Journal of Life Sciences
Volume 6, Number 10, October 2012 (Serial Number 54)
David Publishing Company www.davidpublishing.com
Publication Information
Journal of Life Sciences is published monthly in hard copy (ISSN 1934-7391) and online (ISSN 1934-7405) by David Publishing Company located at 9460 TELSTAR AVE SUITE 5, EL MONTE, CA 91731, USA.
Aims and Scope
Journal of Life Sciences, a monthly professional academic journal, covers all sorts of researches on molecular biology, microbiology, botany, zoology, genetics, bioengineering, ecology, cytology, biochemistry, and biophysics, as well as other issues related to life sciences.
Editorial Board Members
Dr. Stefan Hershberger (USA), Dr. Suiyun Chen (China), Dr. Farzana Perveen (Pakistan), Dr. Francisco Torrens (Spain), Dr. Filipa João (Portugal), Dr. Masahiro Yoshida (Japan), Dr. Reyhan Erdogan (Turkey), Dr. Grzegorz Żurek (Poland), Dr. Ali Izadpanah (Canada), Dr. Barbara Wiewióra (Poland), Dr. Valery Lyubimov (Russia), Dr. Amanda de Moraes Narcizo (Brasil), Dr. Marinus Frederik Willem te Pas (The Netherlands), Dr. Anthony Luke Byrne (Australia), Dr. Xingjun Li (China), Dr. Stefania Staibano (Italy), Dr. Wenle Xia (USA), Hamed Khalilvandi-Behroozyar (Iran).
Manuscripts and correspondence are invited for publication. You can submit your papers via Web Submission, or E-mail to [email protected] or [email protected]. Submission guidelines and Web Submission system are available at http://www.davidpublishing.com.
Editorial Office
9460 TELSTAR AVE SUITE 5, EL MONTE, CA 91731, USA Tel: 1-323-9847526, Fax: 1-323-9847374
E-mail:[email protected], [email protected]
Copyright©2012 by David Publishing Company and individual contributors. All rights reserved. David Publishing Company holds the exclusive copyright of all the contents of this journal. In accordance with the international convention, no part of this journal may be reproduced or transmitted by any media or publishing organs (including various websites) without the written permission of the copyright holder. Otherwise, any conduct would be considered as the violation of the copyright. The contents of this journal are available for any citation. However, all the citations should be clearly indicated with the title of this journal, serial number and the name of the author.
Abstracted / Indexed in
Database of EBSCO, Massachusetts, USA Chemical Abstracts Service (CAS), USA Cambridge Scientific Abstracts (CSA), USA
Chinese Database of CEPS, American Federal Computer Library center (OCLC), USA Ulrich’s Periodicals Directory, USA
Chinese Scientific Journals Database, VIP Corporation, Chongqing, China Universe Digital Library S/B, Proquest
Subscription Information
Price (per year): Print $520, Online $360, Print and Online $680.
David Publishing Company
9460 TELSTAR AVE SUITE 5, EL MONTE, CA 91731, USA Tel: 1-323-9847526, 323-410-1082; Fax: 1-323-9847374 E-mail: [email protected]
David Publishing Company www.davidpublishing.com
J LS
Journal of Life Sciences
Volume 6, Number 10, October 2012 (Serial Number 54)
Contents
Biochemical and Molecular Biology
1077 The Study of Cholesterol Content in Synbiotic Fermented Dairy Products
Ilze Beitane and Inga Ciprovica
1082 Metabolic and Endocrine Responses of Desert-Adapted Mice Reproductive System to Increased Salinity
Elena Bukovetzky, Fuad Fares and Abraham Haim
1094 Influence of Abiotic Elicitors on Accumulation of Thymol in Callus Cultures of Origanum vulgare L.
Abedaljasim M. Aljibouri, Ashwaq S. Abd, Duha M. Majeed and Eman N. Ismail
1100 Low Peptone Dose as Inductor of Alkaline Protease Promoter Used for Invertase Gene Expression in Yarrowia lipolytica
Łukasz Śnieżewski, Ewa Walczak, Zbigniew Lazar and Małgorzata Robak
Biomedicine
1109 In Vitro Study on Virulence Potentials of Burkholderia pseudomallei Isolated from Immunocompromised Patients
Hadeel Tawfiq Al-Hadithi and Rana Muhammad Abdulnabi
1117 The Control of Malaria among PLWHA in Calabar, Cross River State, Nigeria
Patience Edoho Samson-Akpan, Olaide Bamidele Edet, Ekaette Francis Asuquo, Mary Achi Mgbekem and Idang Neji Ojong
1124 Spatiotemporal Distribution of Phlebotomine Sand Flies (Diptera: Psychodidae) in a Focus of Cutaneous Leishmaniasis in Foum Jamâa (Azilal, Atlas of Morocco)
1133 Determination of Fungal Colonization in the Oral Cavity of College Students
Floridia Ricardo, Rodriguez Graciela, Ampuero Verónica and Gonzalez Luis
1142 Preliminary Results of Crayfish Distribution and Diseases in Latvia
Inese Briede
Interdisciplinary Researches
1145 The Effects of Simultaneous Application of Different Organic and Biological Fertilizers on Quantitative and Qualitative Characteristics of Cucurbita pepo L.
Mohsen Jahan, Alireza Koocheki, Mohammad-Kazem Tahami, Mohammad-Behzad Amiri and Mahdi Nassiri-Mahallati
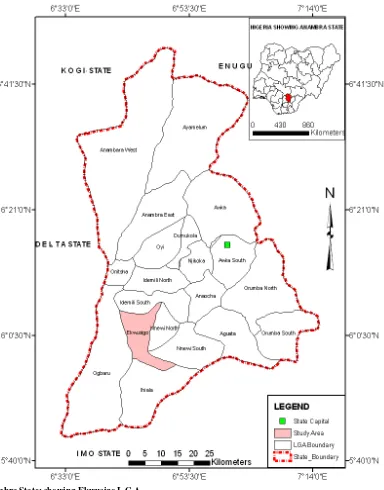
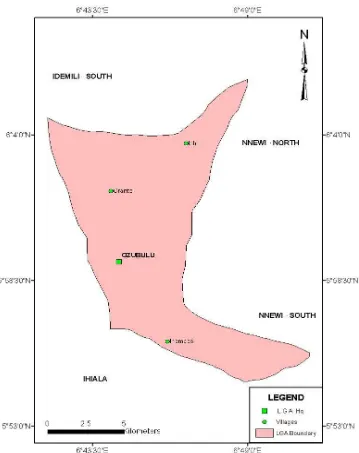
1150 The Impact of Deforestation in Anambra State: The Ekwusigo Example
Joel Ekwutosi Umeuduji and Chukwuma Onyebueke Egbuonu
1158 Contribution of Study Bioecology of the Fauna Chamaerops humilis in the Region of Tlemcen (Algeria)
Damerdji Amina
1167 The Floristic Diversity of the Tlemcen Southern Slope Scrublands (Western Algeria)
Belhacini Fatima and Bouazza Mohammed
1174 Tools for Protein Structure Prediction at the bri-shur.com Web Portal
Sergey Feranchuk, Ulyana Potapova, Vladimir Potapov, Dmitry Mukha, Vladimir Nikolaev and Sergei Belikov
1180 The Elaboration of Horse Meat Products Technology
Аbzhanova Sholpan, Kizatova Мaigul, Мukhtarkhanova Rauan, Тarakbaeva Raushan and
The Study of Cholesterol Content in Synbiotic
Fermented Dairy Products
Ilze Beitane and Inga Ciprovica
Faculty of Food Technology, Latvia University of Agriculture, Jelgava LV-3001, Latvia
Received: April 11, 2012 / Accepted: June 22, 2012 / Published: October 30, 2012.
Abstract: The influence of prebiotics as lactulose as well inulin on the ability of Bifidobacterium lactis to reduce cholesterol in milk was studied during milk fermentation. Pasteurized milk, freeze-dried starter culture Bb-12 (Bifidobacterium lactis, Chr. Hansen, Denmark), inulin—RAFTILINE®HP (ORAFI, Belgium), syrup of lactulose (Duphalac®, the Netherlands) in following concentrations: 0, 1%, 2%, 3%, 4% and 5% were used for experiments. The fermentation process was realized at 37 °C for 16 h. The content of cholesterol was determined according to AOAC Official Method 976.26A. The results showed that it is possible considerable to lower the level of cholesterol in fermented milk using B. lactis. The ability of B. lactis to decrease the level of cholesterol in milk can be influenced with addition of prebiotics. The lower concentration of cholesterol was determined in fermented samples with 4% of lactulose (9.5 mg/100g) and with 1% of inulin (10.4 mg/100g). Evaluating the influence of prebiotics on cholesterol content in fermented milk samples, it is obvious that the influence depends on the type of prebiotics (P > 0.05) and their concentrations (P < 0.05).
Key words: Cholesterol, B. lactis, lactulose, inulin, fermented milk.
1. Introduction
Elevated total cholesterol and LDL (low-density lipoprotein) cholesterol levels are widely represented as a contributory risk factor for the development of artherosclerosis, coronary heart disease and stroke [1-3]. It has been reported that hypercholesterolemia promotes to 45% of heart attacks in Western Europe and 35% in Central and Eastern Europe [4]. In addition, it is known that high cholesterol levels and mortality are close related [5]. Manson et al. [6] have pointed out that even a 1% reduction in serum cholesterol could reduce the risk of coronary heart disease by 2-3%. Therefore it is important to control the cholesterol intake by food and to use the products with the ability to lowering the blood cholesterol level. Fermented milk products
Inga Ciprovica, Ph.D., Prof., research field: dairy science and technology. E-mail: [email protected].
Corresponding authors: Ilze Beitane, Ph.D., Assist. Prof., research field: functional dairy products. E-mail: [email protected].
have been recommended as dietary supplements because of their hypocholesterolemic effect in humans [7]. Evaluating the relationship between LAB (lactic acid bacteria) and the serum cholesterol, it has found that lactobacilli or bifidobacteria can exhibit hypocholesterolemic properties in humans [8-10]. The possible mechanism it could be, that LAB with active bile salt hydrolase or products containing them have been suggested to lower cholesterol levels through interaction with host bile salt metabolism [11]. Bifidobacteria are one of the most important probiotics associated with human health. They have varied positive influence on human health, inter alia, the lowering of serum cholesterol in blood [10]. Xiao et al. [10] observed
that consumption of Bifidobacterium milk leads to a
The Study of Cholesterol Content in Synbiotic Fermented Dairy Products 1078
cholesterol level in blood by regular intake. One of the possibilities is to add probiotics and prebiotics in dairy products. The numerous studies indicate that bifidobacteria have the ability to assimilate cholesterol [12-14] which should be promoted with adding prebiotics. There are limited studies [12] about the influence of prebiotics on the ability of bifidobacteria to reduce the level of cholesterol in fermented milk. Therefore the task of the research was to investigate the influence of lactulose and
inulin on the ability of Bifidobacterium lactis to
reduce cholesterol level in fermented dairy product during milk fermentation.
2. Materials and Methods
Pasteurized milk with fat content 2.5% and the
strain of Bifidobacterium lactis (Bb-12, Chr.Hansen,
Denmark) was used for experiments. During the experiments, the culture was maintained at -18 °C. As
prebiotics were used inulin RAFTILINE®HP (ORAFI,
Belgium) with polymerization degree ≥ 5 and degree
of purity 99.5% and syrup of lactulose (Duphalac®,
the Netherlands) with following composition (%): lactulose—no less than 67, lactose—less than 6, galactose—less than 10.
Different lactulose and inulin concentrations (1%; 2%; 3%; 4% and 5%) were added individually to 100
g of milk. Bifidobacterium lactis was inoculated with
2 mL of milk suspension (106 cfu/mL) and cultured at
37 °C for 16 h. The control sample was prepared without the prebiotics for comparing with the obtained results.
The level of cholesterol was determined according to AOAC Official Method 976.26A.
The differences at the level of cholesterol were analyzed using the analysis of variance (ANOVA).
t-test was applied to compare the mean values, and
P-value at 0.05 was used to determine the significant
differences. Experiments were carried out in triplicate.
3. Results and Analysis
Cholesterol is included in the membrane of fat globules, and it makes up to 95% of the total sterol content [15], the others 5% are cholesteryl esters. The cholesterol content in milk is within the range from 0.09 g/L to 0.22 g/L, on average 0.16 g/L [16]. The cholesterol content in milk is possible to decrease by different techniques as cholesterol distillation, complexes with cyclodextrins as well microorganisms [13].
Consequently, ability of Bifidobacterium lactis to
decrease the cholesterol level in fermented milk, as well as the content of decreased cholesterol level influenced by added prebiotics was studied.
The level of cholesterol in milk, control and in fermented milk samples with different concentrations of lactulose and inulin is presented in Fig. 1.
Fig. 1 The level of cholesterol in milk, control and in fermented milk samples with different concentrations of lactulose and inulin. 18.0
10.4 10.510.9 11.5 11.2
The research results showed that it is considerable possible to lower the level of cholesterol in milk using
B. lactis. The ability of B. lactis to decrease the level of cholesterol in milk can be induced with adding the prebiotics. The lower content of cholesterol was determined in fermented milk samples with 4% of lactulose (9.5 mg/100g) and with 1% of inulin (10.4 mg/100g). It confirms the conclusions of Palframan et al. [17] that a greater bifidogenic effect of inulin was obtained in 1% concentration. It indicates on the
relationship between the multiply of B. lactis and the
assimilation of cholesterol in milk. In preliminary studies were established the ability of inulin and
lactulose to promote the growth of B. lactis in milk
(Table 1).
The results showed that the highest amount of B.
lactis did not provide the lowest level of cholesterol in fermented milk with prebiotics. It is linked with the activity of cholesterol esterase to catalyze the hydrolysis of cholesteryl esters [19], thereby it should be decreased the level of cholesterol in fermented milk. The beneficial influence of lactulose on cholesterol level in fermented milk can be explained with the ability of lactulose to promote the growth of bifidobacteria comparing with other prebiotics [20, 21].
Evaluating the research data it should be concluded that influence of different prebiotics is not significant
(P > 0.05). The type of prebiotics has not significant
influence on the content of cholesterol in milk. The significant decrease was determined in fermented milk
samples with different concentration of prebiotics (P <
0.05). There is established between control sample and fermented milk sample with 4% of lactulose.
Summarizing the research results it should be induced that the considerable decrease of cholesterol content should be reached up to 25% during milk fermentation. This tendency is possible to facilitate by using appropriate prebiotics.
4. Discussion
In scientific articles there are achievements that consumption of fermented dairy products significantly decreases the cholesterol level in blood serum [22, 23] and bifidobacteria have the ability to lower serum cholesterol level in humans [14]. Manning et al. [24] have indicated the ability of lactic acid bacteria to decrease the total and LDL cholesterol level in blood. Whereas Kiessling et al. [25] in study with human reported about increase of high-density lipoprotein
(HDL) level but no reduction in total cholesterol (P =
0.001) in subjects fed yoghurt with Lactobacillus
acidophilus and B. longum. Contradictory data in literature show the necessity to continue the research in this field, because the mechanism how it happens is not quite clear yet. The production of bile salt hydrolase has been suggested as one of possible mechanisms [26].
The effect of lactic acid bacteria is inconsistent; there is possible the significant decrease of cholesterol content and also unchangeable cholesterol content. It depends mainly on the bacteria species used for fermentation [27]. Pereira’s [12] research has
confirmed that depending on the species of bacteria
the decrease of the cholesterol content is possible from 0.4% to 47% in the selective culture mediums. Zhao
et al. [28] reported that Lactobacillus acidophilus was
Table 1 The influence of the concentrations of lactulose and inulin on the growth rate of B. lactis in fermented milk samples, lg cfu·mL-1 [18].
Concentrations(%) Lactulose Inulin
1 8.5 b 8.6 b
The Study of Cholesterol Content in Synbiotic Fermented Dairy Products 1080
effective in reducing cholesterol level in MRS medium. Whereas Ziarno et al. [13] have indicated
that Lactobacillus acidophilus and Bifidobacterium
spp. in fermented milk are able to assimilate cholesterol from 18% to 38%.
The results of the research have shown that it is possible to achieve a considerable decrease of the
cholesterol content if B. lactis is used for milk
fermentation. The obtained results have confirmed with the statements expressed in literature about the ability of lactic acid bacteria and bifidobacteria to decrease the cholesterol content in milk [29].
Consequently, it may be maintained, that B. lactis is
able to influence the cholesterol content in fermented milk. It is obvious that the influence depends on the
type of the used prebiotics (P > 0.05) and their
concentrations (P < 0.05). A parallel may be drawn
with information described in literature. Delzenne et al. [30] have indicated to ability of inulin to suppress the synthesis of triglycerides, so decreasing the cholesterol level in blood. Roberfroid [31] reported if 2.5% of fructo-oligosaccharides were added to yogurt it was possible to facilitate the decrease of cholesterol in blood. Similar tendencies were obtained in the research where the cholesterol content in fermented milk samples was considerable decreased by adding
lactulose (P < 0.05) and inulin (P > 0.05). In general
the research results confirm the achievements reported in literature about the ability of bifidobacteria, in this
case of B. lactis, to assimilate the content of
cholesterol in milk.
5. Conclusions
The ability of B. lactis to decrease the level of
cholesterol in fermented milk should be induced with adding the prebiotics. The lower content of cholesterol was determined in fermented milk samples with 4% of
lactulose (9.5 mg·100g-1) and with 1% of inulin (10.4
mg·100g-1). It is obvious that the influence depends on
the type of the used prebiotics (P > 0.05) and their
concentrations (P < 0.05).
Acknowledgments
Publication and disamination of research results has been made due to the funding of the ERAF Project “Promotion of scientific activities of LLU”, Contract Nr. 2010/0198/2DP/2.1.1.2.0/10/APIA/VIAA/020.
References
[1] S.R.B.M. Eussen, C.J.M. Rompelberg, O.H. Klungel, J.C.H. van Eijkeren, Modelling approach to simulate reductions in LDL cholesterol levels after combined intake of statins and phytosterols/-stanols in humans, Lipids in Health and Disease [Online], 10 (2011) 187, http.//www.lipidworld.com/content/10/1/187 (accessed Oct. 21, 2011).
[2] N. Xie, Y. Cui, Y.N. Yin, X. Zhao, J.W. Yang, Z.G. Wang, et al., Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet, BMC Complementary and Alternative Medicine [Online], 11 (2011) 53, http.//www.biomedcentral.com/1472-6882/11/53
(accessed July 3, 2011).
[3] I.A.A. El-Gawad, E.M. El-Sayed, S.A. Hafez, H.M. El-Zeini, F.A. Saleh, The hypocholesterolaemic effect of milk yoghurt and soy-yoghurt containing bifidobacteria in rats fed on a cholesterol-enriched diet, International Dairy Journal 15 (2005) 37-44.
[4] P.S. Yusuf, S. Hawken, S. Ôunpuu, T. Dans, A. Avezum, F. Lanas, et al., Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study, Lancet 364 (9438) (2004) 937-952.
[5] C.L. Chen, T.M. Pan, Red mold dioscorea: A potentially safe traditional function food for the treatment of hyperlipidemia, Food Chemistry 134 (2012) 1074-1080. [6] J.E. Manson, H. Tosteson, P.M. Ridker, S. Satterfield, P.
Herbert, G.T. O’Connor, et al., The primary prevention of myocardial infarction, The New England Journal of Medicine 326 (21) (1992) 1406-1416.
[7] G.V. Mann, A factor in yoghurt which lowers cholesterolaemia in man, Atherosclerosis 26 (1977) 335-340.
[8] M. Agerbaek, L.U. Gerdes, B. Richelsen, Hypocholesterlaemic effect of a new fermented milk product in healthy middle-aged men, European Journal of Clinical Nutrition 49 (5) (1995) 346-352.
(1999) 43-50.
[10] J.Z. Xiao, S. Kondo, N. Takahashi, K. Miyaji, K. Oshida, A. Hiramatsu, et al., Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers, Journal of Dairy Science 86 (7) (2003) 2452-2461.
[11] I. De Smet, P. De Boever, W. Verstraete, Cholesterol lowering in pigs through enhanced bacterial bile salt hydrolase activity, British Journal of Nutrition 56 (2) (1998) 185-194.
[12] D.I.A. Pereira, G.R. Gibson, Effect of consumption of probiotics and prebiotics on serum lipid levels in humans, Critical Reviews in Biochemistry and Molecular Biology 37 (2002) 259-281.
[13] M. Ziarno, E. Sekul, A.A. Lafraya, Cholesterol assimilation by commercial yoghurt starter cultures, ACTA Scientiarum Polonorum Technologia Alimentaria 6 (2007) 83-94.
[14] D.A. Russell, R.P. Ross, G.F. Fitzgerald, C. Stanton, Metabolic activities and probiotic potential of bifidobacteria, International Journal of Food Microbiology 149 (2011) 88-105.
[15] R.G. Jensen, R.W. Clark, Lipid composition and properties, in: N.P. Wong (Ed.), Fundamentals of Dairy Chemistry, Elsevier Applied Food Sciences, London, 1988, pp. 171-213.
[16] V. Piironen, I. Toivo, A.M. Lampi, New data for cholesterol contents in meat, fish, milk, eggs and their products consumed in Finland, Journal of Food Composition and Analysis 15 (2002) 705-713.
[17] R.J. Palframan, G.R. Gibson, R.A. Rastall, Effect of pH and dose on the growth of gut bacteria on prebiotic carbohydrates in vitro, Anaerobe 8 (2002) 287-292.
[18] I. Beitane, I. Ciprovica, Prebiotics—The influencing factors on growth and survival of probiotics in milk, Proceedings of the Latvia University of Agriculture 21 (316) (2008) 42-50.
[19] D.Y. Hui, J.A. Kissel, Sequence identity between human pancreatic cholesterol esterase and bile salt-stimulated milk lipase, Biomedical Division 276 (1990) 131-134. [20] R.A. Rastall, V. Maitin, Prebiotics and synbiotics:
Towards the next generation, Current Opinion in Biotechnology 13 (2002) 490-496.
[21] C.E. Rycroft, M.R. Jones, G.R. Gibson, R.A. Rastall, A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides, Journal of Applied Microbiology 91 (2001) 878-887.
[22] G.V. Mann, A. Spoerry, Studies of surfactant and cholesteremia in the Massai, American Journal of Clinical Nutrition 27 (1974) 464-469.
[23] A.M.P. Gomes, F.X. Malcata, Bifidobacterium spp. and
Lactobacillus acidophilus: Biological, biochemical, technological and therapeutical properties relevant for use as probiotics, Trends in Food Science & Technology 10 (1999) 139-157.
[24] T.S. Manning, G.R. Gibson, Prebiotics, Best Practice & Research Clinical Gastroenterology18 (2004) 287-298. [25] G. Kiessling, J. Schneider, G. Jahreis, Long-term
consumption of fermented dairy products over 6 months increases HDL cholesterol, European Journal of Clinical Nutrition 56 (2002) 843-849.
[26] M.P. St-Onge, E.R. Farnworth, P.J.H. Jones, Consumption of fermented and nonfermented dairy products: Effects on cholesterol concentrations and metabolism, American Journal of Clinical Nutrition 71 (2000) 674-681.
[27] H. Oberman, L. Libudzisz, Fermented milks, in: B.J.B. Wood (Ed.), Microbiology of Fermented Foods, 2nd ed., Vol. 1, Blackie Academic & Professional, London, 1998, pp. 308-351.
[28] R. Zhao, J. Sun, H. Mo, Y. Zhu, Analysis of functional properties of Lactobacillus acidophilus, World Journal of Microbiology and Biotechnology 23 (2007) 195-200. [29] Ch. Daly, G.F. Fitzgerald, L. O’Connor, R. Davis,
Technological and health benefits of dairy starter cultures, International Dairy Journal 8 (1998) 195-205.
[30] N.M. Delzenne N.N. Kok, Biochemical basis of oligofructose-induced hypolipidaemia in animal models, Journal of Nutrition 129 (1999) 1467-1470.
Journal of Life Sciences 6 (2012) 1082-1093
Metabolic and Endocrine Responses of Desert-Adapted
Mice Reproductive System to Increased Salinity
Elena Bukovetzky1, Fuad Fares1 and Abraham Haim1, 2
1. Department of Evolutionary and Environmental Biology, University of Haifa, Mount Carmel, Haifa 31905, Israel
2. The Israeli Center for Interdisciplinary Studies in Chronobiology, University of Haifa, Mount Carmel, Haifa 31905, Israel
Received: March 12, 2012 / Accepted: April 27, 2012 / Published: October 30, 2012.
Abstract: From an evolutionary point of view, reproduction timing is an important adaptation which enables the transfer of genetic properties, thus enabling species continuation. Rodents inhabiting arid environments need reliable cues for triggering their reproduction. Results of previous studies showed that increased dietary salinity plays an important role as an ultimate regulator for desert adapted rodents’ reproductive system. The authors aimed discovering pathways by which high salinity can affect the reproductive system and metabolic status of desert adapted common spiny mice, Acomys cahirinus. Mice were challenged with osmotic stress, water source salinity increased gradually from 0.9% - 5% NaCl under short days (SD) and long days (LD). The authors assessed leptin and free fatty acid (FFA) levels using ELISA while, SYBR green technology was used for relative receptor expression (RQ) of target genes. Results revealed that serum levels of the hormone leptin were significantly (P < 0.05) reduced in salinity treated (ST) mice. Levels of FFA were significantly (P < 0.05) increased in LD- and SD-ST-males. In ST-SD females a significant increase (P < 0.05) in expression levels of leptin (Ob-Rt) mRNA receptor gene, in ovaries was noted. Aldosteron (Nr3c2) and vasopressin (AVP) mRNA receptor expression genes levels were significantly (P < 0.05) increased in both LD- and SD- ST- males.
Key words:Acomys cahirinus, salinity, desert-adapted, AVP, Nr3c2, Ob-Rt receptor genes, leptin, FFA.
1. Introduction
Mammals are known to use various environmental cues to forecast the occurrence of the optimal season for reproduction. One such cue most commonly used by them is day length changes (photoperiod) [1, 2], which is a common cue in predictable ecosystems with minimal year to year changes in climatic conditions. Desert regions are unpredictable and therefore, additional cues for successful breeding are needed. Because of unpredictable climatic changes, desert species could be “misled” if they entirely rely only on photoperiod. However, photoperiod is used as an initial cue for breeding regulation of desert-adapted rodents [3-5].
Corresponding author: Elena Bukovetzky, Ph.D. candidate, research fields: environmental biology and endocrinology. E-mail: [email protected].
concentration in plants elevate as the dry season progresses since the last water input into the ecosystem. It was suggested that increased dietary salinity could be used as an ultimate signal for reproductive system regulation of the desert adapted
golden spiny mouse Acomys russatus [4]. The results
of previous studies showed a strong negative effect of high salt diet on the reproductive status of
desert-adapted males and females of A. russatus but
not on a mesic population of common spiny mice
Acomys cahirinus [5]. Sheep females, kept on high salt intake diet during pregnancy showed hormonal changes, which can negatively affect the offspring via a mechanism called fetal programming [6]. High salinity gradually treated rodents showed a significant
decrease in body mass (Wb). It has previously been
suggested that high salt consumption decrease food intake in sheep [9].
Another mechanism by which the increased dietary salinity affects reproduction in rodents could be associated with the hormones vasopressin [4, 5] and the hormone leptin, the latter produced by adypocites,
which regulates Wb, metabolism and reproduction [10, 11].
Salinity treatment to desert adapted A. russatus,
revealed a significant decrease in Wb, progressive
reproductive hiatus (decreasing number of estrous cycles and relative decreased testis mass), which was coupled with a massive (about 80%) reduction of white adipose tissue (WAT) mass, compared with their control group [5].
The primary role of WAT commonly referred to as “fat” in mammals [12], is to store free fatty acids (FFA) as triglycerides (triacylglycerol) during periods of caloric excess (lipogenesis) and to mobilize these reserves and release free fatty acids for ATP synthesis via the Kreb’s Cycle during periods when expenditure of calories exceeds the intake (lipolysis) [12]. WAT is now recognised as a multifunctional organ; in addition to the central role of lipid storage, it has a major endocrine function secreting several hormones, notably leptin. Clearly, the emerging picture of WAT
as an endocrine and immunological organ incorporates cross-talk and co-stimulation of a multitude of hormonal signals [13]. It is possible that localized hormonal positive and negative feedback loops interact with the tissue itself and/or through additional pathways.
By the identification of leptin, the adipocyte-derived hormone, and the leptin gene (Lep Ob), followed by its cognate receptor Ob-Rt located within the ventral medial hypothalamus (VMH), paved the way for this area of research. Leptin is a 167 amino acid residue hormone almost exclusively derived from WAT that shares structural similarities with cytokines. The primary effects of leptin involve the mobilization of lipolytic pathways of energy expenditure as it signals to the hypothalamus that energy sufficiency has been met [14].
In rodents it is well established that leptin gene expression and plasma levels are proportional to total body WAT stores [15]. Recently, it has been reported that leptin has a role in the early onset of reproduction [16, 17]. In mice it was noted that leptin serves as a permissive signal for the reproductive system [18]. Results of studies on bats have shown that serum leptin levels are positively correlated with WAT mass and negatively correlated with the reproductive function [19, 20]. Leptin has been shown to promote sexual maturation in various rodent species and its role in reproduction has been investigated at various sites within the hypothalamo-pituitary-gonadal axis [21]. It was suggested that the pituitary-derived leptin acts on the gonads and many other organs equipped with leptin receptor through endocrine function in addition to paracrine action with pituitary hormone secretory cells [22].
Metabolic and Endocrine Responses of Desert-Adapted Mice Reproductive System to Increased Salinity
1084
pharmacological studies have suggested that aldosterone may promote adipogenesis [24, 25]. It was noted that chronic exposure to aldosterone induces morphological, biochemical and molecular markers of adipose conversion by stimulating the adipogenic transcriptional program [26].
The results of a study carried out on obese prone (OP) rats [27], revealed that high-salt intake induced an increase in the size of WAT but a reduction in number of adipocytes, accompanied by twofold increase in circulating leptin. It was suggested that high sodium chloride content diet could modulate leptin levels, independently of obesity. In accordance with this recently conceived concept regarding leptin physiology [28], rodents treated with a high salt (HS)
diet were resistant to metabolic effects of leptin: as Wb,
and adipose mass-reducing [29, 30], but leptin’s sympatho-excitatory actions still remains intact. The accumulated knowledge so far brings us to test the follow hypothesis: if leptin, vasopressin and aldosterone are involved in breeding regulation of desert-adapted rodents than the three hormones will have a direct effect through their receptors on the
gonads or indirect effect of these hormones via WAT.
In order to establish the metabolic status of the tested mice we also detected serum levels of free fatty acid (FFA) and leptin in males and females of a desert
adapted population of common spiny mice, A.
cahirinus acclimated to short day (SD) or long day (LD) photoperiods.
2. Materials and Methods
Studied mice were recruited from a desert adapted
laboratory colony of A. cahirinus, maintained at
Oranim campus, University of Haifa. Mice in the colony are descendants of individuals originally captured on the western Dead Sea shores. All tested mice, at the beginning of acclimation, were adults, aged four months. In the colony, mice were maintained at an ambient temperature of 26 ± 2 °C under a photoperiod regime of 12L:12D. In order to
avoid bias results related to Wb, both SD and LD
acclimated groups had a similar average Wb(34-36 ± 3
g) at the beginning of experiments. Ethical clearance for the use of animals was provided by The Ethical Committee, University of Haifa [8].
2.1 Acclimation to Increasing Salinity under Different Photoperiod Regimes
36 individuals (18 females and 18 males) were acclimated to short days (SD, 8L:16D, lights on from 07:00 a.m.) while 26 individuals (12 females and 14 males) were acclimated to long days (LD, 16L:8D, lights on from 07:00 a.m.) inside a controlled environmental cabinet (158 × 77 × 74 cm; Meditest 600/1300, Austria) for three weeks. As these individuals were used also in for measuring other variables for further information on acclimation groups [8].
2.2 Sacrificing Animals and Collecting Samples
At the end of acclimation to the highest dietary salinity (5% NaCl) mice were, anesthetized by injecting a cocktail of Ketamin (10 mg/kg) and Rampoone (100 mg/kg), and then sacrificed by decapitation. All animals were sacrificed between 10:00 and 12:00 as reported earlier for our studied individuals [8]. (For both photoperiod groups three hours after lights were on). Blood samples were collected and centrifuged at 3,000 rpm for 10 min. Serum was collected and stored at -20 °C for analyzing serum leptin hormone level and FFA. WAT and gonads were removed and weighed. The different organs mass was determined by using an analytical scale (1907 MP8 Sartorius, Germany) and
calculated as percent of Wb. Organs were stored at
-20 °C in RNA later (Beit Haemek, Israel) for RNA stability.
2.3 Determination of Serum Leptin Levels
according to manufacturer’s instructions. This assay employs the quantitative sandwich enzyme immunoassay technique. The product of these
enzymatic reactions determined spectrophotometrically, by ELISA reader (Power
Wave XS, BioTek Gen 5) at a wavelength of 450 nm.
2.4 Determination of Serum FFA Levels
In the assay used, fatty acids are converted to their CoA derivates, which are subsequently oxidized with the concomitant generation of color/fluorescence. C-8 (octanoate) and longer fatty acids can be easily quantified by colorimetric (570 nm) method with palmitic acid employed as a standard (Free Fatty Acid Quantification Kit, BioVision, USA) according to manufacturer’s instructions.
2.5 Total RNA Extraction and Reverse Transcription
The reverse transcription-polymerase chain reaction (RT-PCR) has become a standard tool in quantification of gene expression analysis studies [31]. Total RNA was extracted from mice testis using RNeasy Mini Kit (Qiagen, Germany). For mRNA purification from testis and RNA extraction from WAT the authors used EZ-RNA kit (Biological Industries, Israel, Beit Haemek Ltd.). Isolated total RNA was quantified photometrically at a wavelength of 260 nm. Quality of RNA was verified
by loading 1 μL of total RNA onto a RNA 6,000
Nano Chip using the Nano Assay 600 Bioanalyzer (Agilent, Waldbronn, Germany) following the manufacturer’s instructions. Single stranded cDNA
was generated out of 0.5 μg total RNA using a High
Capacity cDNA RT Kit (Applied Biosystems, UK). The obtained cDNA was stored (-20 °C) for further analysis.
2.6 Receptors Detection
Testis and WAT were tested for the expression of aldosteron (Nr3c2: For reference we used NCBI sequence NM_001083906.1) and vasopressin (AVPr:
For reference we used NCBI sequence NM_016847.2) receptor genes. The expression of leptin receptor was measured only in gonads (Ob-Rt: For reference we used NCBI sequence NM_146146.2). For reference housekeeping gene GAPDH (glyceraldehyde-3- phosphate dehydrogenase: NM_008084.2 as reference sequence) was used.
2.7 Primer Design
All primer sets on exon-exon junction site, were
designed to have a Tm of approximately 60 °C, to
have a GC content of approximately 50%, and to generate a PCR amplicone less than 150 bps. Finally, BLAST searchers were performed on primer pair sequences using the NCBI database to check for uniqueness. Primer sets and identifiers are provided (Appendix 1).
2.8 qRT-PCR Amplification
Metabolic and Endocrine Responses of Desert-Adapted Mice Reproductive System to Increased Salinity
1086
2.9 Statistical Analysis
All values are given as mean ± SEM, measured values of control and experimental groups were
compared using two-tailed independent t test on SPSS
15.0.1 for Windows. For group comparison one way ANOVA was used. Post-Hoc test was conducted using LSD. Difference in mean values were
considered significant when P < 0.05. Actual
probability values were given for each comparison.
3. Results and Analysis
The metabolic status of SD- and LD-acclimated males and females (control) as well as ST-males and females was estimated from serum FFA and leptin concentrations. The relation between WAT as metabolic and endocrine tissue is assessed from the correlation between FFA and leptin (Figs. 1 and 2).
In order to assess these relations with the reproductive system, the response to increased salinity in diet is evaluated from the mRNA receptors expression of Nr3c2, AVP and Ob-Rt in the gonads (Figs. 3 and 4).
In addition, the role of photoperiod effect on metabolic and reproduction molecular response was
highlighted, in both sexes of desert-adapted A.
cahirinus population by studying receptors activation in the gonads.
Because of massive reduction in Wb, and WAT
mass as result of salinity treatment, WAT could only be collected from LD and SD control groups mRNA receptor expression using our primers only Aldosterone, but not AVP was detected. However, no significant differences between the two photoperiod acclimated groups were noted.
3.1 Female Responses to Photoperiod Manipulations and ST Treatment
FFA levels were significantly elevated in ST-LD-acclimated group, compared with the
ST-SD-mice (P < 0.05; F1,11, = 4.589). ST caused a
decrease in serum leptin levels in both, LD- and SD-acclimated females, compared with their control
groups (P < 0.05). There was a trend to increase leptin
levels (~ 25%) in SD-acclimated females, compared with the LD-females (Table 1A). However, FFA levels in LD- and SD-acclimated (control) females were similar. There was a strong positive correlation
(R2 = 0.48, P < 0.05) between leptin and FFA serum
levels in SD-acclimated females (Fig. 1).
As ovaries of ST-LD-acclimated females were atrophied we could only compare between ovaries of SD- and ST-SD-females. Expression of Nr3c2 mRNA was not affected by ST in SD-acclimated females while, a strong effect of ST was noted in
Fig. 1B Correlations between serum FFA levels (nmol/µL) and leptin levels (pg/mL) of control short day acclimated (SD control) and salinity treated (SD-ST) desert-adapted female common spiny mice Acomys cahirinus. R2 = 0.48, *P < 0.05 (Pearson) for SD-control group, R2 = 0.27 for SD-ST group.
Fig. 2A Correlations between serum FFA levels (nmol/µL) and leptin levels (pg/mL) of control long day acclimated (LD control) and salinity treated (LD-ST) desert-adapted male common spiny mice Acomys cahirinus. R2 = 0.63; *P < 0.01 (Pearson) for LD-control group, R2 = 0.51, *P < 0.05 (Pearson) for LD-ST group.
Metabolic and Endocrine Responses of Desert-Adapted Mice Reproductive System to Increased Salinity
1088
Fig. 3 Receptors mRNA expression (relative quantification—RQ) in ovaries of short day (SD control) acclimated and salinity treated (SD-ST) desert adapted female common spiny mice A. cahirinus. Nr3c2: aldosterone receptor gene expression; AVP: vasopressine receptor gene expression; Ob-Rt: leptin receptor gene expression. *P < 0.05 in SD-ST females, compared with SD-control ones.
Fig. 4 Receptors mRNA expression (Relative quantification—RQ) in testis of short and long day (SD, LD-control) acclimated and salinity treated (SD-ST, LD-ST) desert adapted female common spiny mice A. cahirinus. Nr3c2: aldosterone receptor gene expression; AVP: vasopressine receptor gene expression; Ob-Rt: leptin receptor gene expression. *P < 0.05 in treated groups, compared with their controls.
Table 1A Leptin and FFA concentration in serum (± SEM) of short and long day acclimated (SD, LD-control) and salinity treated (SD-ST, LD-ST) desert-adapted female common spiny mice Acomys cahirinus.
Treatment Leptin levels (pg/mL) FFA levels (nmol/µL)
LD female 2,700 ± 120 0.045 ± 0.01
LD-ST female 2,000 ± 80* 0.078 ± 0.015*
SD female 3,600 ± 100 0.045 ± 0.02
SD-ST female 2,600 ± 95* 0.042 ± 0.015*
Leptin (pg/mL) and FFA (nmol/μL) concentration (n = 9 in each SD group) and (n = 6 in each LD group). Leptin levels in LD-ST and SD-ST females compared with their control *P < 0.05. FFA serum levels in LD-ST females compared with their control, *P < 0.05. FFA serum levels in SD-ST females compared with the LD-ST ones *P < 0.05, (F1,12 = 1.34).
Table 1B Leptin and FFA serum levels (± SEM) of short and long day acclimated (SD, LD control), SD and LD salinity treated (SD-ST, LD-ST) desert adapted males of common spiny mice Acomys cahirinus.
Treatment Leptin levels (pg/mL) FFA levels (nmol/µL)
LD male 3,500 ± 115 0.04 ± 0.01
LD-ST male 2,300 ± 100* 0.072 ± 0.01*
SD male 3,200 ± 178 0.04 ± 0.01
SD-ST male 2,400 ± 89* 0.091 ± 0.015*
Leptin (pg/mL) and FFA (nmol/μL) concentration (n = 9 in each SD group) and LD (n = 6 in each LD group). Leptin levels in LD-ST and SD-ST males compared with their control, *P < 0.05. FFA serum levels in LD-ST and SD-ST males compared with their control *P < 0.05.
RQ
mRNA receptors expression genes of AVP and Ob-Rt,
which were significantly (P < 0.05) increased in
ST-SD females compared with their controls (Fig. 3).
3.2 Male Responses to Photoperiod Manipulations
and ST Treatment
No effect of photoperiod was noted, as under both LD and SD acclimation FFA serum levels were
similar. However, ST caused a significant (P < 0.05)
increase in serum FFA and a significant decrease (P <
0.05) in leptin levels, in both, LD and SD group compared with their control groups (Table 1B). The correlations between leptin and FFA serum levels
were significant and negative for both, ST-LD (R2 =
0.51, P < 0.05) and ST-SD (R2= 0.65, P < 0.01) mice.
The same correlation was noted as positive and
significant (R2 = 0.63, P < 0.01) in LD-acclimated
mice (Fig. 2).
The mRNA receptor expression of Nr3c2 was affected
by ST in both, LD- and SD- males. A significant (P <
0.05) increase in mRNA receptor expression genes was noted in both Nr3c2 and AVP under both photoperiod regimes. However, Ob-Rt mRNA
receptor expression genes increased significantly (P <
0.05) by ST only under LD-acclimation. ST under SD-acclimation had no effect on testis Ob-Rt mRNA receptor expression genes (Fig. 4).
4. Discussion
4.1 Metabolic Response to ST
According to the results of previous studies, ST
induces a dramatically Wb decrease [4, 5] in
desert-adapted A.russatus. A massive reduction in Wb
and WAT mass was noted in ST-SD males where Wb
decreased significantly (P < 0.001) by 26% ± 0.1%,
while in ST-LD males the loss was only of 13% ±
0.03% (P < 0.01). In SD-ST females a Wb loss was of
only 17% ± 0.03% but still significant (P < 0.01),
while for LD-ST females a 3% ± 0.2% significant (P
< 0.01) decrease in Wb from their initial values as
reported earlier [8] for A. cahirinus, individuals used
in the present study. Some other previous studies showed that dietary sodium chronic restriction has been related to increased WAT mass in rats [32]. WAT development depends on a balance between food consumption and energy expenditure [33]. It was also revealed that high salt dietary consumption significantly decreased feed intake in sheep [9, 34]. The results of our study, in addition to the progressive
decrease in Wb, revealed an almost complete
abolishment of WAT in ST-mice as reported earlier[8].
WAT reduction and Wb values decrease in our study
could not be explained by reduced energy intake, as ST mice consumed the same amount of food as the control mice throughout the experimental period [8]. We tested adipocytes lipid mobilization resulted from ST compared with their control. This process known as lipolysis, consists of Triacylglycerole (TAG) hydrolysis, FFA and glycerol release, which represent an important mechanism for controlling WAT mass and metabolism [35]. ST males showed an increase in FFA release, during both, LD and SD acclimation compared with their controls. ST-LD-acclimated females showed increased FFA release compared with ST-SD females (Table 1A). Chronic salt loading may increase dysfunction of fat cells in lean and obese alike [35]. A significant linear positive relationship between leptin levels and WAT was noted in previous study where lower serum leptin levels in cold acclimated animals could act as a starvation signal [36]. It was also shown that ST induced hyperleptinemia and it may stimulate the lipolytic process by a direct action [37].
Metabolic and Endocrine Responses of Desert-Adapted Mice Reproductive System to Increased Salinity
1090
and FFA levels were significant, in both, males and females (Figs. 1 and 2). These correlations were positive in LD and SD-acclimated groups (controls), but negative, in ST groups. Combining our results with Ref. [35], we suggest that ST may alter (directly or indirectly) the secretion function and metabolic activity of WAT.
4.2 Reproductive Response to ST
Previous studies on leptin receptors (leptin-R) in rodents have demonstrated the expression of leptin-R gene in the hypothalamus, ovary, uterus, testis and pituitary by reverse transcriptase polymerase chain reaction (RT-PCR) [39]. In our study using the same method we detected a significant increase in expression levels of mRNA receptors genes for aldosterone (Nr3c2) in testis of ST-males under the
two photoperiods (P < 0.05, Fig 4). ST caused a
significant increase (P < 0.05, Fig. 4) in expression
level of vasopressin (AVP) mRNA receptor genes in testis under both photoperiods while for females only
SD-ST could be measured (P < 0.05, Fig 3). High
gene expression levels of leptin (Ob-Rt) mRNA
receptors were noted in SD-ST females (P < 0.05,
Fig 3) while in males only in LD-ST individuals (P <
0.05, Fig. 4), compared with their controls. We suggest that ST caused a significant decrease of leptin levels in serum and for this reason; leptin receptors sites expressed on gonads were not occupied by the hormone.
The authors have no data on receptors gene expression in ovaries of ST-LD females, as there ovaries were atrophied. Yet the authors can suggest that ST had an effect on metabolism and reproductive ability of ST-LD-acclimated females, as leptin levels were decreased under both photoperiod acclimations regimes. It is important to note that ingestion of salt has a dramatic effect on sheep reproductive capacity [6].
Aldosterone and vasopressin are two essential hormones for controlling the water balance and
osmoregulation [40] on the one hand and they affect the reproductive ability of desert adapted rodents on the other [8]. The AVP gene is now known to be expressed in a number of peripheral organs such as the adrenal glands, ovaries, and testes [41]. Ivell [42] reported that AVP mRNA is detectable in the rat testis. It is now well documented that aldosterone receptor are also expressed in non-epithelial tissues, including the cardiovascular and central nervous systems as well as on the white adipose tissue [43].
The results of our study show that a part of the involvement of these two hormones in osmoregulation, they are also possibly involved in inhibiting reproduction under osmolarity stress as there serum levels increased also mRNA receptor expression genes increase. These increases suggest an involvement of molecular mechanism in the gonads as a response to increased osmolarity stress.
Human WAT cells secrete mineralocorticoid- releasing factors [44]. The AVP receptors (V1b and V2) were attributed in mice to lipid metabolism; expression of the two genes was also noted in the heart, liver, kidney, skeletal muscle, BAT, and WAT. The V1a receptor was expressed in all tissues examined, but the V1b receptor was expressed only in WAT, while the V2 receptor in mice was expressed only in the kidney [45]. We searched for AVP and Nr3c2 mRNA receptor genes expression in WAT. For expression of receptors we had compared only SD- and LD-acclimated control males, as WAT was abolished due to ST. There was no detectable AVP mRNA receptor gene expression, but only of Nr3c2. There was no significant difference in expression levels between LD- and SD-acclimated groups.
5. Conclusions
resources in the environment, as an ultimate cue. The idea of two signals was first suggested by Louw and Seely [46]. Therefore, our results on the endocrine and molecular levels are of importance for the proposed idea as they show that hormones regulating water balance and osmoregulation (vasopressin, aldosterone) as well as energy storage (leptin) through receptors presented on the gonads are involved in reproduction of desert-adapted rodents. Together, with our earlier results [8] we can conclude that photoperiod on the one hand and availability of water and food resources on the other are involved in the comprehensive activation of the reproductive system in the desert
adapted population of A.cahirinus.
Acknowledgments
The authors thank the ISF (Israel Academy of Science and Humanities) for financial support through a grant to Abraham Haim and Fuad Fares. We also would like to thank Ms. Lilach Ashkenazi for her help and constructive comments. We thank Ms. Nina Dinov and the staff of the department of Biology at Oranim Campus for their assistance in maintaining the animals. Authors also thank anonymous advisors for their constructive comments on an earlier version of this paper.
References
[1] L.R. Jones, The effect of photoperiod and temperature on testicular growth in captive black-bulled magpies, Condor 88 (1986) 91-93.
[2] R.J. Nelson, J. Dark, I. Zucker, Influence of photoperiod, nutrition and water availability on reproduction of male California voles (Microtus californicus), Journal of Reproduction and Fertility 69 (1983) 473-477.
[3] C. Desjardins, F.H. Bronson, J.L. Blank, Genetic selection for photoperiodic responsiveness in deer mice, Nature 322 (1986) 172-173.
[4] U. Shanas, A. Haim, Diet salinity and vasopressin as reproduction modulators in the desert-dwelling golden spiny mouse (Acomys russatus), Physiology and Behavior 81 (2004) 645-650.
[5] T. Wube, A. Haim, F. Fares, Effect of increased dietary salinity on the reproductive status and energy intake of xeric and mesic populations of the spiny mouse, Acomys,
Physiology and Behavior 96 (2009) 122-127.
[6] S.N. Digby, M.A. Chadwick, D. Blache, Salt intake and reproductive function in sheep, Animal 5 (8) (2011) 1207-1216.
[7] N. Tian, K.D. Thrasher, P.D. Gundy, M.D. Hughson, R.D. Jr Manning, Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension, Hypertension 45 (2005) 934-939.
[8] E. Bukovetzky, H. Schwimmer, F. Fares, A. Haim, Photoperiodicity and increasing salinity as environmental cues for reproduction in desert adapted rodents, Hormones and Behavior 61 (2012) 84-90.
[9] D. Blache, M.S. Grandison, D.G. Masters, R.A. Dynes, M.A. Blackberry, G.A. Martin, Relationships between metabolic endocrine systems and voluntary feed intake in Merino sheep fed a high salt diet, Australian Journal of Agriculture Research 47 (2007) 544-550.
[10] L.J. Spicer, Leptin: A possible metabolic signal affecting reproduction, Domestic Animal Endocrinology 21 (2001) 251-270.
[11] D.A. Zieba, M. Amstalden, G.L. Williams, Regulatory roles of leptin in reproduction and metabolism: A comparative review, Domestic animal endocrinology 29 (2005) 166-185.
[12] R.S. Ahima, Metabolic actions of adipocyte hormones: Focus on adiponectin, Obesity 14(1) (2006) 9S-15S. [13] P. Trayhurn, I.S. Wood, Adipokines: Inflammation and
the pleiotropic role of white adipose tissue, British Journal of Nutrition 92 (2004) 347-355.
[14] E.E. Kershaw, J.S. Flier, Adipose tissue as an endocrine organ, The Journal of Clinical Endocrinology and Metabolism 89 (6) (2004) 2548-2556.
[15] J. Hiraoka, K. Hosoda, Y. Ogawa, K. Ikeda, Y. Nara, H. Masuzaki, et al., Augmentation of obese (ob) gene expression and leptin secretion in obese spontaneously hypertensive rats (obese SHR or Koletsky rats), Biochemistry Biophysics Research Community 231 (1997) 582-285.
[16] F.F. Chehab, K. Mounzik, R. Lu, M.E. Lim, Early onset of reproductive function in normal female mice treated with leptin, Science 275 (1997) 88-90.
[17] R.S. Ahima, J. Dusday, S.N. Flier, D. Prabakaran, J.S. Flier, Leptin accelerates the onset of puberty in normal female mice, Journal of Clinical Investigation 99 (1997) 391-395.
[18] I.A. Barash, C.C. Cheung, D.S. Weigle, H. Ren, E.B. Kabigting, J.L. Kuijper, et al., Leptin is a metabolic signal to the reproductive system, Endocrinology137 (7) (1996) 3144-3147.
Metabolic and Endocrine Responses of Desert-Adapted Mice Reproductive System to Increased Salinity
1092
(2007) 165-176.
[20] A. Banerjee, K.J. Meenakumari, A. Krishna, Role of leptin in delayed embryonic development in the Indian short-nosed fruit bat, Cynopterus sphinx, General and Comparative Endocrinology 168 (2010) 36-45.
[21] I.J. Clarke, B.A. Henry, Leptin and reproduction, Reviews of Reproduction 4 (1999) 48-55.
[22] M. Sone, H. Nagata, S. Takekoshi, R.Y. Osamura, Expression and localization of leptin receptor in the normal rat pituitary gland, Cell Tissue Research 305 (2001) 351-356.
[23] M.C. Zennaro, D. Le Menuet, S. Viengchareun, F. Walker, D. Ricquier, M. Lombes, Hibernoma development in transgenic mice identifies brown adipose tissue as a novel target of aldosterone action, Journal of Clinical Investigation 101 (1998) 1254-1260.
[24] H. Hauner, G. Entenmann, M. Wabitsch, D. Gaillard, G. Ailhaud, R. Negrel, E.F. Pfeiffer, Promoting effects of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium, Journal of Clinical Investigation 84 (1989) 1663-1670. [25] C.M. Rondinone, D. Robbard, M.E. Baker, Aldosterone
stimulates differentiation of mouse 3T3-L1 cells into adipocytes, Endocrinology 132 (1993) 2421-2426. [26] M. Caprio, B. Feve, A. Claes, S. Viengchareum, M.
Lombes, M.C. Zennaro, Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis, FASEB Journal 21 (2007) 2185-2194. [27] A.D. Dobrian, S.D. Schriver, T. Lynch, R.L. Prewitt,
Effect of salt on hypertension and oxidative stress in a rat model of diet-induced obesity, American Journal of Physiology, Renal Physiology 285 (4) (2003) 19-28. [28] A.L. Mark, M.L. Correia, K. Rahmouni, W.G. Haynes,
Selective leptin resistance: A new concept in leptin physiology with cardiovascular implications, Journal of Hypertension 20 (2002)1245-1250.
[29] N. Levin, C. Nelson, A. Gurney, R. Vandlen, F. de Sauvage, Decreased food intake does not completely account for adiposity reduction after ob protein infusion, Proceedings of the National Academy of Sciences of the United States of America 93 (4) (1996) 1726-1730. [30] N. Barzilai, J. Wang, D. Massilon, P. Vuguin, M.
Hawkins, L. Rossetti, Leptin selectively decreases visceral adiposity and enhances insulin action, The Journal of clinical investigation 100 (12) (1997) 3105-3110.
[31] S.A. Bustin, Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems, Journal of Molecular Endocrinology29 (2002) 23-39. [32] P.O. Prada, M.M. Okamoto, L.N.S. Furukawa, U.F.
Machado, J.C. Heimann, M.S. Dolnikoff, High- or low-salt diet from weaning to adulthood: Effect on insulin sensitivity in Wistar rats, Hypertension 35 (2000)
424-429.
[33] D.B. Hausman, M. DiGirolamo, T.J. Bartness, G.J. Hausman, R.J. Martin, The biology of white adipocyte proliferation, Obesity Review 2 (2001) 239-254. [34] D.G. Masters, A.J. Rintoul, R.A. Dynes, K.L. Pearce,
H.C. Norman, Feed intake and production in sheep fed diets high in sodium and potassium, AustralianJournal of Agricultural Research 56 (5) (2005) 427-434.
[35] D. Langin, Control of fatty acid and glycerol release in adipose tissue lipolysis, Comptes Rendus Biologies 329 (2006) 598-607.
[36] X.Y. Zhang, D.H. Wang, Energy metabolism, thermogenesis and body mass regulation in Brandt’s voles (Lasiopodomys brandtii) during cold acclimation and rewarming, Hormone and Behavior 50 (2006) 61-69.
[37] C.A. Siegrist-Kaiser, V. Pauli, C.E. Juge-Aubry, O. Boss, A. Pernin, W.W. Chin, et al., Direct effects of leptin on brown and white adipose tissue, Journal of Clinical Investigation 100 (1997) 2858-2864.
[38] M.H. Fonseca-Alaniz, L.C. Brito, C.N. Borges-Silva, J. Takada, S. Andreotti, F.B. Lima, High dietary sodium intake increases white adipose tissue mass and plasma leptin in rats, Obesity 15 (2007) 2200-2208.
[39] P.L. Zamorano, V.B. Mahesh, L.M. De Sevilla, L.P. Chorich, G.K. Bhat, D.W. Brann, Expression and localization of the leptin receptor in endocrine and euroendocrine tissues of the rat, Neuroendocrinology 65 (1997) 223-228.
[40] J.D. Louden, Regulation of fluid and electrolyte balance, Anaesthesia and Intensive Care Medicine 10 (6) (2009) 279-285.
[41] D.L. Lefebvre, H.H. Zingg, Novel vasopressin gene- related transcripts in rat testis, Molecular Endocrinology 5 (5) (1991) 645-652.
[42] R. Ivell, Vasopressin and oxytocin gene expression in the mammalian ovary and testis, in: S. Jard, R. Jamison (Eds.), Colloque Inserm 208, John Libbey Eurotext, Paris, 1991, pp. 31-38.
[43] J.W. Funder, Mineralocorticoid receptors: Distribution and activation, Heart Failure Review 10 (2005) 15-22. [44] M. Ehrhart-Bornstein, V. Lamounier-Zepter, A. Schraven,
J. Langenbach, H.S. Willenberg, A. Barthel, et al., Human adipocytes secrete mineralocorticoid-releasing factors, Proceedings of the National Academy of Sciences of the United States of America 100 (24) (2003) 14211-14216.
[45] M. Hiroyama, T. Aoyagi, Y. Fujiwara, J. Birumachi, Y. Shigematsu, K. Kiwaki, et al., Hypermetabolism of fat in V1a vasopressin receptor knockout mice, Molecular Endocrinology 21 (1) (2007) 247-258.
Appendix 1
GenBank: Genetic sequence database at the National Center for Biotechnical Information (NCBI): GenBank ID Glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) NM_008084.2
GAPDH-F: 5’-AGGTCGGTGTGAACGGATTTG-3’; GAPDH-R: 5’-TGTAGACCATGTAGTTGAGGTCA-3’; Aldosterone receptor gene (Nr3c2) NM_001083906.1
Nr3c2-F: 5’-GAAGAGCCCCTCTGTTTGCAG-3’; Nr3c2-R: 5’-TCCTTGAGTGATGGGACTGTG-3’; Vasopressin receptor gene (AVP) NM_016847.2
AVP-F: 5’-CAATTTCGTTTGGACCGATTC-3’; AVP-R: 5’-GGGTTGCAGCAGCTGTTCA-3’; Leptin receptor gene (Ob-Rt) NM_146146.2
Journal of Life Sciences 6 (2012) 1094-1099
Influence of Abiotic Elicitors on Accumulation of Thymol
in Callus Cultures of
Origanum vulgare
L.
Abedaljasim M. Jasim Al-Jibouri, Ashwaq S. Abd, Duha M. Majeed and Eman N. Ismail
Biotechnology Research Center, Al-Nahrain University, Baghdad 10072, Iraq
Received: May 05, 2012 / Accepted: July 20, 2012 / Published: October 30, 2012.
Abstract: Callus cultures of Origanum vulgare L. were established from leaf discus on Murashige and Skoog (MS) medium containing different levels of growth regulators, i.e., 2,4-Dichlorophenoxyacetic acid (2,4-D), Naphthalene acetic acid (NAA), Benzyl Adenine (BA) and Kinetin (Kn) and incubated under dark condition. Callus tissues were employed to study the influence of abiotic elicitors on the production of thymol. Constant weights of callus (300 mg) were cultured on accumulation medium treated separately with each one of elicitors used (50 g/L sucrose, 200 mg/L NaCl and 50 or 100 mg/L proline). The fresh and dry weights of callus were recorded after six weeks. The result indicated that maximum production of fresh and dry callus weight were 1,014 mg and 46.20 mg respectively achieved at 0.5 mg/L 2,4-D and 3 mg/L BA adding to the medium. Dry callus tissues were extracted with 70% methanol and analyzed by HPLC to determine the concentrations of thymol. The addition of abiotic elicitors to MS medium caused significant reduction in fresh weight of callus compared with control treatment. The concentration of thymol in the callus cultured on control treatment was 146.6 ppm. The data showed that 50 or 100 mg/L proline produced the highest yield of thymol 181.48 ppm and 174.58 ppm respectively, followed by sucrose 162.9 ppm, whereas the treatment with NaCl caused reduction in thymol concentration to percentage of 50.56% compared with the control.
Key words:Origanum vulgare L., thymol production, callus culture, abiotic elicitors.
1. Introduction
Origanum vulgare L. is a member of the Lamiaceae family (Labiatea), commonly named oregano, wild marjoram, marzanjosh or mardaqoush, which grows abundantly on stony slopes and in rocky mountain areas at a wide range of altitude [1]. The Origanum species, which are rich in essential oils, have been used for thousands of years as spices
and as local medicines. Arial parts of Origanum
vulgare are used in respiratory tract disorders such as cough or bronchial catarrh (as expectorant and spasmolitic agents), in gastrointestinal disorders (as choleretic, digestive, eupeptic and spasmolitic agents) as oral antiseptic, in urinary tract disorders (as diuretic and antiseptic) [2] and modulates blood
Corresponding author: Abedaljasim M. Jasim Al-Jibouri, Ph.D., assistant professor, research field: plant biotechnology. E-mail: [email protected].
sugar and lipids [3]. The main components of essential oil of Oregano plants are thymol, carvacrol,
p-cymen and β-pinen [1, 4, 5]. Essential oil is a
mixture of volatile compounds produced in small quantities as secondary metabolites from aromatic and medicinal plants [6]. Thymol is a most valuable crystalline phenol [7], have many biological activities, i.e. anti-oxidant [8], anti-cancer [9, 10], anti-mutagenic [11], anti-inflammatory [12], anti-microbial [13, 14], and anti-fungal activity [15].
The accumulation of secondary metabolites in plant tissue cultures has been obtained from various medicinal plants. The production of hyoscyamine and scopolamine has been reported in Callus cultures of
Datura metel L. [16]. High in vitro production of
indole alkaloids from Catharanthus roseus tissue
culture has also been reported [17]. In vitro studies in
induced to study the effect of different type of nutrient media and various growth regulators on different morphological responses [1], Arafeh et al. [6] studied
the content of essential oil in intact plants (in vitro and
ex vitro), callus, and cell cultures in Origanumvulgare
and O. syriacum. The production of volatile oils
has been shown in callus culture of Origanum
vulgare [18].
The advantages of producing secondary compounds using tissue culture technique are more reliable, simpler, predictable, easy isolation and efficient
compared with in vivo production. In addition, cell
culture can yield a source of defined standard phytochemicals in large volume and as technique could be a good model to evaluate various elicitors effects.
Secondary metabolite synthesis may be enhanced in
vitro by controlling the composition of the culture medium and the environment, certain secondary compounds are produced by the plant under the influence of various biotic and abiotic factors that are known as “elicitors” [19].
The main objective of present study was to examine
the effect of exogenous elicitors on biomass
production and accumulation of thymol in callus
tissue of Origanumvulgare L. under dark condition.
2. Materials and Methods
2.1 Plant Material
The plants of Origanumvulgare L., were collected
at the flowering stage in April 2010 from the Botanical Garden of Biotechnology Research Center, Al-Nahrain University, Baghdad, Iraq.
Taxonomic identification of plant material was conferred by Prof. Dr. Ali Al-Musawi, Department of Biology, Baghdad University. Leaf explants were taken and washed with tap water for 30 min, then immersed in 3% of sodium hypochlorite and added 2-3 drops of Tween 20 for 10 min, finely these leaves were washed once with 70% ethanol and several times with distilled sterile water.
2.2 Callus Induction and Maintenance
Sterilized leaf segments (0.5 cm2) were cultured on
solidified MS medium [20] supplemented separately with 0.5 mg/L or 1 mg/L 2,4-D, 0.5 mg/L or 1 mg/L NAA, 0.5 mg/L 2,4-D and 3 mg/L BA and 0.5 mg/L 2,4-D and 3 mg/L Kn. All cultures were performed in the dark condition at 25 ± 2 °C. Data were recorded after 6 weeks on fresh and dry weight of callus. The callus production was selected and maintained on fresh media (supplemented with 0.5 mg/L 2,4-D and 3 mg/L BA which produced highest yield of callus) to study the effect of abiotic elicitors on thymol production.
2.3 Abiotic Elicitors Used
Equal amount of callus (300 mg) was cultured on maintenance medium (0.5 mg/L 2,4-D and 3 mg/L BA), three abiotic elicitors have been added to medium separately, 50 mg/L sucrose, 200 mg/L sodium chloride (NaCl) and amino acid proline at two concentrations 50 mg/L or 100 mg/L. All cultures were incubated under dark condition at 25 ± 2 °C. Fresh and dry weights of callus were recorded after 6 weeks of incubation and thymol content were determined.
2.4 Thymol Extraction
Dry callus (200 mg) of each treatment were grounded and macerated over night in 10 mL of 70% methanol at room temperature with periodical mixing, then filtrated and concentrated at 45 °C to get 5 mL of sample. All samples were filtrated through a 0.22 µm Millipore filter and then stored in dark at 4 °C until used [21, 22].
2.5 HPLC Analysis
Thymol contents were determined using HPLC
instrument (Cecil Company, England). ODC (C18)
column (25 cm × 4.6 mm, partial size 5 µm) was used
Influence of Abiotic Elicitors on Accumulation of Thymol in Callus Cultures of Origanum vulgare L. 1096
out at 254 nm. The quantitative determination was carried out through the use of external standard method.
2.6 Statistical Analysis
All experiments were done with minimum of 20 replicates per treatment. Significance of treatment effect was determined by using analysis of variance
(ANOVA) followed by LSD test (P ≤ 0.05) to
determine significant differences among treatment means.
3. Results and Discussion
3.1 Callus Induction
The results showed that significant differences between the growths regulators in fresh and dry
weight of callus produced from leaf explants of O.
vulgare (Fig. 1). The supplementation of MS medium with 0.5 mg/L 2,4-D and 3 mg/L BA gave the maximum average of fresh and dry weights of callus compared with other supplementations, reached 1014 mg and 46.20 mg respectively which significant differences of all treatments except the treatment of 0.5 mg/L .This callus was healthy, white in color and compact in texture. Medium supplemented with 0.5 mg/L 2,4-D also showed high callus weight but it was
gelatinous in texture. The control treatment gave lowest fresh and dry weight of callus (118 mg and 16.5 mg) respectively which significant differences with all treatment. The obtained results were in agreement with El-Gengaihi et al. [1] who reported that supplementation of auxin and cytokinin (0.5 mg/L NAA and 3 mg/L BA) to MS medium gave the best
results of callus induction form Origanum species.
Similarly to the authors’ results, Stojakowska et al. [23]
reported that callus tissue of Inula helenium was
obtained from leaf explants when cultured on solidified MS medium containing 1 mg/L 2,4-D and 3
mg/L Kn. Arafeh et al. [6] mentioned that best callus
induction in O. vulgare was obtained at lower levels
of 2,4-D (0.5 mg/L or 1 mg/L) added to medium. The result appeared that callus initiation and production were dependent on the presence of auxin and cytokinin, which stimulate both cell division and cell elongation [24].
3.2 Effects of Abiotic Elicitors
The result in Fig. 2 illustrated the adding of abiotic elicitors such as 50 mg/L proline to medium produced highest average of fresh and dry weight of callus reached 1,357 mg and 110.4 mg respectively, which are significantly differences with all treatments except
0 200 400 600 800 1000 1200
118
978
885
750 728
1014 862
16.5 43.14 42.5 41.7 39.7 46.2 39.8
weights
mg
Plant regulatores
Fresh weight