Sri Lanka 2017
Acronyms
AD Auto disable
AEFI Adverse events following immunization
AFP Acute flaccid paralysis
BCG Bacillus Calmette-Guérin vaccine
CES Coverage evaluation survey
cMYP Comprehensive multi-year plan
CRS Congenital rubella syndrome
DHS Demographic health survey
DT Diphtheria tetanus toxoid, pediatric
DTP Diphtheria – tetanus – pertussis vaccine
DTP-Hib-HepB Pentavalent vaccine
DTP-Hib-HepB3 3rd dose pentavalent vaccine
EPI Expanded programme on immunization
GDP Gross domestic product
HCW Health care worker
HepB Hepatitis B vaccine
Hib Haemophilus influenzae type b
HPV Human papilloma virus
IgM Immunoglobulin M
IPV Inactivated poliovirus vaccine
JE Japanese encephalitis
JE_Live-Atd JE live attenuated vaccine
JRF WHO UNICEF joint reporting form
LB Live birth
M Measles
MCV1 First dose measles containing vaccine
MCV2 Second dose measles containing vaccine
MICS Multiple indicator cluster survey
MMR Measles mumps rubella vaccine
MNT Maternal and neonatal tetanus
MR Measles rubella vaccine
NCIP National committee on immunization practices
NID National immunization day
NTAGI National technical advisory group on immunization
NPEV Non-polio enterovirus
NT Neonatal tetanus
OPV Oral poliovirus vaccine
bOPV Bivalent OPV
tOPV Trivalent OPV
PCV Pneumococcal conjugate vaccine
SEAR WHO South-East Asia Region
SIA Supplementary immunization activities
SNID Subnational immunization day
Td Tetanus diphtheria toxoid; older children, adults
TT Tetanus toxoid
TT2+ 2 or more doses TT
VDPV Vaccine derived poliovirus
VPD Vaccine preventable diseases
WCBA Women of child bearing age
Contents
Impact of rouine immunizaion
Page
No.
EPI history 5
Basic informaion 2016 Table 1 5
Immunizaion schedule 2016 Table 2 5
Naional immunizaion coverage 1980 - 2016 Figure 1 6
Immunizaion system highlights Table 3 6
DTP3 coverage, diphtheria and pertussis cases 1980 - 2016 Figure 2 7
Reported cases of vaccine preventable diseases 2011 - 2016 Table 4 7
DTP-Hib-HepB3 coverage by district 2015 Figure 3 7
DTP-Hib-HepB3 coverage by district 2016 Figure 4 7
Towards measles eliminaion and rubella/congenital rubella
syndrome control
Page
No.
MCV1 and MCV2 coverage, measles and rubella cases, 1980-2016 Figure 10 11
MCV supplementary immunizaion aciviies Table 7 11
MCV1 coverage by district 2015 Figure 11 12
MCV1 coverage by district 2016 Figure 12 12
MCV2 coverage by district 2015 Figure 13 12
MCV1 coverage by district 2016 Figure 14 12
Immunity against measles – immunity proile by age in 2016 Figure 15 12
Subnaional risk assessment for measles and rubella Figure 16 12
Sporadic and outbreak associated measles cases by month 2011 - 2016 Figure 17 13
Immunizaion status of conirmed (laboratory and Epi linked) measles outbreak
associated cases by age 2011 – 2016 Figure 18 13
Quality of ield and laboratory surveillance for measles and rubella 2012 - 2016 Table 8 14
Performance of laboratory surveillance 2012 - 2016 Table 9 14
WHO supported laboratory network for VPD surveillance Figure 19 15
Maternal and neonatal tetanus eliminaion is sustained
Page
No.
TT2+ coverage and NT cases 1980 - 2016 Figure 5 8
Polio-free status is maintained
Page
No.
AFP surveillance indicators 2011 - 2016 Table 5 9
Non-polio AFP rate by district 2015 Figure 6 9
Non-polio AFP rate by district 2016 Figure 7 9
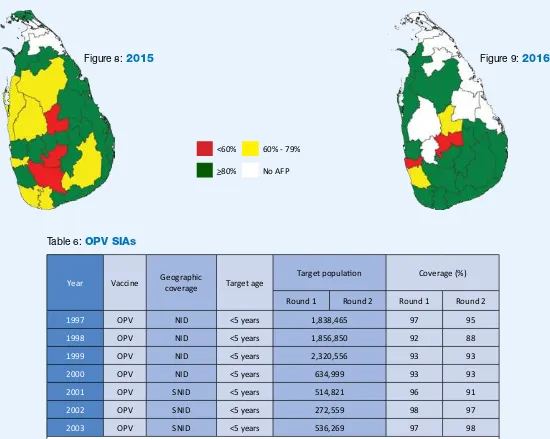
Adequate stool specimen collecion percentage by district 2015 Figure 8 10
Adequate stool specimen collecion percentage by district 2016 Figure 9 10
WHO South-East Asia Region
EPI history
• EPI launched in 1978
• Inacivated JE vaccine introduced in 1988 and live JE vaccine naionwide in 2011
• Rubella vaccine introduced in 1996
• Vitamin A supplementaion added in 2000
• MR vaccine and aTd vaccine introduced in 2001
• HepB vaccine introduced in 2003
• DTP-Hib-HepB vaccine introduced in 2008
• MMR vaccine introduced in 2011
• IPV introduced in 2015
• Naionwide fIPV introduced in 2016
• tOPV to bOPV switched on 30 April 2016.
Source: cMYP 2017-2021 and EPI/MOH
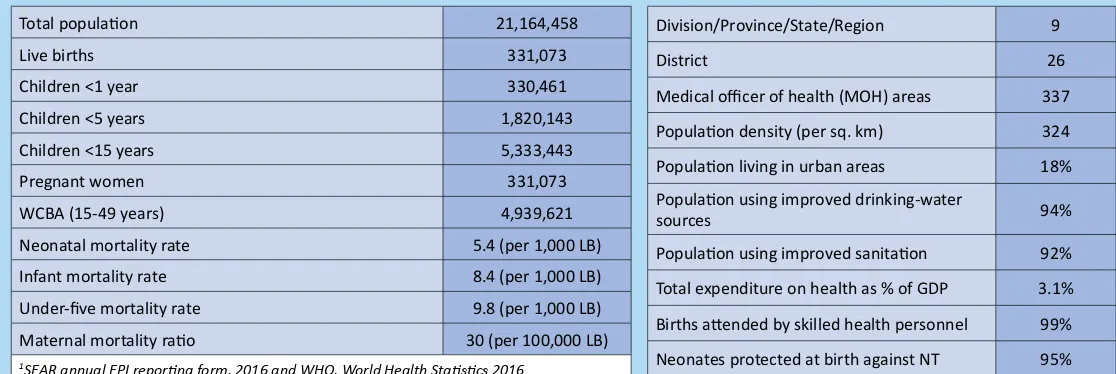
Table 1:
Basic information
12016
Total populaion 21,164,458
Live births 331,073
Children <1 year 330,461
Children <5 years 1,820,143
Children <15 years 5,333,443
Pregnant women 331,073
WCBA (15-49 years) 4,939,621
Neonatal mortality rate 5.4 (per 1,000 LB)
Infant mortality rate 8.4 (per 1,000 LB)
Under-ive mortality rate 9.8 (per 1,000 LB)
Maternal mortality raio 30 (per 100,000 LB)
1SEAR annual EPI reporing form, 2016 and WHO, World Health Staisics 2016
Division/Province/State/Region 9
District 26
Medical oicer of health (MOH) areas 337
Populaion density (per sq. km) 324
Populaion living in urban areas 18%
Populaion using improved drinking-water
sources 94%
Populaion using improved sanitaion 92%
Total expenditure on health as % of GDP 3.1%
Births atended by skilled health personnel 99%
Neonates protected at birth against NT 95%
Table 2:
Immunization schedule, 2016
Vaccine Age of administraionBCG Birth
DTP-Hib-HepB 2 months, 4 months and 6 months
OPV 2 months, 4 months, 6 months, 18 months and 5 years
IPV (fIPV) 2 months and 4 months
JE_LiveAtd 1 year
MMR 9 months and 3 years
DTP 18 months
DT 5 years
aTd 12 years
TT Pregnant women (2 doses in 1st pregnancy and 1 dose in subsequent 3 pregnancies)
Vitamin A 6 to 36 months Source: WHO/UNICEF JRF, 2016
Figure 1:
National immunization coverage, 1980-2016
Source: WHO/UNICEF esimates of naional immunizaion coverage, July 2017 revision
Table 3:
Immunization system highlights
cMYP for immunizaion 2017-2021
NTAGI fully funcional
Spending on vaccines inanced by the government no data
Spending on rouine immunizaion programme inanced by the government 74%
Updated micro-plans that include aciviies to improve immunizaion coverage 26 districts (100%)
Naional policy for health care waste management including waste from immunizaion aciviies in place
Naional system to monitor AEFI in place
Most recent EPI CES EPI coverage survey Nuwara Eliya district 2016
>80% coverage for DTP-Hib-HepB3 26 districts (100%)
>90% coverage for MCV1 26 districts (100%)
>10% drop-out rate for DTP-Hib-HepB1 to DTP-Hib-HepB3 no district
Source: WHO/UNICEF JRF, 2016
1980 1985 1990 1995 2000 2005 2010 2014 2015 2016
BCG 61 71 84 90 98 99 99 99 99 99
DTP3 46 70 86 93 99 99 99 99 99 99
OPV 46 71 86 92 99 99 99 99 99 99
MCV1 20 80 87 99 99 99 99 99 99
<70% 70% - 79% 80% - 89% >90%
Figure 3:
2015
Figure 4:
2016
Source: SEAR annual EPI reporing form, 2016 (administraive data) Source: SEAR annual EPI reporing form, 2015 (administraive data)
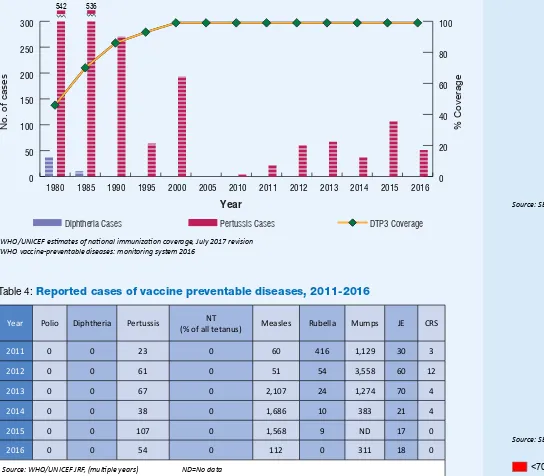
Figure 2:
DTP3 coverage
1, diphtheria and pertussis cases
2, 1980-2016
Year
Diphtheria Cases Pertussis Cases DTP3 Coverage
%
1WHO/UNICEF esimates of naional immunizaion coverage, July 2017 revision 2WHO vaccine-preventable diseases: monitoring system 2016
Table 4:
Reported cases of vaccine preventable diseases, 2011-2016
Year Polio Diphtheria Pertussis NT(% of all tetanus) Measles Rubella Mumps JE CRS
2011 0 0 23 0 60 416 1,129 30 3
Source: WHO/UNICEF JRF, (muliple years) ND=No data
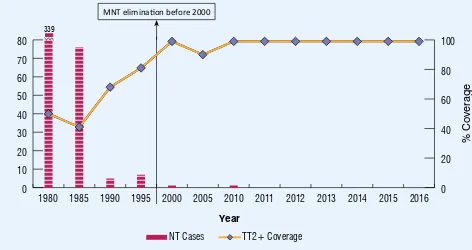
1980 1985 1990 1995 2000 2005 2010 2011 2012 2013 2014 2015 2016
NT Cases TT2+ Coverage 0
1 WHO/UNICEF JRF, Country oicial esimates, 1980-2015
2WHO vaccine-preventable diseases: monitoring system 2015 & JRF 2015
Maternal and neonatal tetanus elimination is sustained
MNT eliminaion before 2000
© WHO/Sri Lanka/K Reidey
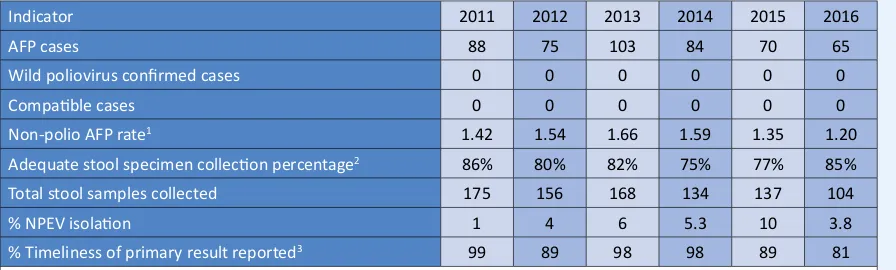
Table 5:
AFP surveillance performance indicators, 2011-2016
Indicator 2011 2012 2013 2014 2015 2016
AFP cases 88 75 103 84 70 65
Wild poliovirus conirmed cases 0 0 0 0 0 0
Compaible cases 0 0 0 0 0 0
Non-polio AFP rate1 1.42 1.54 1.66 1.59 1.35 1.20
Adequate stool specimen collecion percentage2 86% 80% 82% 75% 77% 85%
Total stool samples collected 175 156 168 134 137 104
% NPEV isolaion 1 4 6 5.3 10 3.8
% Timeliness of primary result reported3 99 89 98 98 89 81
1Number of discarded AFP cases per 100,000 children under 15 years of age.
2Percent with 2 specimens, at least 24 hours apart and within 14 days of paralysis onset. 3Results reported within 14 days of sample received at laboratory.
Figure 6:
2015
Figure 7:
2016
Polio-free status is maintained
The last laboratory conirmed polio case due to WPV was reported in November 1993.
Non-polio AFP rate by district
<1 1 – 1.99
>2 No non-polio AFP case
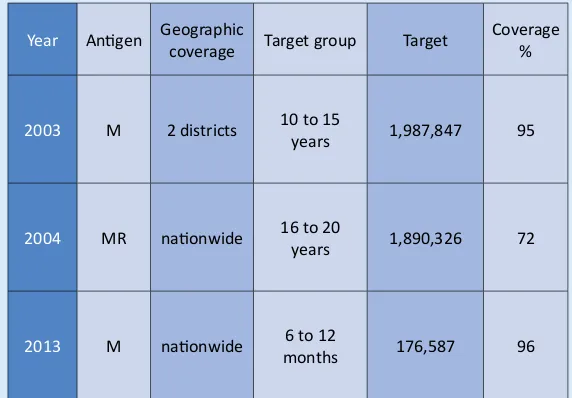
Table 6:
OPV SIAs
Year Vaccine Geographic
coverage Target age
Target populaion Coverage (%)
Round 1 Round 2 Round 1 Round 2
1997 OPV NID <5 years 1,838,465 97 95
1998 OPV NID <5 years 1,856,850 92 88
1999 OPV NID <5 years 2,320,556 93 93
2000 OPV NID <5 years 634,999 93 93
2001 OPV SNID <5 years 514,821 96 91
2002 OPV SNID <5 years 272,559 98 97
2003 OPV SNID <5 years 536,269 97 98
Source: NCCPE reports and WHO/UNICEF JRF
Adequate stool specimen collection % by district
Figure 9:
2016
Figure 8:
2015
<60% 60% - 79%
>80% No AFP
Towards measles elimination and rubella/CRS control
Figure 10:
MCV1 and MCV2 coverage
1, measles and rubella cases
2, 1980-2016
Measles Cases Rubella MCV1 Coverage MCV2 Coverage
2016
No. of cases
Year
1WHO/UNICEF esimates of naional immunizaion coverage, July 2017 revision 2WHO vaccine-preventable diseases: monitoring system 2016
Table 7:
MCV SIAs
Year Anigen Geographic
coverage Target group Target
Coverage %
2003 M 2 districts 10 to 15
years 1,987,847 95
2004 MR naionwide 16 to 20
years 1,890,326 72
2013 M naionwide 6 to 12
months 176,587 96
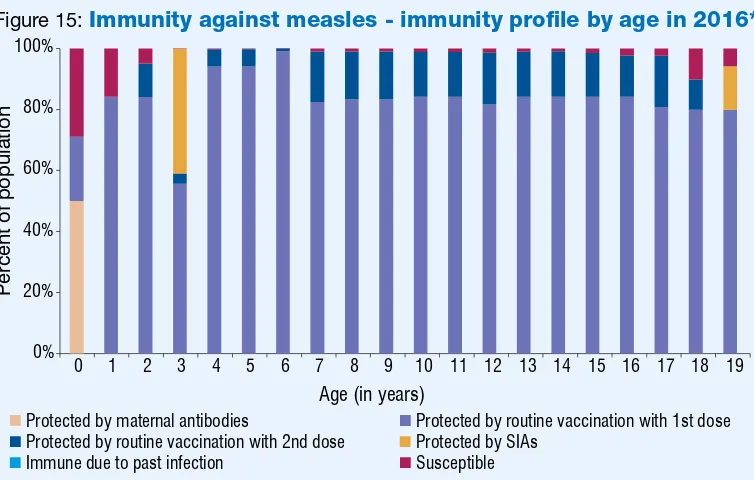
0%
Percent of population
Age (in years)
Protected by maternal antibodies Protected by routine vaccination with 1st dose
Protected by routine vaccination with 2nd dose Protected by SIAs
Immune due to past infection Susceptible
Figure 15:
Immunity against measles - immunity profile by age in 2016*
* Modeled using MSP tool ver 2 assuming the schedule and MCV coverage remain unchanged in 2016.
<80% 80% - 89% 90% - 94% >95%
Source: SEAR annual EPI reporing form, 2016 (administraive data)
Figure 12:
2016
Figure 11:
2015
Source: SEAR annual EPI reporing form, 2015 (administraive data)
Figure 14:
2016
Figure 13:
2015
Source: SEAR annual EPI reporing form, 2016 (administraive data)
Source: SEAR annual EPI reporing form, 2015 (administraive data)
Figure 16:
Sub-national risk assessment - measles and rubella
MCV1 coverage by district
MCV2 coverage by district
Source: developed using WHO risk assessment tool based on JRF & ARF data base
Figure 17:
Sporadic and outbreak associated measles cases* by month, 2011-2016
Outbreak associated measles
No of cases
0
*Includes laboratory conirmed and epidemiologically linked cases Source: SEAR Monthly VPD reports
Figure 18:
Immunization status of confirmed (laboratory and EPI linked) measles
outbreak associated cases, by age, 2011-2016
> 15 years
10-14 years
5-9 years
1-4 years
< 1 year
> 15 years
10-14 years
5-9 years
1-4 years
< 1 year
> 15 years
10-14 years
5-9 years
1-4 years
< 1 year
> 15 years
10-14 years
5-9 years
1-4 years
< 1 year
> 15 years
10-14 years
5-9 years
1-4 years
< 1 year
> 15 years
10-14 years
5-9 years
1-4 years
< 1 year
2011 2012 2013 2014 2015 2016
0
Table 8:
Surveillance performance indicators for measles and rubella, 2012-2016
Year
No. of suspect
ed measles
Case classiicaion (number) Indicators
Measles Rubella
Disc
ar
ded non-measles
non-rubella c
ases
Annual incidence of con
irmed measles c
ases per
million t
ot
al popula
ion
Annual incidence of con
irmed rubella c
ases per
oporion of all suspect
ed
measles and rubella c
ases
tha
t ha
ve had an adequa
te
in
ves
ig
aion iniia
ted within
48 hour
s of noiic
aion
Disc
ar
ded non-measles
non-rubella incidence per 100,000 t
ot
al popula
ion
Pr
oporion of subna
ional
measles non-rubella c
ases
per 100,000 t
ot
al popula
ion
Pr
oporion of sub-na
ional
sur
veillance units r
eporing
Source: SEAR annual EPI reporing form (2012-2016) ND=No data
Year
Serum specimen collect
ed fr
Serum specimen receiv
ed in
labor
a
tor
y
within 5 da
ys of
collecion Specimen posiiv
e f
or
measles IgM Specimen posiiv
e f
or
rubella IgM
% R
esults within
4 da
tecion Genotypes de
tect
*this includes 5 rubella Igm posiive samples that were subsequently excluded as they are post vaccinaion and one sample that was a repeat sample Source: SEAR annual EPI reporing form (2012-2016) ND=No data
Figure 19:
WHO supported laboratory network for VPD surveillance
Medical Research Insitute, Colombo • Naional polio laboratory
• Naional measles and rubella laboratory • Naional Japanese encephaliis laboratory • Rotavirus laboratory
For contact or feedback:
Expanded Programme on Immunizaion Ministry of Health, Colombo, Sri Lanka
Tel: +94 11 2695112; Fax: +94-11-2696583 Email: samithag@hotmail.com
www.health.gov.lk
Immunizaion and Vaccine Development (IVD)
WHO-SEARO, IP Estate, MG Marg, New Delhi 110002, India Tel: +91 11 23370804, Fax: +91 11 23370251
Email: SearEpidata@who.int