Mycorrhizal inoculation enhances growth and nutrient uptake of
micropropagated apple rootstocks during weaning in commercial
substrates of high nutrient availability
A. Schubert
∗, G. Lubraco
Dipartimento Colture arboree, Universita’ di Torino, via Leonardo da Vinci 44, 10095 Grugliasco (TO), Italy
Received 31 May 1999; received in revised form 1 December 1999; accepted 23 March 2000
Abstract
Apple (Malus pumila L.) plants of the rootstock clone MM106 were micropropagated from axillary buds and, after rooting, were transplanted into pots containing three different commercial-type peat-based substrates, fertilized before planting with about 100 mg soluble P per liter (about 300 mg kg−1wet substrate). Plants were inoculated at transplant with the arbuscular
mycorrhizal fungus Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe. After 112 days of growth, roots were heavily colonized by mycorrhizal fungi. Inoculation increased P uptake in all substrates. Plant growth was enhanced by inoculation in two of the substrates. During the growth period, the P content of the substrate was severely depleted, and this may explain the intense root colonization and nutrient uptake enhancement observed in such nutrient-rich substrates. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Phosphorus; Malus; Glomus; Plant propagation
1. Introduction
In vitro culture of fruit plants is an established method of plant propagation, now applied very com-monly because of advantages such as the absence of pathogens and the high numbers of plants which can be obtained in a relatively short time and reduced space. Clonal apple rootstocks are routinely used in apple or-chard plantings in the world and are mostly obtained by micropropagation (Zimmermann, 1991).
Inoculation with arbuscular mycorrhizal (AM) fungi of micropropagated fruit plants at transplant ex vitro improves growth and nutrient uptake during
∗Corresponding author. Tel.:+39-011-6708654; fax:+39-011-6708658.
E-mail address: [email protected] (A. Schubert)
the weaning stage, yielding plants of larger size and improved commercial characteristics (Lovato et al., 1992; Cordier et al., 1996). The apple plant is com-monly infected by AM fungi (Koch et al., 1981), and apple plants grown in soils of low or high nutrient availability show significant growth enhancements if inoculated with AM fungi (Plenchette et al., 1981; Morin et al., 1994).
Although the effects of AM inoculation on micro-propagated apple plants in mineral soils are relatively well known, little information is available on the ef-fects of AM inoculation in the artificial substrates which are used in commercial weaning of micropropa-gated plants. Commercial fruit plant micropropagation normally makes use of peat-based substrates, contain-ing no mineral soil, enriched in N, P, and K (Preece and Sutter, 1991). The high chemical fertility of these
substrates is designed to improve plant growth, but it can have negative effects on the growth of the mycor-rhizal fungi, which show slow development and little infection potential in soils of high P content (Smith and Read, 1997).
In this work, we studied the development of the fungus and of the plant partner in apple plants in-oculated with AM fungi. Inoculation and weaning were carried out in pots containing commercial growth substrates.
2. Materials and methods
Apple (Malus pumila L.) rootstocks (clone MM106) were micropropagated from axillary buds. Buds were sampled from surface-sterilized young shoots and were cultured for 60 days in a proliferation medium containing Murashige and Skoog (MS) salts and vita-mins, and the following hormones: indolebutyric acid 0.1 mg l−1, benzylaminopurine 1 mg l−1 and GA3
0.1 mg l−1. After proliferation, single shoots were sub-cultured for 30 days into a rooting medium containing MS salts and vitamins, and indoleacetic acid 2 mg l−1. All media contained 30 g l−1sucrose and 6% agar.
After rooting (about four roots per plant, about 15 mm long) plants were transplanted into 7 cm×7 cm, 280 ml plastic pots. Transplant was made in con-ditions of high ambient humidity to reduce dam-age to the plantlets. At transplant, pots were filled with different substrates, composed of mixtures of a peat-based commercial substrate (TRIOHUM Pot-grond P, Klasmann–Deilmann GmbH, Geeste, Ger-many), acid sphagnum peat (SF 40101, Juaskvea, Finland) and perlite (Agripan 100, Italy). The sub-strates were named PT (Potgrond P 100%), PE (Pot-grond P/perlite 60/40 v/v) and TC (acid sphagnum peat, added at pot filling with 3 g l−1 solid Ca(OH)2
to reach pH 6.7, and with 3 g l−1 of a fertilizer (Ni-trophoska Gold), containing 15% slow-release N, 9% P2O5, 15% K, and 2% Mg). Substrates were wetted
before filling the pots. The main characteristics of the substrates are presented in Table 1.
At transplant, plants were inoculated with the AM fungus Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe (isolate BEG 12), in the form of a mixture of spores, soil, and infected clover roots. Ten grams of inoculum were placed in each planting hole about
Table 1
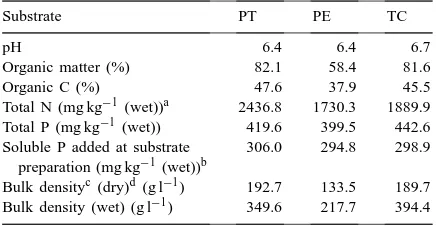
Chemical analysis of the substrates used in the experiment (PT: commercial peat-based, fertilized substrate; PE: PT sub-strate/perlite 60/40 v/v and TC: acid sphagnum peat amended with a slow-release fertilizer)
Substrate PT PE TC
pH 6.4 6.4 6.7
Organic matter (%) 82.1 58.4 81.6
Organic C (%) 47.6 37.9 45.5
Total N (mg kg−1(wet))a 2436.8 1730.3 1889.9 Total P (mg kg−1(wet)) 419.6 399.5 442.6 Soluble P added at substrate
preparation (mg kg−1(wet))b
306.0 294.8 298.9
Bulk densityc(dry)d (g l−1) 192.7 133.5 189.7 Bulk density (wet) (g l−1) 349.6 217.7 394.4
aWet: substrate added with water to soil capacity.
bIn substrates PT and PE soluble P was added at substrate preparation by the producer, in TC it was added at pot filling.
cDensity measurements were made on substrate subjected to the same compression as in pots.
dDry: substrate dried at 105◦C.
1 cm below the roots, for a total of 80 propagules per plant, measured with the most probable number method (Porter, 1979). Non-inoculated roots received 10 g of the autoclaved soil used for inoculum produc-tion (Olsen P 8 mg kg−1).
content per treatment was assessed on two replicate pools of dried shoots and substrates from 24 pots.
The experiment was set up with a randomized block design, each block containing six replicate plants per treatment, for a total of eight blocks. Data were processed by analysis of variance, and averages were separated by the Duncan test.
3. Results
3.1. Root mycorrhizal colonization and plant growth
At the end of the experiment the roots of non-inoculated plants were not colonized by the AM fun-gus. Inoculated plants were mycorrhizal, and root colonization was (averages±S.E.): 72.8±1.5% in the substrate PT, 84.0±2.2% in PE, and 82.5±3.2% in TC.
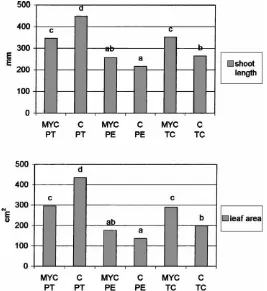
Fig. 1. Shoot length (above) and total leaf area (below) of micropropagated apple plants, inoculated with the AM fungus Glomus mosseae (MYC) or non-inoculated (C) in three different substrates (PT, PE and TC) during the weaning stage. Values marked by the same letters do not differ significantly at p=0.05.
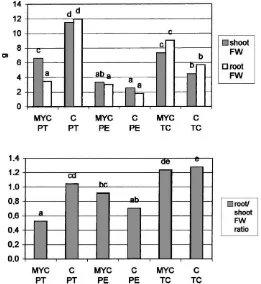
Fig. 2. Shoot and root fresh weight (above) and root/shoot fresh weight ratio (below) of micropropagated apple plants, inoculated with the AM fungus Glomus mosseae (MYC) or non-inoculated (C) in three different substrates (PT, PE and TC) during the weaning stage. Values marked by the same letters do not differ significantly at p=0.05.
substrates, while it was significantly lower in mycor-rhizal plants in the PT substrate (Fig. 2).
3.2. Plant and soil nutrient content
Fungal inoculation significantly affected the P con-tent of plants, which was higher in mycorrhizal than in non-inoculated plants in all substrates, the differ-ence being significant in substrates PT and PE (Fig. 3). Plants grown on the TC substrate had significantly lower P contents than plants grown on the other sub-strates.
The total P content of the substrates decreased dur-ing the experiment: 112 days after transplant, in inoc-ulated pots total P content was reduced, on an average of the three substrates, by about 94%, and only about 7% of the lost P was found in the plant tissues. In non-inoculated pots about 32% P was recovered in the substrate after plant cultivation, and 6% was found in the plant tissues (Table 2).
4. Discussion
The results of this experiment show that inoculation with AM fungi induced root fungal colonization and increased nutrient content of apple micropropagated plants in three commercial, nutrient-rich substrates. Most of the published experiments on inoculation of micropropagated plants have been carried out in substrates relatively poor in nutrients, with available P contents not higher than 100 mg kg−1. In the case
Fig. 3. Concentration of P in the dry shoots of micropropagated apple plants, inoculated with the AM fungus Glomus mosseae (MYC) or non-inoculated (C) in three different substrates (PT, PE and TC) during the weaning stage. Values marked by the same letters do not differ significantly at p=0.05.
and shoot P concentration was significantly higher in mycorrhizal than in non-mycorrhizal plants. Thus stable mycorrhizal inoculation can be obtained in such substrates, giving micropropagated plantlets the added values of increased nutrient content, increased growth, and potential defence against root pathogens. An intense root colonization and an enhancement of plant P content are usually not expected in soils of high soluble P content, as root infection by AM fungi is slowed down by soil P availability (Smith and Read, 1997). We measured the total P content of the substrate and of the plant at the beginning and at the end of the experiment. On an average only about 7% of the total P present in the pots was incorporated into the plants, and this percentage increases to about 10% of the sol-uble P present in the substrates as a fertilizer. However at the end of the experiment total P content per pot was much lower than at transplant, its values ranging
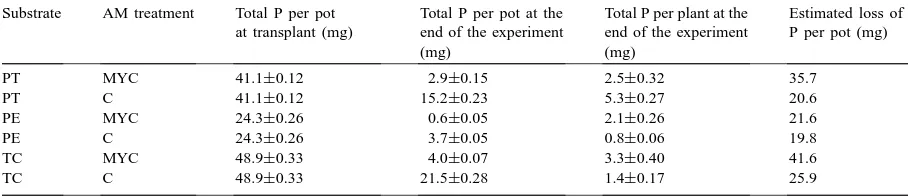
Table 2
Total P content per pot in three different substrates (PT, PE and TC), inoculated (MYC) or non-inoculated (C) with the AM fungus Glomus
mosseae, at transplant and at the end of the experiment, total P content per plant at the end of the experiment, and estimated loss of P
during plant cultivation (means±S.E.)
Substrate AM treatment Total P per pot at transplant (mg)
Total P per pot at the end of the experiment (mg)
Total P per plant at the end of the experiment (mg)
Estimated loss of P per pot (mg)
PT MYC 41.1±0.12 2.9±0.15 2.5±0.32 35.7
PT C 41.1±0.12 15.2±0.23 5.3±0.27 20.6
PE MYC 24.3±0.26 0.6±0.05 2.1±0.26 21.6
PE C 24.3±0.26 3.7±0.05 0.8±0.06 19.8
TC MYC 48.9±0.33 4.0±0.07 3.3±0.40 41.6
TC C 48.9±0.33 21.5±0.28 1.4±0.17 25.9
between 13 and 54 mg kg−1wet substrate. Thus AM
fungi and plant roots, originally introduced into a soil containing high amounts of soluble P, were exposed for part of the experiment to relatively low P concen-trations. This can explain the high rate of root colo-nization and the increase in P content in mycorrhizal plants.
inoculated pots, where more than the soluble P added at substrate preparation was lost from the soil than in control pots which lost nearly the same amount added as soluble fertilizer. The inoculation with mycorrhizal fungi has been shown to enhance, although indirectly, the solubilization of organic P (Joner and Jakobsen, 1994), and this may have provided more soluble P in inoculated pots, which could be leached by irrigation water.
Mycorrhizal colonization and the enhancement of nutrient uptake increased growth of both roots and shoots only in two out of three substrates: in the sub-strate PT control plants grew more than inoculated plants. The growth enhancement of AM inoculation can be due to factors other than improved P uptake, such as improved water relations (Graham and Syvert-sen, 1984) and protection against root pathogens (Lin-derman, 1994). Although inoculation seems in general to be effective to obtain micropropagated, mycorrhizal plants, it is not possible to easily predict whether a growth response can be obtained during weaning in a given commercial substrate.
Acknowledgements
The authors wish to thank Prof. E. Barberis for use-ful discussion on the interpretation of substrate anal-ysis data.
References
Cordier, C., Trouvelot, A., Gianinazzi, S., Gianinazzi-Pearson, V., 1996. Arbuscular mycorrhiza technology applied to micro-propagated Prunus avium and to protection against
Phytophthora cinnamomi. Agronomie 16, 679–688.
Giovannetti, M., Mosse, B., 1980. An evaluation of techniques for measuring vesicular–arbuscular mycorrhizal infection of roots. New Phytol. 84, 489–500.
Graham, J.H., Syvertsen, J.P., 1984. Influence of vesicular– arbuscular mycorrhiza on the hydraulic conductivity of roots of two Citrus rootstocks. New Phytol. 97, 277–284.
Joner, E.J., Jakobsen, I., 1994. Contribution by two arbuscular mycorrhizal fungi to P uptake by cucumber (Cucumis sativus L.) from32P-labelled organic matter during mineralisation in soil. Plant Soil 163, 203–209.
Koch, B.L., Covey, R.P., Larsen, H.J., 1981. Response of apple seedlings in fumigated soil to phosphorous and vesicular–arbuscular mycorrhiza. HortScience 17, 232–233. Linderman, R.G., 1994. Role of VAM in biocontrol. In: Pfleger,
F.L., Linderman, R.G. (Eds.), Mycorrhizae and Plant Health. APS Press, St Paul, MN, pp. 1–26.
Lovato, P., Guillemin, J.P., Gianinazzi, S., 1992. Application of commercial endomycorrhizal fungal inoculants to the establishment of micropropagated grapevine rootstock and pineapple plants. Agronomie 12, 873–880.
Morin, F., Fortin, J.A., Hamel, C., Granger, R.L., Smith, D.L., 1994. Apple rootstock response to vesicular–arbuscular mycorrhizal fungi in a high phosphorous soil. J. Am. Soc. Hort. Sci. 119, 578–583.
Page, A.L., Miller, R.H., Keeney, D.R., 1982. Methods of Soil Analysis. American Society of Agronomy, Madison, WI, 1158 pp.
Plenchette, C., Furlan, V., Fortin, J.A., 1981. Growth stimulation of apple trees in unsterilized soils under field conditions with VA mycorrhiza inoculum. Can. J. Bot. 59, 2003–2008. Porter, W.M., 1979. The most probable number method
for enumerating infective propagules of vesicular–arbuscular mycorrhizal fungi in soil. Aust. J. Soil Res. 17, 515–519. Preece, J.E., Sutter, E.G., 1991. Acclimatization of
micro-propagated plants to the greenhouse and the field. In: Debergh, P.C., Zimmermann, R.H. (Eds.), Micropropagation. Kluwer Academic Publishers, Dordrecht, pp. 71–93.
Smith, S.E., Read, D.J., 1997. Mycorrhizal Symbiosis. Academic Press, San Diego, CA, 605 pp.