial activity in soils. Biol. Fertil. Soils 27, 374–379.
Doran, J.W., Zeiss, M.R., 2000. Soil health and sustainability: managing the biotic component of soil quality. Appl. Soil Ecol. 15, 3–11.
Ekenler, M., Tabatabai, M.A., 2002.b-Glucosaminidase activity of soils: effect of cropping systems and its relationship to nitrogen mineralization. Biol. Fertil. Soils 36, 367–376.
Elsgaard, L., Andersen, G.H., Eriksen, J., 2002. Measurement of arylsulphatase activity in agricultural soils using a simplified assay. Soil Biol. Biochem. 34, 79–82. Fließbach, A., Ma¨der, P., 2000. Microbial biomass and
size-density fractions differ between soils of organic and conventional systems. Soil Biol. Biochem. 32, 757–768. Fließbach, A., Ma¨der, P., 1997. Carbon source utilization by
microbial communities in soils under organic and conventional farming practice. In: Insam, H., Rangger, A. (Eds.), Microbial Communities — Functional versus Structural Approaches. Springer-Verlag Berlin, Germany, pp. 109–120.
Friedel, J.K., Gabel, D., 2001. Nitrogen pools and turnover in arable soils under different durations of organic farming. I. Pool sizes of total soil nitrogen, microbial biomass nitrogen, and potentially mineralizable nitrogen. J. Plant Nutr. Soil Sci. 164, 415–419.
Garcı`a, C., Herna`ndez, T., Costa, F., 1997. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun. Soil Sci. Plan. 28, 123–134. Garzillo, A.M.V., Badalucco, L., De Cesare, F., Grego, S.,
Buonocore, V., 1996. Synthesis and characterization of an acid phosphatase–polyresorcinol complex. Soil Biol. Biochem. 28, 1155–1161.
Gil-Sotres, F., Trasar-Cepeda, C., Leiros, M.C., Seoane, S., 2005. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 37, 877–887. Joergensen, R.G., 1996. The fumigation–extraction method to
estimate soil microbial biomass: calibration of the kEC value. Soil Biol. Biochem. 28, 25–31.
Joergensen, R.G., Brookes, P.C., 1990. Ninhydrin-reactive N measurements of microbial biomass in 0.5 M K2SO4soil extracts. Soil Biol. Biochem. 19, 1023–1027.
Kaur, K., Kapoor, K.K., Gupta, A.P., 2005. Impact of organic manures with and without mineral fertilizers on soil chemical and biological properties under tropical conditions. J. Plant Nutr. Soil Sci. 168, 117–122. Knight, T.R., Dick, R.P., 2004. Differentiating microbial and
stabilizedb-glucosidase activity relative to soil quality. Soil Biol. Biochem. 36, 2089–2096.
Lagomarsino, A., Di Tizio, A., Marinari, S., Moscatelli, M.C., Mancinelli, R., Grego, S., in press. Soil organic matter pools under different system management and tillage level in a three-year crop rotation. Agrochimica.
Lal, R., Kimble, J.M., 1997. Conservation tillage for carbon sequestration. Nutr. Cycl. Agroecosyst. 49, 243–253. Ma¨der, P., Fließbach, A., Dubois, D., Gunst, L., Fried, P., Niggli, U.,
2002. Soil fertility and biodiversity in organic farming. Science 296, 1694–1697.
Mancinelli, R., Campiglia, E., Di Tizio, A., Lagomarsino, A., Grego, S., 2007. The effect of organic and conventional cropping systems on CO, emission from agricultural soils: preliminary results. Ital. J. Agron. 2, 151–155.
Marinari, S., Mancinelli, R., Campiglia, E., Grego, S., 2006. Chemical and biological indicators of soil quality in organic and conventional farming systems in Central Italy. Ecol. Indicators 6, 701–711.
Masciandaro, G., Ceccanti, B., 1999. Assessing soil quality in different agro-ecosystems through biochemical and
chemico-structural properties of humic substances. Soil Tillage Res. 51, 129–137.
Melero, S., Ruiz Porras, J.C., Herencia, J.F., Madejon, E., 2006. Chemical and biochemical properties in a silty loam soil under conventional and organic management. Soil Tillage Res. 90, 162–170.
Mijangos, I., Pe´rez, R., Albizu, I., Garbisu, C., 2006. Effects of fertilization and tillage on soil biological parameters. Enzyme Microb. Technol. 40, 100–106.
Miller, M., Palojarvi, A., Rangger, A., Reeslev, M., Kjioller, A., 1998. The use of fluorogenic substrates to measure fungal presence and activity in soil. Appl. Environ. Microbiol. 64, 613–617.
Monokrousos, N., Papatheodorou, E.M., Diamantopoulos, J.D., Stamou, G.P., 2006. Soil quality variables in organically and conventionally cultivated field sites. Soil Biol. Biochem. 38, 1282–1289.
Nannipieri, P., 1994. The potential use of soil enzymes as indicators of productivity, sustainability and pollution. In: Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Grace, P.R. (Eds.), Soil Biota: Management and Sustainable Farming Systems. CSIRO, Australia, pp. 238–244.
Ndiaye, E.L., Sandeno, J.M., McGrath, D., Dick, R.P., 2000. Integrative biological indicators for detecting change in soil quality. Am. J. Altern. Agric. 15, 26–36.
Parham, J.A., Deng, S.P., 2000. Detection, quantification and characterization ofb-glucosaminidase activity in soil. Soil Biol. Biochem. 32, 1183–1190.
Pascual, J.A., Garcı´a, C., Hernandez, T., 1999. Lasting
microbiological and biochemical effects of the addition of municipal solid waste to an arid soil. Biol. Fertil. Soils 30, 1– 6.
Powlson, D.S., 1994. The soil microbial biomass: before, beyond and back. In: Ritz, K., Dighton, J., Giller, G.E. (Eds.), Beyond The Biomass. John Wiley & Sons, Chichester, UK, pp. 3–20. Puglisi, E., Del Re, A.A.M., Rao, M.A., Gianfreda, L., 2006.
Development and validation of numerical indexes integrating enzyme activities of soils. Soil Biol. Biochem. 38, 1673–1681.
Reeves, D.W., 1997. The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Tillage Res. 43, 131–167.
Schjonning, P., Elmholt, S., Munkholm, L.J., Debosz, K., 2002. Soil quality aspects of humid sandy loams as influenced by organic and conventional long-term management. Agric. Ecosyst. Environ. 88, 195–214.
Sinsabaugh, R.L., Antibus, R.K., Linkins, A.E., McClaugherty, C.A., Rayburn, L., Repert, D., Weiland, T., 1993. Wood decomposition: nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 74, 1586– 1593.
Sparling, G.P., 1997. Soil microbial biomass, activity and nutrient cycling as indicators of soil health. In: Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R. (Eds.), Biological Indicators of Soil Health. CAB International, Wallingford, pp. 97–119.
Stanford, G., Smith, S.J., 1972. Nitrogen mineralization potentials of soils. Soil Sci. Soc. Am. Proc. 36, 465–472. Stark, C., Condron, L.M., Stewart, A., Di, H.J., O’Callaghan, M.,
2007. Influence of organic amendments on microbial soil properties and processes. Appl. Soil Ecol. 35, 79–93. Trevors, J.T., 1984. Effect of substrate concentration, inorganic
nitrogen, O2 concentration, temperature and pH on dehydrogenase activity in soil. Plant Soil 77, 285–293. Tu, C., Koenning, S.R., Hu, S., 2003. Root-parasitic nematodes
enhance soil microbial activities and nitrogen mineralization. Microb. Ecol. 46, 134–144.
organic inputs and straw mulching. Soil Biol. Biochem. 38, 247–255.
Tu, C., Louws, F.J., Creamer, N.G., Mueller, J.P., Brownie, C., Fager, K., Bell, M., Hu, S., 2006b. Responses of microbial biomass and N availability to transition strategies from conventional to organic farming systems. Agric. Ecosyst. Environ. 113, 206–215.
Vance, E.D., Brookes, P.C., Jenkinson, D.S., 1987. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707.
Waldrop, M.P., Balser, T.C., Firestone, M.K., 2000. Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 32, 1837–1846.
Wang, W.J., Smith, C.J., Chen, D., 2004. Predicting soil nitrogen mineralization dynamics with a modified double
exponential model. Soil Sci. Soc. Am. J. 68, 1256.
Wardle, D.A., Ghani, A., 1995. A critique of the microbial metabolic quotient (qCO2) as a bioindicator of disturbance
and ecosystem development. Soil Biol. Biochem. 27, 1601– 1610.
Wick, B., Ku¨hne, R.F., Vielhauer, K., Vlek, P.L.G., 2002. Temporal variability of selected soil microbiological indicators under different soil quality conditions in SW-Nigeria. Biol. Fertil. Soils 35, 155–167.
Winding, A., Hund-Rinke, K., Rutgers, M., 2005. The use of microorganisms in ecological soil classification and assessment concepts. Ecotox. Environ. Saf. 62, 230–248. Zaman, M., Di, H.J., Cameron, K.C., 1999. A field study of gross
rates of N mineralization and nitrification and their relationships to microbial biomass and enzyme activities in soils treated with dairy effluent and ammonium fertilizer. Soil Use Manage. 15, 188–194.