www.elsevier.com/locate/jinsphys
Procathepsin and acid phosphatase are stored in
Musca domestica
yolk spheres
Paulo E.M. Ribolla
a,*, A. Tania Bijovsky
b, Antonio G. de Bianchi
aaDepartamento de Bioquı´mica, Instituto de Quı´mica, Universidade de Sa˜o Paulo, 05508-900 Sa˜o Paulo, SP, Brazil bDepartamento de Parasitologia, Instituto de Cieˆncias Biome´dicas, Universidade de Sa˜o Paulo, 05508-900 Sa˜o Paulo, SP, Brazil
Received 26 October 1999; accepted 30 June 2000
Abstract
Yolk spheres present in mature invertebrate oocytes are composed of yolk proteins and proteolytic enzymes. In the fly Musca domestica, yolk proteins are degraded during embryogenesis by a cathepsin-like proteinase that is stored as a zymogen. An acid phosphatase is also active in the yolk spheres during Musca embryogenesis. In this paper we show that procathepsin and acid phosphatase are initially stored by a different pathway from the one followed by yolk protein precursors. Both enzymes are taken up by the oocytes and transitorily stored into small vesicles (lysosomes) surrounding the early yolk spheres. Fusion of both structures, the early yolk spheres and lysosomes, creates the mature yolk spheres.2001 Elsevier Science Ltd. All rights reserved.
Keywords:Cathepsin; Acid phosphatase; Yolk proteins; Oocytes;Musca domestica; Immunocytochemistry
1. Introduction
Insect oocyte maturation is marked by intense traffic of proteins from the fat bodies to the oocyte (Chia and Morrison, 1972; Huebner et al., 1975; Bast and Telfer, 1976; Waring and Mahowald, 1979; Brennan et al., 1982). These proteins, the yolk protein precursors, have an important role as an amino acid source for building new tissues during embryogenesis. Besides the accumu-lation of yolk proteins, several enzymes and/or proen-zymes are stored. These enproen-zymes are activated during embryogenesis to digest the protein reserve. In Bombyx mori (Indrasith et al., 1988) and Rhodnius prolixus
(Nussenzveig et al., 1992), a trypsin-like and a cathep-sin-like protease are involved in yolk hydrolysis. In Dro-sophila melanogasteronly cathepsin-like activities were detected during embryogenesis (Medina et al., 1988). Besides functioning in yolk protein degradation during embryogenesis, cathepsin-like enzymes are active during insect metamorphosis (Takahashi et al., 1993) and are found in hemocyte cell lines (Tryselius and Hultmark,
* Corresponding author (at Departamento de Parasitologia). Tel.: +55-11-818-7274; fax:+55-11-818-7417.
E-mail address:[email protected] (P.E.M. Ribolla).
0022-1910/01/$ - see front matter2001 Elsevier Science Ltd. All rights reserved. PII: S 0 0 2 2 - 1 9 1 0 ( 0 0 ) 0 0 1 1 4 - 1
1997). In other organisms, cathepsin-like enzymes have been described in situations of cell rennovation, such as frog metamorphosis (Komukai et al., 1986) and tumor cell invasion (Berquin and Sloane, 1996).
Yolk spheres containing yolk proteins are the most abundant cytoplasmic inclusion in insect eggs (Roth and Porter, 1964). Although the compartmentalization of yolk proteins is well documented, relatively few studies have determined the intracellular localization of the enzymes involved in their degradation during embryogenesis. In some insects, proteases are stored as proenzymes in the mature egg. In Aedes aegypti, beside vitellogenin, two enzymes are synthesized in the fat bod-ies and transported via hemolymph to the developing oocyte, a carboxypeptidase (VCP) and a 44-kDa protein (44KP). After internalization, both pro-enzymes are stored in the mature yolk bodies around the periphery of the crystallized vitellogenin (vitellin) (Hays and Raikhel, 1990; Cho et al., 1991; Chen et al., 1995; Snigirevskaya et al., 1997a). In the tick Ornitodoros moubata, the reduction in pH that occurs in the yolk spheres after fert-ilization (Fagotto 1990a,b, 1991) activates a procathep-sin L present inside the eggs. Recently, Giorgi et al. (1997) identified a cysteine pro-protease inside yolk spheres of Blatella germanica.
hydrolyzed during Musca domestica embryogenesis by a cathepsin-like proteinase, which is present in mature oocytes as a proenzyme (Ribolla et al., 1993; Ribolla and de Bianchi, 1995). The procathepsin is auto-acti-vated after fertilization by a reduction in pH in the yolk granule. An acid phosphatase is also active during
Musca embryogenesis, however its function is not yet
understood (Ribolla et al., 1993). In this study we used ultrastructural immunolabeling of procathepsin and yolk proteins and cellular fractionation techniques to visualize the process of internalization and storage of the Musca domesticayolk protein precursors, procathepsin and acid phosphatase, by oocytes.
2. Materials and methods
2.1. Animals
Musca domestica flies were reared as described by
Ribolla et al. (1993). The vitellogenic stages ofM. dom-esticaoocytes (S1 to S10) were determined according to
Adams (1974).
2.2. Ovaries and oocytes
Ovaries were dissected from CO2anesthetized flies in
dissection buffer (25 mM HEPES–NaOH, pH 7.2, con-taining 200 mM NaCl, 50 mM KCl and 5 mM EDTA). The ovarioles were dissected under a binocular micro-scope, and the oocytes were separated from the terminal follicle of each ovariole.
2.3. Cell fractionation
About 80 oocytes were gently broken in 1 ml of section buffer. Most chorions floated and were dis-carded. 500 µl of the resulting suspension were layered over a sucrose gradient. The gradient was made in two steps. First, two solutions with 0 and 1.5 M sucrose on PBS (3.5 ml each) were continuously mixed to obtain a linear gradient. After that, 3 ml of a 2 M sucrose solution on PBS was layered on the bottom of the gradient. After ultracentrifugation (28,000g, 1 h, 4°C, rotor RPS 55-T2
(Hitachi Koki Co., Tokyo, Japan)), fractions were manu-ally collected from the top of the gradient with a pipette. The collected fractions were numbered from the top to the bottom. Protein content was measured by the Coom-assie blue method (Spector, 1978).
2.4. Hydrolase assays
Assays for both enzymes were carried out in wells of microtiter plates. Assays for cathepsin were executed using N-α-carbobenzoxy-l-lysine ρ-nitrophenyl ester
(CLN) as substrate (Bajkowski and Frankfater, 1975).
The samples were diluted 6-fold in 0.2 M sodium acetate pH 4.6, containing 10 mM EDTA and 10 mM DTT. Aliquots were incubated at room temperature with 1.56 mM CLN and rates of hydrolysis were analyzed at 340 nm using a microtiter plate reader (Titertek Multiskan, MCC 340, Flow Laboratories, Lugano, Switzerland).
The acid phosphatase assay was conducted as a modi-fication of the method described by Purcell et al. (1988) by dilution of the samples (1:5, v/v) in 0.2 M sodium acetate buffer, pH 4.6, containing 1.25 mM ρ -nitro-phenyl phosphate (ρ-NPP). The reactions were termin-ated with 50µl of 4 M NaOH and the freeρ -nitrophenol-ate ion was determined by measuring its absorbance at 410 nm using a microtiter plate reader.
2.5. Yolk protein partial purification and anti-sera production
After mature oocytes were cell fractionated (described above) it was possible to recover yolk protein from a pellet formed at the bottom of the tube. There were no cellular organelles in this pellet as visualized by electron microscopy and the yolk protein polypeptides were rela-tively pure as analyzed by SDS–PAGE (data not shown). The pellet protein was solubilized with PBS and utilized for the production of yolk protein anti-serum.
The mouse anti-yolk protein serum was obtained by subcutaneous injection of 50µg ofMuscayolk protein emulsified with complete Freund adjuvant. A second injection with the same amount of protein emulsified with incomplete Freund adjuvant was administered 1 week later. After 1 more week, mouse blood was col-lected. The resulting serum was added to 0.1% (w/v) NaN3 and stored at220°C until needed.
Rabbit anti-cathepsin serum was the same as described by Ribolla and de Bianchi (1995).
2.6. Electron microscopy
Small fragments of S7 (immature) and S10 (mature)
ovaries were fixed by immersion in 0.5% glutaraldehyde, 4% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.2) containing 1 mM CaCl2for 24 h at 4°C. Postfixation
was performed with 1% OsO4 in the same buffer for 1
h followed by 2% aqueous uranyl for 2 h. The samples were washed in cacodylate buffer, dehydrated in graded ethanol, transferred to propylene oxide, and embedded in Spurr’s resin. Thin sections (60–70 nm), stained with uranyl acetate and lead citrate, were examined with a JEOL 100 CX electron microscope.
2.7. Immunocytochemistry
7.2) containing 1 mM CaCl2. After fixation, the
frag-ments were embedded in L.R. White, and placed in gela-tin capsules for polymerization at 37°C.
Silver sections placed onto Formvar-coated nickel grids were washed in TBS (0.02 M Tris(hydroxymethyl)aminomethane, 0.15 M NaCl, pH 8.2) and incubated for 1 h on a drop of TBS containing 1% bovine serum albumin (BSA/TBS). The sections were incubated at 4°C overnight with the primary anti-bodies diluted 1:100 in 1% BSA in TBS. Samples were washed several times in the same buffer and incubated for 1 h on a drop of 15 nm gold-conjugated goat anti-rabbit serum (Amersham, UK) at room temperature. After incubation, the grids were washed several times, fixed in 2.5% glutaraldehyde in cacodylate buffer, stained, and examined as described below.
For double labeling, the grids were first incubated in a mixture of 1:100 diluted primary antibodies (rabbit anti-cathepsin, mouse anti-yolk proteins) at 4°C overnight, followed by incubation for 1 h in 5 nm gold-conjugated goat anti-mouse serum (Amersham, UK) and another hour in 15 nm gold-conjugated goat anti-rabbit serum (Amersham, UK).
3. Results
3.1. Procathepsin and acid phosphatase activities during oocyte maturation
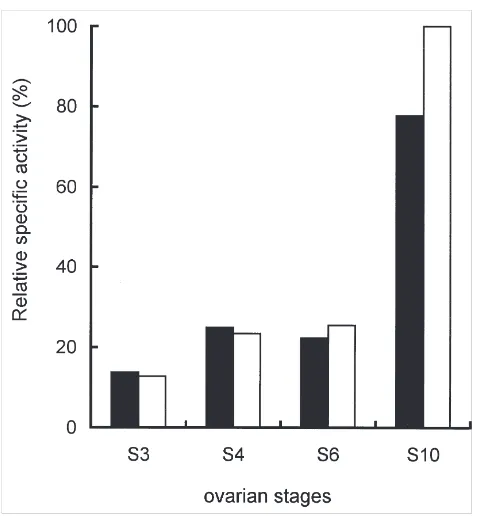
Procathepsin and acid phosphatase activities were determined in ovaries dissected from flies at different vitellogenic stages (Fig. 1). Cathepsin activity was meas-ured after full activation at pH 4.6. Activity profiles sug-gest that both enzymes are stored preferentially between S6 and S10, following a pattern similar to that for yolk
proteins (de Bianchi et al., 1985).
3.2. Yolk proteins and procathepsin localization during oocyte maturation
Sections of immature (S7) ovaries reveal yolk protein
precursors in follicle cells, in the extracellular space between them, and at the surface of the oocyte. The yolk proteins are internalized via coated pits along the oocyte plasma membrane and transferred to the early yolk spheres (Fig. 2).
At this stage of development, the early yolk spheres consist of a homogeneous dense main body containing yolk proteins (Fig. 3). Some of them have, at their per-iphery, a less electrondense region containing procathep-sin (Fig. 4). Procathepprocathep-sin, but not yolk protein, is also present in smaller, heterogeneous lysosome-like granules (Fig. 4).
Fig. 1. Cathepsin and acid phosphatase activities duringMusca dom-esticavitellogenesis. The amount of cathepsin (black) and acid phos-phatase (white) activities were determined at different ovary stages after full activation of cathepsin by incubation at pH 4.6 for 5 h. The activity values (arbitrary units) were divided by the amount of protein. The maximum value was assigned the 100% value.
3.3. Yolk proteins and procathepsin localization in the mature oocytes
Different cell inclusions can be distinguished in S10
oocytes (Fig. 5): Type 1 corresponds to glycogen stored in electron translucent aggregates that contain small ves-icles and thin filaments surrounded by mitochondria; Type 2 inclusions are lipid droplets; and Type 3 are the membrane-limited yolk spheres, highly electron dense when stained with uranyl/lead due to their high protein content. At this final stage of vitellogenesis, it is also possible to find smaller and more heterogeneous gran-ules, resembling lysosomes.
Double labeling of S10oocytes revealed that most yolk
spheres contain both yolk proteins and procathepsin (Figs. 6, 7). Lysosome-like granules, containing mainly procathepsin, are frequently seen in close proximity to the yolk spheres (Figs. 6, 7).
3.4. Fractionation by gradient centrifugation of the particulate materials obtained from mature oocytes
To purify yolk spheres, S10oocytes were mechanically
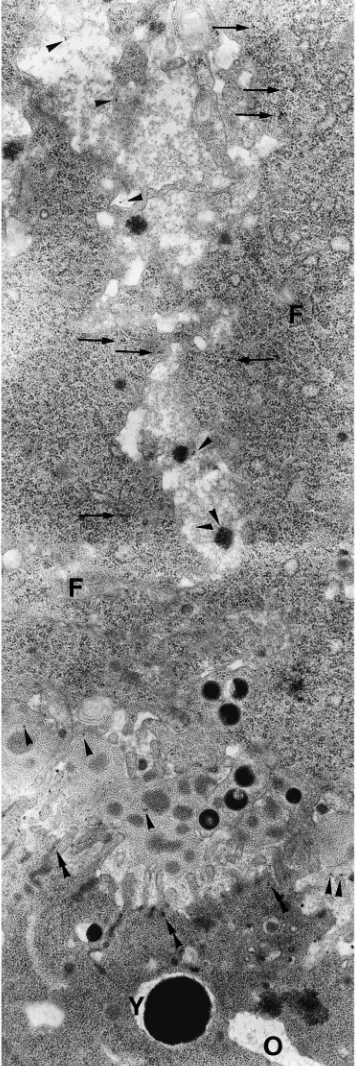
Fig. 2. Immunocytochemistry of S7 oocytes. The internalization of
yolk protein into the immature oocytes was observed with an anti-yolk protein serum. Yolk protein (arrowheads) is present in the space between the follicular cells (F) and between them and the oocyte (O)(perioocyte space). The figure also shows the presence of yolk pro-tein inside the follicular cells (arrows). After internalization (double arrowheads), the protein is accumulated into dense yolk spheres (Y). ×19,000.
Fig. 3. Immunocytochemistry of S7 oocytes with anti-yolk protein
serum. The labeling is distributed on the main body of yolk spheres. ×15,000.
Fig. 4. Immunocytochemistry of S7 oocytes with anti-cathepsin
serum. Both the main body of yolk spheres and the periphery regions as well as heterogeneous granules (arrows) show specific label. ×15,000.
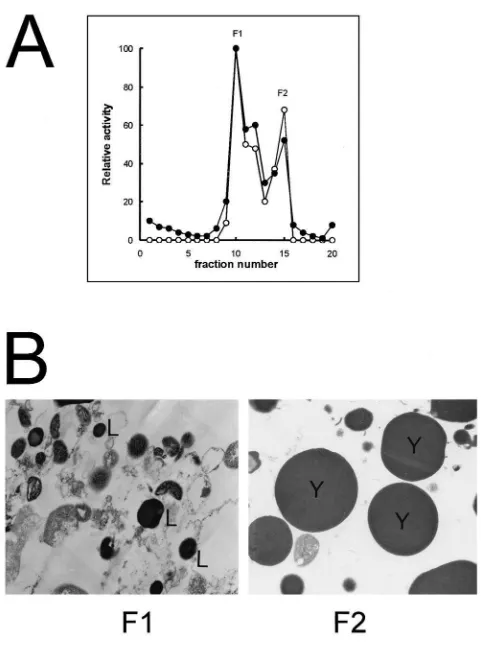
were determined for each fraction. Cathepsin and acid phosphatase activities have similar sedimentation pat-terns and concentrate in the F1 and F2 regions (Fig. 8A). Electron microscopic analysis of the Musca oocyte subcellular fractions revealed that F1 (gradient fraction 10), which contains enzymatic activities, is composed of lysosome-like particles and abundant mitochondria (Fig. 8B), while F2 (gradient fraction 15) which also contains enzymatic activities, consists predominantly of large yolk spheres (Fig. 8B).
4. Discussion
We previously demonstrated thatMuscayolk proteins are synthesized by the fat bodies and also by the ovaries (de Bianchi et al., 1985). Oocyte immunochemical analyses (Fig. 2) are in agreement with the earlier obser-vation and indicate that within the ovaries, the follicle cells are the site of yolk protein synthesis.
Fig. 5. General aspect of mature oocyte cytoplasm showing the different cell inclusions. Electron translucent aggregates containing small vesicles and thin filaments (1) and surrounded by mitochondria (M). 2: Lipid drops. 3: Membrane-limited yolk spheres. L: Lysosomes.×21,400.
Fig. 6. Double labeling of S10oocytes. Most of the yolk spheres
con-tain both yolk proteins (smaller particles, 5 nm) and cathepsin (larger particles, 15 nm). Smaller granules (L) containing mainly cathepsin are frequently seen in close proximity with the yolk spheres.×54,000.
Rhodnius prolixus (Oliveira et al., 1989). Our
ultra-structural analysis of S7 ovaries indicates that, after
internalization, yolk proteins are incorporated into the early yolk spheres, which probably are modified late endosomes (Snigirevskaya et al., 1997a).
Fig. 7. Double labeling of S10oocytes. Most of the yolk spheres
Fig. 8. Subcellular fractionation ofMusca domesticaS10oocytes. A:
Cellular content of S10oocyte was submitted to sucrose gradient
ultra-centrifugation. The gradient was fractionated and cathepsin activity (+–+) and acid phosphatase activity ( • – • ) of the fractions were determined. F1 and F2 are the main fractions containing enzymatic activities. B: Electron microscopy analysis of fractions F1 and F2, obtained from sucrose gradient. The regions of the sucrose gradient containing enzymatic activities (F1 and F2) were analyzed by electron microscopy. L: lisosomes; Y: yolk spheres.×12,500.
In Musca domestica, a procathepsin is present in the mature oocytes, and its activation is dependent on a reduction in pH during embryogenesis, after which the enzyme is responsible for yolk protein degradation (Ribolla et al., 1993; Ribolla and de Bianchi, 1995). An acid phosphatase is also present in Musca mature oocytes (Ribolla et al., 1993) as was described for
Dro-sophila melanogaster (Sawicki and MacIntyre, 1978)
and Aedes aegypti (Raikhel, 1984). The synthesis site
for Musca domestica cathepsin is the ovary, as shown
by Northern blot experiments (Ribolla, 1996; manuscript in preparation).
The relative quantities of cathepsin and acid phospha-tase in the ovaries increase, along with yolk protein pre-cursors (de Bianchi et al., 1985) between S6 and S10.
However, immunocytochemical data suggest that the enzymes are internalized into the oocyte by a different pathway when compared with yolk proteins. Both enzymes are apparently sequestered by developing oocytes and included into lysosome-like vesicles. These
vesicles contain cathepsin and acid phosphatase, but not yolk proteins. Our results strongly suggest that lyso-some-like structures fuse with immature yolk spheres forming the mature yolk spheres. InDrosophilaoocytes, Golgi-derived vesicles surround the associated body in alpha-1 yolk spheres (Giorgi et al., 1993). The authors suggested that vesicles are lysosomes being targeted to the yolk spheres. The inclusion of inactive proteolytic enzymes into storage granules was also demonstrated in rat parathyroid cells (Hashizume et al., 1993). Cathep-sins B and H were localized inside the parathyroid hor-mone-containing granules and in lysosomes near stor-age granules.
It is an interesting question why two or more cargo proteins (proteolytic enzymes and yolk proteins), which are directed to the same final destination, are routed via different cell pathways. Vitellogenin receptors are recycled inAedes aegyptioocytes (Snigirevskaya et al., 1997b). Receptor recycling probably requires a decrease in the vesicle’s pH to dissociate the receptors and ligands (Desbuquois et al., 1992; Suzuki et al., 1997). It was also demonstrated by us that cathepsin is activated by a decrease in pH (Ribolla and de Bianchi, 1995). Since cathepsin activation occurs only after fertilization and fertilization occurs only after oocyte maturation, this enzyme is possibly routed by a different pathway than yolk proteins and is only incorporated in the mature yolk sphere after sorting and recycling of yolk protein recep-tors has been completed.
Mature oocytes must contain in their cytoplasm at least three structures harboring yolk proteins and/or lyso-somal enzymes: the mature yolk spheres containing both the yolk proteins and the enzymes, lysosome-like struc-tures containing only enzymes and immature yolk spheres containing yolk proteins but no enzymes.
The immunocytochemstry experiments suggest that procathepsin is uniformly distributed as mature granules, a situation that is different from what occurs in Aedes aegypti where procathepsin is stored around the periph-ery of the yolk granule (Cho et al., 1999).
The activity profile obtained from S10ovaries’ cellular
fractionation (Fig. 8A) shows that at this stage, cathepsin and acid phosphatase are predominantly present at the lysosome fraction (F1) and not as mature granules (F2). This result indicates that probably fusion between imma-ture granules and lysosomes occurs even after fertiliz-ation during embryogenesis.
Acknowledgements
is a staff member of Departamento de Parasitologia, ICB, USP. A.G.B. is a staff member of Departamento de Bioquı´mica, IQ, USP and recipient of a CNPq fellow-ship.
References
Adams, T.S., 1974. The role of juvenile hormone in housefly ovarian follicle morphogenesis. Journal of Insect Physiology 20, 263–276. Bajkowski, A.S., Frankfater, A., 1975. Specific spectrophotometric
assay for cathepsin B1. Analytical Biochemistry 68, 119–127.
Bast, R.E., Telfer, R.H., 1976. Follicle cell protein synthesis and its contribution to the yolk of the Cecropia moth oocyte. Developmen-tal Biology 52, 83–97.
Berquin, I.M., Sloane, B.F., 1996. Cathepsin B expression in human tumors. Advances in Experimental Medicine and Biology 389, 281–294.
Brennan, M.D., Weiner, A.J., Goralski, T.J., Mahowald, A.P., 1982. The follicle cells are a major site of vitellogenin synthesis in Droso-phila melanogaster. Developmental Biology 89, 225–236. Chen, J.S., Hays, A.R., Snigirevskaya, E.S., Raikhel, A.S., 1995.
Mos-quito cathepsin B-like thiol protease is a yolk protein precursor produced by the fat body, accumulated by oocytes and activated during embryogenesis. Journal of Cellular Biochemistry 21A, 215. Chia, W.K., Morrison, P.E., 1972. Autoradiographic and ultrastructural studies on the origin of yolk protein in the housefly,Musca dom-esticaL. Canadian Journal of Zoology 50, 1569–1576.
Cho, W.-L., Deitsch, K.W., Raikhel, A.S., 1991. An extraovarian pro-tein accumulated in mosquito oocytes is a carboxypeptidase acti-vated in embryos. Proceedings of the National Academy of Sciences USA 88, 10821–10824.
Cho, W.L., Tsao, S.M., Hays, A.R., Walter, R., Chen, J.S., Snigirev-skaya, E.S., Raikhel, A.S., 1999. Mosquito cathepsin B-like pro-tease involved in embryonic degradation of vitellin is produced as a latent extraovarian precursor. Journal of Biological Chemistry 274 (19), 13311–13321.
de Bianchi, A.G., Coutinho, M., Pereira, S.D., Marinotti, O., Targa, H.J., 1985. Vitellogenin and vitellin ofMusca domestica: quantifi-cation and synthesis by fat bodies and ovaries. Insect Biochemistry 15, 77–84.
Desbuquois, B., Lopez, S., Janicot, M., Burlet, H., De Galle, B., Fouque, F., 1992. Role of acidic subcellular compartments in the degradation of internalized insulin and in the recycling of the internalized insulin receptor in liver cells: in vivo and in vitro stud-ies. Diabetic Medicine 18, 104–112.
Fagotto, F., 1990a. Yolk degradation in tick eggs: I. Occurrence of a cathepsin L-like acid proteinase in yolk spheres. Archives of Insect Biochemistry and Physiology 14, 217–235.
Fagotto, F., 1990b. Yolk degradation in tick eggs: II. Evidence that cathepsin L-like proteinase is stored as a latent, acid-activable pro-enzyme. Archives of Insect Biochemistry and Physiology 14, 237–252.
Fagotto, F., 1991. Yolk degradation in tick eggs: III. Developmentally regulated acidification of the yolk spheres. Development Growth and Differentiation 33, 57–66.
Giorgi, F., Lucchesi, P., Morelli, A., Bownes, M., 1993. Ultrastructural analysis ofDrosophilaovarian follicles differing in yolk polypep-tide (yps) composition. Development 117, 319–328.
Giorgi, F., Yin, L., Cecchettini, A., Nordin, J.H., 1997. The vitellin-processing protease ofBlattella germanicais derived from a pro-protease of maternal origin. Tissue and Cell 29, 293–303. Hays, A.R., Raikhel, A.S., 1990. A novel protein produced by the
vitellogenic fat body and accumulated in mosquito oocytes. Roux’s Archives of Development Biology 199, 114–121.
Hashizume, Y., Waguri, S., Watanabe, T., Kominami, E., Uchiyama, Y., 1993. Cysteine proteinases in rat parathyroid cells with special reference to their correlation with parathyroid hormone (PTH) in storage granules. Journal of Histochemistry and Cytochemistry 41, 273–282.
Huebner, E., Tobe, S.S., Davey, K.G., 1975. Structural and functional dynamics of oogenesis inGlossina austeni: vitellogenesis with spe-cial reference to the follicular epithelium. Tissue and Cell 7, 535–558.
Indrasith, L.S., Sasaki, T., Yamashita, O., 1988. A unique protease responsible for selective degradation of a yolk protein inBombyx mori. Journal of Biological Chemistry 263, 1045–1051.
Kindle, H., Ko¨nig, R., Lanzrein, B., 1988. In vitro uptake of vitellog-enin by follicles of the cockroachNauphoeta cinerea: comparison of artificial media with hemoymph media and the role of vitellog-enin concentration and juvenile hormone. Journal of Insect Physi-ology 34, 541–548.
Komukai, M., Kobayashi, K., Horiuchi, S., 1986. Autolysis in the tail muscles of metamorphosing tadpoles. Comparative Biochemistry and Physiology B 85, 55–59.
Medina, M., Leon, P., Vallejo, C.G., 1988.Drosophilacathepsin B-like proteinase: a suggested role in yolk degradation. Archives of Biochemistry and Biophysics 263, 355–363.
Nussenzveig, R.H., Oliveira, P.L., Masuda, H., 1992. Identification of yolk platelet-associated hydrolases in the oocytes ofRhodnius pro-lixus. Archives of Insect Biochemistry and Physiology 21, 253– 262.
Oliveira, P.L., Petretsky, M.D.A., Masuda, H., 1989. Vitellin pro-cessing and degradation during embryogenesis in Rhodnius pro-lixus. Insect Biochemistry 19, 489–493.
Purcell, J.P., Quinn, T.M., Kunkel, J.G., Nordin, J.H., 1988. Corre-lation of yolk phosphatase expression with the programmed proteo-lysis of vitellin inBlattella germanicaduring embryonic develop-ment. Archives of Insect Biochemistry and Physiology 8, 237–251. Raikhel, A.S., 1984. Accumulations of membrane-free clathrin-like lat-tices in the mosquito oocyte. European Journal of Cell Biology 35, 279–283.
Raikhel, A.S., 1987. Monoclonal antibodies as probes for processing of yolk protein in the mosquito; a high-resolution immunolocaliz-ation of secretory and accumulative pathways. Tissue and Cell 19, 515–529.
Ribolla, P.E.M., 1996. Aspectos bioquı´micos e estruturais da vitelog-eˆnese e embriogvitelog-eˆnese de Musca domestica. Tese de Doutorado, Instituto de Quı´mica, USP, Brasil.
Ribolla, P.E.M., De Bianchi, A.G., 1995. Processing of procathepsin from Musca domesticaeggs. Insect Biochemistry and Molecular Biology 25, 1011–1017.
Ribolla, P.E.M., Daffre, S., De Bianchi, A.G., 1993. Cathepsin B and acid phosphatase activities during Musca domestica embryogen-esis. Insect Biochemistry 23, 217–223.
Ro¨hrkasten, A., Ferenz, H.-J., 1985. In vitro study of selective endo-cytosis of vitellogenin by locust oocytes. Wilhelm Roux Archives of Developmental Biology 194, 411–416.
Roth, T.F., Cutting, J.J., Atlas, S.B., 1976. Protein transport: a selective membrane mechanism. Journal of Supramolecular Structure 4, 527–548.
Roth, T.F., Porter, K.R., 1964. Yolk protein uptake in the oocyte of mosquitoAedes aegyptiL. L. Cell Biology 20, 313–318. Sawicki, J.A., MacIntyre, R.J., 1978. Localization at the ultrastructural
level of maternality derived enzyme and determination of the time of paternal gene expression for acid phosphatase-1 inDrosophila melanogaster. Development Biology 63, 47–58.
Snigirevskaya, E.S., Hays, A.R., Raikhel, A.S., 1997a. Secretory and internalization pathways of mosquito yolk protein precursors. Cell and Tissue Research 290, 129–142.
Internal-ization and recycling of vitellogenin receptor in the mosquito oocyte. Cell and Tissue Research 290, 175–183.
Spector, T., 1978. Refinament of the Coomassie blue method of protein quantification. Analytical Biochemistry 86, 142–146.
Suzuki, K., Doi, T., Imanishi, T., Kodama, T., Tanaka, T., 1997. The conformation of the alpha-helical coiled coil domain of macro-phage scavenger receptor is pH dependent. Biochemistry 36, 15140–15146.
Takahashi, N., Kurata, S., Natori, S., 1993. Molecular cloning of
cDNA for the 29 kDa proteinase participating in decomposition of the larval fat body during metamorphosis ofSarcophaga peregrina
(flesh fly). FEBS Letters 334, 153–157.
Tryselius, Y., Hultmark, D., 1997. Cysteine proteinase 1 (CP1), a cathepsin L-like enzyme expressed in theDrosophila melanogaster