252 (2000) 199–219
www.elsevier.nl / locate / jembe
Acute and acclimated digestive responses of the cockle
Cerastoderma edule (L.) to changes in the food quality and
quantity
II. Enzymatic, cellular and tissular responses of the digestive
gland
a ,* b a a b
I. Ibarrola , M. Etxeberria , J.I.P. Iglesias , M.B. Urrutia , E. Angulo
a
´ ´ ´
Departamento de Biologıa Animal y Genetica, Facultad de Ciencias, Universidad del Paıs Vasco /Euskal
Herriko Unibertsitatea, Apartado 644, E-48080 Bilbao, Spain
b
´ ´
Departamento de Zoologıa y Dinamica Celular Animal, Facultad de Ciencias, Universidad del Paıs
Vasco /Euskal Herriko Unibertsitatea, Apartado 644, E-48080 Bilbao, Spain Received 15 February 2000; received in revised form 14 March 2000; accepted 31 May 2000
Abstract
Cockles Cerastoderma edule were fed two different concentrations of two diets with different qualities which were achieved by mixing different proportions of ashed silt particles with cells of the microalgae Tetraselmis suecica. After 3 days (acute response) and 11 days (acclimated response) of exposure to the diets, we analysed the digestive activity of the digestive gland using cyto-histological and enzymatic techniques. We measured (i) the volumetric fraction of digestive and basophilic cells in digestive tubules, (ii) the diverticular radius and the thickness of digestive epithelia, (iii) the stereological parameters characterizing the lysosomal system and, (iv) dry weight, soluble protein content and specific and total amylase, cellulase, laminarinase, and protease activities of the digestive gland. In the conditions of the present study, specific cellulase and laminarinase activities in the digestive gland of cockles were correlated with the volumetric fraction of basophilic cells (r50.672 and 0.642, respectively), whereas the specific protease was highly correlated (r50.866) with lysosomal volume density. The implications of these correla-tions are discussed in relation to the feeding and absorptive parameters reported in the preceding publication. In the acute response, adjustments of the synthesis of constituents of the lysosomal / proteolytic system of the digestive cells seemed to be the only mechanism operating at the digestive level to respond to the changes in food availability. Lysosomal volume density increased with rising ingestion rate of organic matter, however, the occurrence of a limit in this short-term tissular response would account for the recorded trade-off between absorption efficiency and ingestion rate with different food qualities. With regard to acclimation, food quality determined the
*Corresponding author. Tel.: 134-94-601-2728.
E-mail address: [email protected] (I. Ibarrola).
nature of the response of the digestive gland. With low quality diets, a time-dependent capability of the digestive gland for intensifying lysosomal / proteolytic production explains the increase of food absorption rates that result from higher filtration and ingestion rates. With high quality food, digestive acclimation differed with food particle concentration: with low rations, in spite of constant morphometrical and stereological parameters, the significant changes in the absorptive balance of biochemical components suggests the existence of an increased production of lysosomes that promotes an accelerated turn-over rate of the digestive epithelia. With high food concentrations, this response was coupled with increased activities of cellulase and laminarinase enzymes, probably as a consequence of higher rates of enzyme secretions from basophilic cells. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Basophilic cells; Bivalves; Cockles; Digestive cells; Digestive enzymes; Lysosomes
1. Introduction
The digestive gland of bivalves consists of blind-ending tubules connected with the stomach through a branched system of primary and secondary ducts. The tubules comprise two kind of cells: digestive and basophilic. Intracellular digestion and absorption of food particles entering into the digestive gland takes place within the lysosomes of the digestive cells. Four steps in the intracellular digestive process have been described (see Owen (1970, 1974) and Morton (1983) for review): (a) production of primary lysosomes, (b) fusion of primary lysosomes with pinocytic vesicles to form heterolysosomes where intracellular digestion and absorption of food occurs, (c) accumulation of indigestible, electrodense residual bodies, and (d) release of the residual bodies to form ‘digestive spherules’ (apical part of the digestive cells filled with residual bodies), which are excreted via stomach and intestine and finally evacuated through the faeces. Such endogenous organic matter might account for part of the so-called metabolic faecal losses (Hawkins et al., 1983; Hawkins and Bayne, 1984), a negative element of the digestive process which has been recognized to limit energy gain in mussels (Hawkins and Bayne, 1985; Hawkins et al., 1990) and cockles (Navarro and Iglesias, 1993; Navarro et al., 1994).
On the other hand, basophilic cells appear as darkly stained pyramidal cells lying in regular groups. In early studies, these cells were considered immature cells capable of renovating digestive epithelia at the reconstituting phase (see Morton (1983) for review). However, since mitosis has been rarely observed in the digestive gland, this role is unclear. Conversely, histochemical (Mathers, 1973; Palmer, 1979) and ultrastructural (Owen, 1970) studies showing highly developed Golgi complexes suggested the possibility that basophilic cells are involved in the synthesis and secretion of extracellu-lar digestive enzymes.
in alternated and endogenously synchronized phases of (i) food filtration, (ii) extracellu-lar digestion (in the stomach) and, (iii) intracelluextracellu-lar digestion (within the gland). These studies brought great information regarding anatomy, morphology and histochemistry of the digestive system but they fail to provide information on the physiological control of the digestive function since none of them analyzed the changes of the parameters characterizing intra- or extracellular digestive activity in bivalves fed different foods.
The aim of this study was to identify the cellular mechanisms involved in the acute and acclimated changes of the functional gut capacity of cockles treated to different food qualities and quantities. We performed a histological study quantifying the volume density of lysosomes, digestive and basophilic cells, the thickness of digestive epithelia and the radium of digestive tubules together with the determination of the digestive enzyme activities of cockles fed four diets (two different qualities supplied at two different rations) for 3 and 11 days. Details on the characteristics of the diets and recorded feeding rates, absorption efficiencies and absorption rates (of total organic matter and biochemical components) have been reported in the preceding publication (I. Feeding and absorption).
2. Materials and methods
2.1. Experimental set-up and manipulation of cockles
A total of 140 cockles (ranging in size 24–28 mm) were collected from an intertidal mud-flat in the Mundaka estuary (Biscay, N. Spain) during February 1993. In the laboratory, 120 cockles were divided in four groups, each fed one of the four diets described in the experimental protocol (Ll, Lh, Hl and Hh), and the remaining 20 cockles were submitted to starvation (S). Further experimental details on experimental set-up were reported in the preceding contribution. After 3 days and 11 days of exposure to the diets, digestive gland and crystalline styles of 15 cockles from each experimental condition were excised. The enzyme activities of the digestive gland were determined in 10 individuals, whereas the remaining five individuals were used for histological measurements.
2.2. Histological measurements
2.2.1. Preparation of digestive glands
2.2.2. Stereology of the digestive tubes
A Weibel reticule (Multipurpose test system M-168) was used to calculate the volumetric fractions of the different cell types in the digestive tubes. Two sections cut from different layers, and five randomly selected areas per section were counted for each individual (i.e. 10 measurements per individual). The volumetric fractions of digestive (VF ) and basophilic (VF ) cells were determined. In addition, we determined theD B volumetric fraction corresponding to apical cell fragments detached from digestive cells of disintegrating tubules (VFCF).
2.2.3. Planimetric determinations
For each individual digestive gland, a total of 50 tubule sections were drawn with the aid of a drawing tube coupled to a Nikon Optiphot microscope. We used an objective lens of 3100. Five sections were randomly selected from each 10 paraffin section belonging to two different portions of the gland (50 mm apart). The profiles of the 50 sections were recorded with a Watanabe DT1000 digitizer and the resulting planimetric measurements were calculated with a personal computer following the method of
´
Marigomez (1989) which is based in the geometrical transformation of the tubule section shapes. Two parameters are obtained: mean diverticular radius (MDR) and mean epithelial thickness (MET).
Simultaneously, the tubules were classified according to their morphological appear-ance. Four different tubule types were distinguished following the standards of Morton (1983): holding (H), absorptive (A), disintegrating (D) and reconstituting (R).
2.2.4. Stereology of the lysosomal system
Lysosomes were stained following the method of Moore (1976) as described in Cajaraville et al. (1991) which is based on the cytochemical reaction for b-glucuronid-ase. The enzymatic reaction yields a purple-colored product. Light microscopic images were then obtained by coupling an image processing system comprising a B&W-CCD TV camera, a HR-TV colour screen and a personal computer (AT-PC), to the Leitz light microscope and using an objective lens of 3100. The area corresponding to the lysosomes in purple colour and the rest of cytoplasmic matter of the digestive cells were manually segmented by assigning them different colours on the monitor. Computerized measurements of the areas corresponding to different colours allows computation of the following parameters (Lowe et al., 1981): lysosomal volume density (VD, lysosomal volume / cytoplasmic volume), lysosomal numerical density (ND, number of lysosomes / cytoplasmic volume), lysosomal surface density (SD, lysosome surface / cytoplasmic volume) and the surface to volume ratio of the lysosomes (S / V).
2.3. Digestive enzyme activities
2.4. Statistical procedures
Significant effects promoted by food quality (ql) and quantity (qn) on histological measurements and enzyme activities in the acute and acclimated responses of cockles were analyzed by performing two factor analysis of variance (Zar, 1984). The arcosin transformation was used to normalize the data corresponding to histological parameters which are expressed as percentage. The effect promoted by acclimation to the different diets was analyzed by testing for significant differences (t-test) between the mean values obtained for the acute and acclimated responses.
Possible functional relationship between enzyme activities and digestive structures was analyzed by calculation of the correlation indexes between specific activities and volume densities of the different digestive structures.
3. Results
3.1. Stereology of the digestive tubule
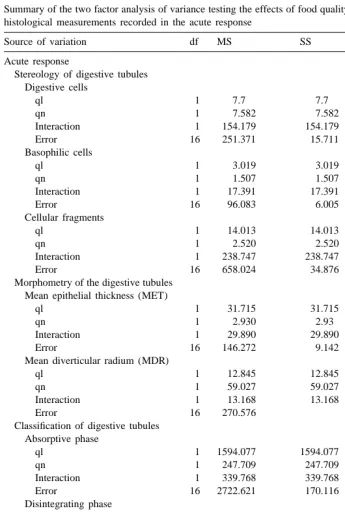
Table 1 shows the results of the stereological analysis. Digestive and basophilic cells account, respectively, for 60–70% and 13–17% of the volume of the digestive tubules irrespective of the feeding condition. Cellular fragments represent a minor proportion of the total volume (maximum value of 11.5%). Significant differences exerted by food quality and quantity on the histological parameters of cockles fed for 3 and 11 days are shown in Table 2. In the acute response, the proportion of digestive cells increased with rising rations of low quality food and decreased with increasing rations of high quality
Table 1
a
Volumetric fractions of the main components of the digestive tubules
Diet VFD VFB VFL VFF.S.
S3 0.65960.060 0.16860.057 0.13960.088 0.03460.024 S11 0.67360.078 0.09960.039* 0.17960.039 0.04760.086 Ll3 0.61060.112 0.13560.036 0.14060.037 0.11560.103 Ll11 0.67260.038 0.14660.023 0.12960.051 0.05360.010 Lh3 0.68360.042 0.16460.032 0.10860.010 0.04560.019 Lh11 0.69460.021 0.15860.023 0.10760.034 0.04160.013 Hl3 0.72360.028 0.16660.026 0.08360.020 0.03060.015 Hl11 0.63960.125 0.14460.023 0.15660.031* 0.06160.094 Hh3 0.61260.051 0.15060.028 0.14160.034 0.09760.065 Hh11 0.65260.066 0.19560.032* 0.12560.078 0.02960.010*
a
VF , volumetric fraction of digestive cells; VF , volumetric fraction of basophilic cells; VF , volumetricD B L
fraction of the light of the tubules; VFF.S., volumetric fraction of fragmentation spherules. Values are means6S.D. n55. S, starved; Ll, low quality food dosed at low ration; Lh, low quality dosed at high ration; Hl, high quality food dosed at low ration; Hh, high quality food dosed at high ration. The subindex 3 and 11 are used to indicate, respectively, acute (3 days) and acclimated (11 days) responses.
*
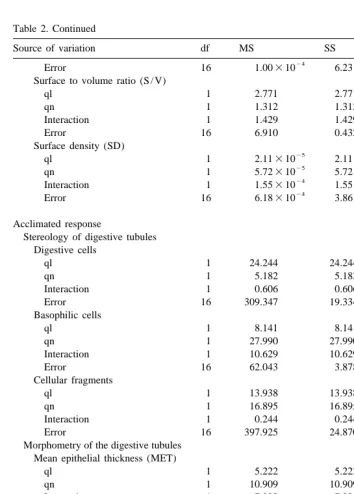
Table 2
Summary of the two factor analysis of variance testing the effects of food quality (ql) and quantity (qn) on the histological measurements recorded in the acute response
Source of variation df MS SS F P
Acute response
Stereology of digestive tubules Digestive cells
ql 1 7.7 7.7 0.490 0.4939
qn 1 7.582 7.582 0.479 0.4987
Interaction 1 154.179 154.179 9.814 0.0064**
Error 16 251.371 15.711
Basophilic cells
ql 1 3.019 3.019 0.503 0.4885
qn 1 1.507 1.507 0.251 0.6232
Interaction 1 17.391 17.391 2.896 0.1081
Error 16 96.083 6.005
Cellular fragments
ql 1 14.013 14.013 0.402 0.5351
qn 1 2.520 2.520 0.072 0.7915
Interaction 1 238.747 238.747 6.846 0.0187*
Error 16 658.024 34.876
Morphometry of the digestive tubules Mean epithelial thickness (MET)
ql 1 31.715 31.715 3.469 0.0810
qn 1 2.930 2.93 0.320 0.5792
Interaction 1 29.890 29.890 3.269 0.0894
Error 16 146.272 9.142
Mean diverticular radium (MDR)
ql 1 12.845 12.845 0.760 0.3964
qn 1 59.027 59.027 3.490 0.0801
Interaction 1 13.168 13.168 0.779 0.3906
Error 16 270.576
Classification of digestive tubules Absorptive phase
ql 1 1594.077 1594.077 9.368 0.0075**
qn 1 247.709 247.709 1.456 0.2452
Interaction 1 339.768 339.768 1.997 0.1768
Error 16 2722.621 170.116
Disintegrating phase
ql 1 1485.398 1485.398 8.429 0.0111*
qn 1 258.337 258.337 1.435 0.2485
Interaction 1 344.118 344.118 1.911 0.1768
Error 16 2881.154 180.072
Interaction 1 2.38310 2.38310 4.667 0.0462*
25 26
Table 2. Continued
Source of variation df MS SS F P
24 26
Error 16 1.00310 6.23310
Surface to volume ratio (S / V)
ql 1 2.771 2.771 6.417 0.0221*
qn 1 1.312 1.312 3.038 0.1005
Interaction 1 1.429 1.429 3.309 0.0877
Error 16 6.910 0.432
Interaction 1 1.55310 1.55310 4.026 0.0620
24 25
Error 16 6.18310 3.86310
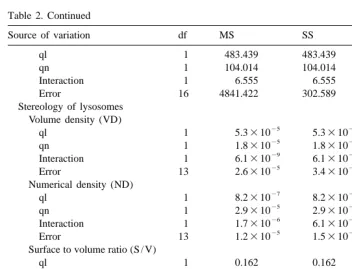
Acclimated response
Stereology of digestive tubules Digestive cells
ql 1 24.244 24.244 1.254 0.279
qn 1 5.182 5.182 0.268 0.611
Interaction 1 0.606 0.606 0.031 0.862
Error 16 309.347 19.334
Basophilic cells
ql 1 8.141 8.141 2.099 0.169
qn 1 27.990 27.990 7.218 0.016*
Interaction 1 10.629 10.629 2.741 0.117
Error 16 62.043 3.878
Cellular fragments
ql 1 13.938 13.938 0.560 0.465
qn 1 16.895 16.895 0.679 0.422
Interaction 1 0.244 0.244 0.010 0.922
Error 16 397.925 24.870
Morphometry of the digestive tubules Mean epithelial thickness (MET)
ql 1 5.222 5.222 0.399 0.537
qn 1 10.909 10.909 0.833 0.375
Interaction 1 7.222 7.222 0.552 0.468
Error 16 209.503 13.094
Mean diverticular radium (MDR)
ql 1 15.247 15.247 0.753 0.398
qn 1 9.091 9.091 0.445 0.514
Interaction 1 57.570 57.570 2.843 0.111
Error 16 324.039 20.252
Classification of digestive tubules Absorptive tubules
ql 1 505.515 505.515 1.805 0.198
qn 1 111.723 111.723 0.399 0.537
Interaction 1 6.856 6.856 0.024 0.877
Error 16 4481.453 280.091
Table 2. Continued
Source of variation df MS SS F P
ql 1 483.439 483.439 1.598 0.224
qn 1 104.014 104.014 0.344 0.566
Interaction 1 6.555 6.555 0.022 0.885
Error 16 4841.422 302.589
Interaction 1 1.7310 6.1310 0.141 0.713
25 24
Error 13 1.2310 1.5310
Surface to volume ratio (S / V)
ql 1 0.162 0.162 0.169 0.688
qn 1 2.166 2.166 2.251 0.157
Interaction 1 0.121 0.121 0.126 0.728
Error 13 0.962 12.507
Interaction 1 6.0310 6.0310 0.013 0.910
25
Error 13 4.6310 0.001
food (Table 1), and thus, the interaction ql3qn appears as a significant factor affecting VFD (Table 2). Consistently, the inverse trend is evident for VFCF. Significant modifications of the volumetric fractions of the digestive tubules of cockles after acclimation to diets have been indicated with asterisks in Table 1. The volumetric fraction of basophilic cells increased significantly in cockles acclimated to Hh diet whereas decreased significantly after prolonged starvation.
3.2. Morphometry of the digestive tubules
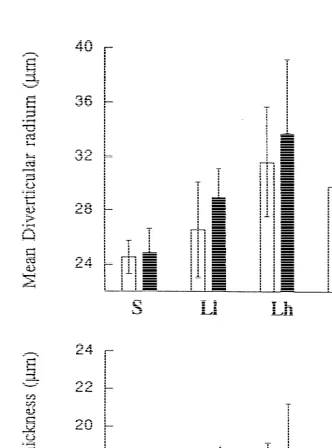
Results of the planimetric analysis of tubules have been plotted in Fig. 1. Two factor variance analysis performed for both short- and long-term responses (Table 2) indicates no significant differences in mean diverticular radium and mean epithelial thickness of tubules from cockles fed on different food qualities and rations.
acclimation, cockles were found to have significantly higher MDR than starved ones in all feeding conditions.
Both epithelial thickness and diverticular radius are one-dimensional measurements characterizing a three-dimensional structure. Thus, slight changes in such measurements are likely to represent large variations in terms of volume. Total volume of a tubule can
3
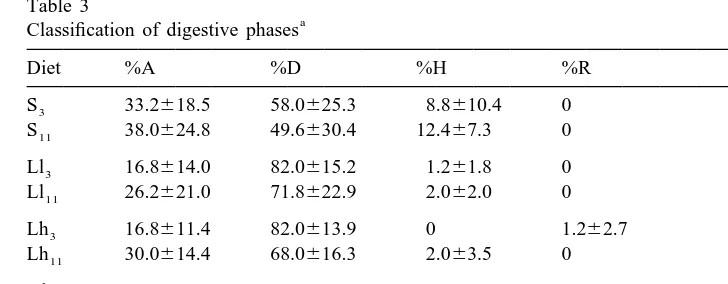
be considered to be proportional to the cubic of the diverticular radius (MDR ). In Fig. 3 2, mean dry weight (shown in Table 5) is plotted against the estimated mean MDR to analyze to what extent the weight increase of the gland could be attributed to the size increment of digestive tubules. As previously reported (Ibarrola et al., 1996, 1998b), the increment of the dry weight of the gland may play a significant role in the enzymatic response of the cockles. A good correlation is evident between both parameters with the exception of cockles acclimated to Lh and Hh whose digestive gland weight lay above the relationship.
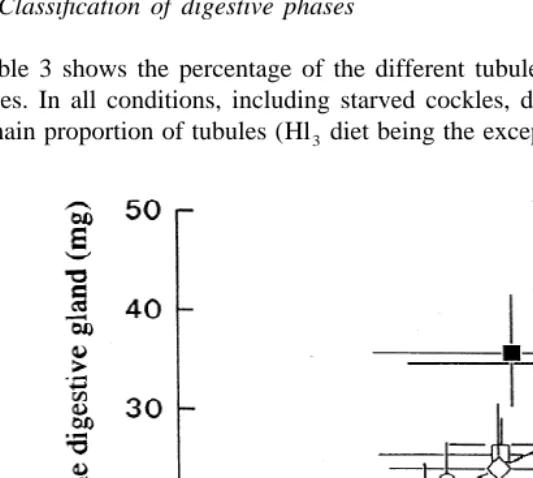
3.3. Classification of digestive phases
Table 3 shows the percentage of the different tubule types in the digestive gland of cockles. In all conditions, including starved cockles, disintegrating tubules account for the main proportion of tubules (Hl diet being the exception in the sense that absorptive3
Fig. 2. Dry weight (mg) of the digestive gland as a function of the estimated volume of a single digestive tubule in cockles fed different diets (Ll, triangles; Lh, rhombs; Hl, circles and Hh, squares) for 3 days (hollow symbols) and 11 days (solid symbols). Estimation of a tubule equivalent volume was calculated as its
3 3
Table 3
Results are expressed as percentage. %A, absorptive phase; %D, disintegrating phase; %H, holding phase; %R, reconstituting phase. Values are means6S.D. n55. S, starved; Ll, low quality food dosed at low ration; Lh, low quality dosed at high ration; Hl, high quality food dosed at low ration; Hh, high quality food dosed at high ration. The subindex 3 and 11 are used to indicate, respectively, acute (3 days) and acclimated (11 days) responses.
tubules are prevalent over disintegrating). Holding and reconstituting tubules accounted only for a marginal proportion. Cockles fed high quality diets for 3 days had significantly higher proportions of tubules in absorptive phase and lower proportions in disintegrating phase than those fed low qualities, thus, food quality appears as a significant factor in variance analysis shown in Table 2. These differences were lost after acclimation (Table 2).
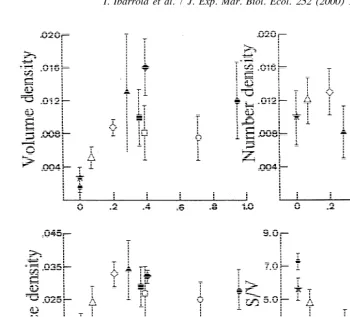
3.4. Stereology of lysosomes
Results of the stereological analysis of lysosomes are shown in Table 4. The presence of food affected significantly the characteristics of the lysosomal system in less than 3 days and, thus, the volume density was significantly lower in starved than in fed cockles. Furthermore, the lysosomal volume density appears to increase with improved feeding condition: cockles fed Ll3 diet had significantly higher (t-test, P,0.005) lysosomal density than starved ones but significantly lower than those fed the remaining diets. Accordingly, Table 2 shows that the interaction term ql3qn affects significantly lysosomal volume density.
Table 4
a
Stereological parameters characterizing the lysosomal system from digestive cells of the digestive gland
Diet VD ND S / V SD
VD, volume density; ND, numerical density; S / V, surface to volume ratio; SD, surface density. Values are means6S.D. n55. S, starved; Ll, low quality food dosed at low ration; Lh, low quality dosed at high ration; Hl, high quality food dosed at low ration; Hh, high quality food dosed at high ration. The subindex 3 and 11 are used to indicate, respectively, acute (3 days) and acclimated (11 days) responses.
*
Indicates significant differences (Fischer test for P50.05) between short- and long-term responses. have analyzed the relationship between lysosomic parameters and ingestion rate of organic matter (reported in the preceding publication). The figure shows that: (a) both volume and surface density of lysosomes rose to saturation with increasing organic ingestion rate, both in the acute and acclimated responses. (b) For a given value of organic ingestion rate, corresponding VD is higher after acclimation to the diet. (c) Increments in lysosomal volume density with rising ingestion rate occurred as a consequence of a strong increment of the lysosomal size (see S / V) and in spite of reduction of the numerical density (ND).
3.5. Enzyme activities in the digestive gland
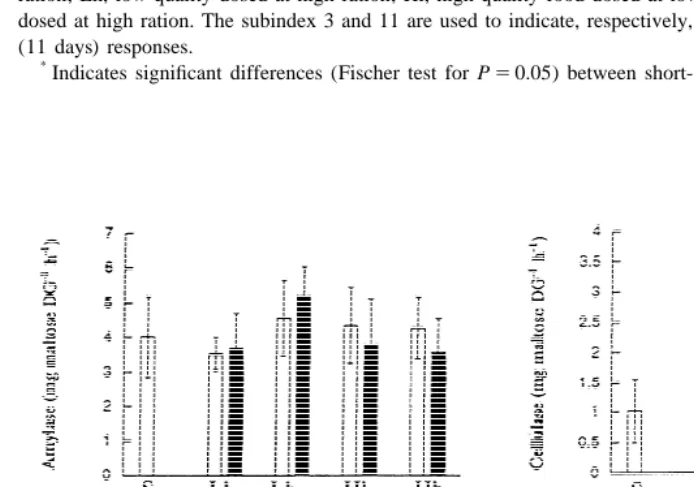
Dry weight, protein contents and specific enzyme activities (mg of end-product per mg protein per hour) of the digestive glands are shown in Table 5. Resulting total activities (mg of end-product per digestive gland per hour) have been plotted in Fig. 4a–d. Significant modifications after acclimation have been indicated with asterisks. Two-factor variance analysis testing for significant effects of food quality and quantity in the acute and acclimated responses are shown in Table 6.
In the acute response, both quantity and quality exerted significant (positive) effects on the dry weight of the digestive gland (Table 6). The changes in the soluble protein content were not statistically significant. Total protease and cellulase activities were significantly higher in cockles fed on high than on low food rations. The increase of protease activity was especially high in cockles fed Lh diet, due to the simultaneous increment of the dry weight of the digestive gland and the specific protease activity. Conversely, no significant changes were recorded for total amylase and laminarinase (Table 6).
Fig. 3. Stereological parameters of the lysosomic system of cockles starved (3 days, solid star; 11 days, asterisks) and fed different diets (Ll, triangles; Lh, rhombs; Hl, squares; Hh, circles) for 3 days (hollow symbols) and 11 days (solid symbols). Values are means6S.D. (n55).
generalized increment of dry weight of the gland, especially in cockles fed concentrated rations, that brought about increased activities of cellulase, laminarinase and proteases but not amylases (see the statistical significance of qn on Table 2). Food quality exerted a further differential effect: increment of cellulase and especially laminarinase activities with increasing food concentrations were more pronounced in cockles acclimated to high quality diets (Hh , see Table 5).11
3.6. Correlation between histological parameters and enzyme activities
Table 5
Dry weight (WDG; mg), percentage of soluble protein (%p) and specific enzyme activities of the digestive 21 21 a
gland (mg of end product mg prot h )
Diet WDG %p Aspc Cspc Lspc Pspc
S3 15.964.6 22.161.1 1.1560.25 0.3160.13 0.3660.26 0.2360.04 Ll3 17.962.6 23.061.8 0.8660.10 0.2760.07 0.2860.11 0.3060.08 Ll11 20.664.1 20.661.7* 0.8760.18 0.3160.13 0.1760.11 0.3760.06 Lh3 24.166.6 21.562.2 0.8660.15 0.3560.09 0.2960.11 0.4360.02 Lh11 35.064.9* 21.860.9 0.6960.10* 0.3460.08 0.2560.08 0.5160.08* Hl3 22.864.0 21.561.9 0.8960.17 0.3160.12 0.2860.12 0.4160.09 Hl11 26.466.1 21.662.9 0.6860.23* 0.2960.12 0.3260.13 0.4260.07 Hh3 25.763.5 21.862.2 0.7760.16 0.3360.09 0.2660.06 0.3260.04 Hh11 35.766.0 21.861.1 0.4660.11* 0.3560.09 0.3860.06* 0.4760.04*
a
Values are means (6S.D.) n510. S, starved, (S11 not determined); Ll, low quality food dosed at low ration; Lh, low quality dosed at high ration; Hl, high quality food dosed at low ration; Hh, high quality food dosed at high ration. The subindex 3 and 11 are used to indicate, respectively, acute (3 days) and acclimated (11 days) responses.
*
Indicates significant differences (Fischer test for P50.05) between short- and long-term responses.
Table 6
Summary of ANOVA testing the effects of food quality (ql) and quantity (qn) on the dry weight, protein content and total enzyme activities of the digestive gland
Source of variation df MS SS F P
Acute response Dry weight
ql 1 104.65 104.65 5.29 0.027*
qn 1 208.39 208.39 10.53 0.002*
Interaction 1 29.41 29.41 1.49 0.231
Error 36 712.74 19.80
Interaction 1 8.60310 8.60310 2.10 0.156
24
Error 36 0.01 4.09310
Total amylase
ql 1 0.62 0.62 0.75 0.392
qn 1 2.16 2.16 2.61 0.116
Interaction 1 2.80 2.80 3.37 0.075
Error 33 27.36 0.83
Total cellulase
ql 1 0.16 0.16 0.51 0.479
qn 1 2.64 2.64 8.18 0.007*
Interaction 1 0.56 0.56 1.75 0.195
Error 33 10.64 0.32
Total laminarinase
24 24 23
ql 1 1.94310 1.94310 6.76310 0.935
qn 1 0.67 0.67 2.34 0.136
Interaction 1 0.44 0.44 1.75 0.223
Error 33 9.47 0.28
Total protease
ql 1 0.09 0.09 0.48 0.494
qn 1 1.48 1.48 7.94 0.008*
Interaction 1 3.75 3.75 20.15 0.000*
Error 33 6.5 0.19
Acclimated response Dry weight
ql 1 103.01 103.01 3.55 0.068
qn 1 1334.56 1334.56 45.96 0.000*
Interaction 1 62.03 62.03 2.13 0.153
Error 34 987.32 29.04
Interaction 1 2.30310 2.30310 0.66 0.421
24
Error 34 0.01 3.47310
Total amylase
ql 1 5.44 5.44 4.89 0.034*
qn 1 4.09 4.09 3.68 0.063
Interaction 1 6.97 6.97 6.26 0.017*
Table 6. Continued
Source of variation df MS SS F P
Total cellulase
ql 1 0.41 0.41 0.73 0.399
qn 1 13.63 13.63 24.35 0.000*
Interaction 1 0.28 0.28 0.50 0.482
Error 34 19.03 0.56
Total laminarinase
ql 1 10.55 10.55 20.61 0.000*
qn 1 13.10 13.10 25.58 0.000*
Interaction 1 0.01 0.01 0.03 0.857
Error 34 17.41 0.51
Total protease
ql 1 1.17 1.17 1.84 0.184
qn 1 29.77 29.77 46.91 0.000*
Interaction 1 2.65 2.65 9.17 0.049*
Error 34 21.57 0.63
(Cspc, Lspcand Pspcfrom Table 5) and lysosomal volume densities (VD from Table 4) as well as the relative abundance of cell types in the digestive gland (FV , FV , from TableB D 1). Since individuals used in enzymatic and histological determinations were different, mean values have been used in the analysis. Results are presented in the form of a correlation matrix in Table 7. Specific protease activity had a high correlation index with lysosome volume density (VD), while specific cellulase and laminarinase activities were correlated with the volume fraction of basophilic cells. A significant negative correlation between specific amylase and lysosomal VD was also found.
4. Discussion
In the present experiment, the digestive gland of cockles has shown to have a great plasticity. Modifications of (i) the volume of lysosomes, (ii) the thickness of the digestive epithelia, (iii) the proportion of basophilic cells, and (iv) the size of digestive diverticula were associated to different extents in the short-term (3 days) and acclimated (11 days) responses of Cerastoderma edule to changes in the food quality and quantity. Two functional relationships might be suggested between the above parameters and digestive enzyme activities: (i) the high correlation index between specific protease
Table 7
Correlation coefficient (r) between cyto-histological structures and specific enzyme activities of the digestive gland (*P,0.05; **P,0.001)
VD FVD FVB
Aspc 20.670* 0.125 20.314
Cspc 0.517 0.351 0.672*
Lspc 20.366 20.132 0.642*
activity and lysosomal volume density suggest that protease activity was associated to lysosomes. This is consistent with early studies (see Reid and Rauchert (1972) for review). Conversely, the negative correlation of specific amylase and laminarinase with lysosomal volume density indicates that, in contrast with protease, the increment of lysosomal volume diluted the pool of such carbohydrases, pointing to a different origin for these enzymes. (ii) Specific cellulase and laminarinase activities were significantly correlated with the volume fraction of basophilic cells suggesting the possibility that this cell type might be responsible for the synthesis of such carbohydrases. This is consistent with some earlier ideas on the secretory function of the basophilic cell (Owen, 1970; Pal, 1971; Mathers, 1973).
We think that the above correlations are key elements of information concerning both acute and acclimated feeding and absorptive responses of cockles to changes in the diet.
4.1. Acute response
Activation of intracellular digestion at low rates of organic ingestion promoted the release of digestive spherules from digestive cells, as shown by the simultaneous increment of the lysosomal volume density, decrease of mean epithelial thickness and increase of the proportion of disintegrating tubules in cockles fed Ll diet as compared with starved ones. With higher rates of organic ingestion (i.e. Lh , Hl and Hh ), the3 3 3 increment of the lysosomal volume density is accompanied with the enhancement of both the radium (MDR) and the epithelial thickness (MET) of the digestive tubules (see Fig. 1). This simultaneous increment of the size and thickness of the digestive tubules with higher ingestion rates reflects the existence of an expansion process based on a size increment of digestive cells, which is likely to result in a volume increase of the whole
3
organ. This is strongly suggested by the high correlation between MDR and the dry weight of the gland. Moreover, the high variability found in histological parameters recorded at high ingestion rates would be interpreted as reflecting a loss of synchroniza-tion of intracellular digessynchroniza-tion phases between individual tubules. This is likely to occur with higher digestive activities promoting a faster completion of these phases and resulting in a continuous release of digestive spherules (Langton, 1975; Robinson and Langton, 1980; Robinson, 1983).
Thus, the increment of both mean values and standard deviations of MET and MDR would reflect the intensification of the intracellular digestion with rising ingestion rate. Two characteristics of this process are relevant to understand short-term behaviour of feeding and absorptive parameters recorded in cockles submitted to different food conditions:
this adaptative response, evident with high quality diets, would have resulted in a decrease of AE with increasing OIR (Table 2 in the preceding contribution), thus, imposing the demand for regulation of ingestion rate by reducing clearance rates. Therefore, on the present occasion, the replenishment of digestive diverticula at high OIR seems to exert the same effect to that elicited by saturation of gut capacity in a previous study (Ibarrola et al., 1998a). This suggests that lysosomal volume of digestive cells might be a good descriptor of the potential capacity of the digestive system of cockles for processing food and, in this respect, would appear strongly related to gut contents;
(ii) the correlation between lysosomal volume density and specific protease activity suggest that increased lysosomal density with higher ingestion rates of organics was not accounted for by a mere enlargement of primary lysosomes resulting from its fusion with pinocytic vesicles; rather, an enhanced synthesis of lysosomal con-stituents seems also to exist. Mathers (1973) reported that food presence induces a general increment of several lysosome-based enzyme activities in the digestive gland of Ostrea edulis. The fact that we measured a greater correlation index of VD with proteases than with carbohydrases indicates that, at least in the conditions of present experiments, proteases are a more significant component of the enzymatic pool than carbohydrases in the digestive cells of cockles. Since we use a non-specific method to assay proteases, our measurements reflect the activities of proteases either concerned with digestive or with other proteolytic (metabolic or autolytic) functions (cathepsin B and D, respectively, as determined by Reid and Rauchert (1972)); therefore, we cannot make any assumption regarding the protease type involved in such response. Whatever it is, however, the important point concerning absorption of biochemical components is that the rapid reaction of the lysosomal–proteolytic system would cause the recorded prevalence of protein absorption (see the preceding contribution).
4.2. Acclimation
completion of the intracellular digestive cycle (Ibarrola et al., 1996, 1998a). The present results showing that increasing lysosomal production is accompanied with reduction of AE greatly reinforces this statement.L
As regards the process of acclimation to high quality diets, the morphometrical parameters of digestive tube and stereological parameters of lysosomes were found to remain unchanged. This contrasts with the dramatic fall in lipid absorption efficiency (see the preceding contribution) suggesting the existence of higher digestive investments. Information from these two sources might be conciliated considering the turn-over rate of digestive epithelia: in conditions of saturated gut capacity, increasing intracellular activity (lysosome production) would accelerate the hydrolysis, increasing gross absorption of food. However, net absorption rate would become restricted because metabolic faecal losses per unit time would also tend to increase due to a faster completion of the digestive cycle which would increase the release rate of apocrine secretions.
Although the above traits are common for cockles acclimated to both Hl and Hh diet, those with Hh diet showed a differential feature: a significant increase of total cellulase and laminarinase activities, probably released by basophilic cells (see Table 7). As regards the effect of such induction in the absorptive balance, the fact that reduction of AEC was clearly lower in cockles acclimated to Hh diet than to Hl in spite of the increased carbohydrate ingestion rate (see Table 5 in the preceding contribution), might be seen as a consequence of such carbohydrase induction. Since in our previous studies, cockles fed high quality diets were found to induce carbohydrase activity faster (less than 3 days; Ibarrola et al., 1996, 1998a), this response might be considered to be the same one but inserted in a different time-course.
To summarize, these results illustrate the ability of cockles to adjust the lysosomal / proteolytic system to cope with increasing food availability, induction of carbohydrases being retarded in this case for comparison with previous experiments. This delay suggests that the acquisition of an optimal status of the digestive diverticula characteris-tic of cockles fed high quality diets would require different times depending on the seasons. Also, it could constitute a factor contributing to the observed differences in the effect elicited by increasing food quality on the absorptive balance of biochemical components between present and previous experiments.
Evidence of seasonal modifications in the functional and physical properties of the digestive gland has been reported for several bivalve species. The following parameters have been shown to experience a more or less intense winter depression: (i) the lysosomal cathepsin B endopeptidase activity involved in the proteolysis of exogenous
´
the predominant activity within the digestive system of bivalves. In consequence, it becomes the digestive resource most flexible to respond to the presence of food. Since this is basically a proteolytic system, utilization of such a digestive mechanism would basically maximize protein absorption.
Acknowledgements
´
I. Ibarrola was supported by a grant from the Plan de Formacion del Personal ´
Investigador (Ministerio de Educacion y Ciencia, Spain). Part of this research was
´ ´
funded by the Comision Interministerial de Ciencia y Tecnologıa (CICYT) of the Spanish Government through Project MAR89-185. [SS]
References
Bayne, B.L., 1993. Feeding physiology of bivalves: time dependence and compensation for changes in food availability. In: Dame, R.F. (Ed.), Bivalve Filter Feeders in Estuarine and Coastal Ecosystem Processes. NATO ASI Series, Vol. G33. Springer-Verlag, pp. 1–24.
Bayne, B.L., Livingstone, D.R., Moore, M.N., Widdows, J., 1976. A cytochemical and a biochemical stress in
Mytilus edulis L. Mar. Pollut. Bull. 7, 221–224.
´
Cajaraville, M.P., Marigomez, J.A., Angulo, E., 1991. Automated measurements of lysosomal structure alterations in oocytes of mussels exposed to petroleum hydrocarbons. Arch. Environ. Contam. Toxicol. 21, 395–400.
´
Etxebarria, M., Cajaraville, M.P., Marigomez, I., 1995. Changes in digestive cell lysosomal structure in mussels as biomarkers of environmental stress in the Urdaibai estuary (Biscay Coast, Iberian Peninsula). Mar. Pollut. Bull. 30, 599–603.
Hawkins, A.J.S., Bayne, B.L., 1984. Seasonal variation in the balance between physiological mechanisms of feeding and digestion in Mytilus edulis (Bivalvia: Mollusca). Mar. Biol. 82, 233–240.
Hawkins, A.J.S., Bayne, B.L., 1985. Seasonal variation in the relative utilization of carbon and nitrogen by the mussel Mytilus edulis. Budgets, conversion efficiencies and maintenance requirements. Mar. Ecol. Prog. Ser. 25, 181–188.
Hawkins, A.J.S., Bayne, B.L., Clarke, K.R., 1983. Co-ordinated rhythms of digestion, absorption and excretion in Mytilus edulis (Bivalvia: Mollusca). Mar. Biol. 74, 41–48.
Hawkins, A.J.S., Navarro, E., Iglesias, J.I.P., 1990. Comparative allometries of gut content, gut passage time and metabolic faecal loss in Mytilus edulis and Cerastoderma edule. Mar. Biol. 105, 197–204. Ibarrola, I., Iglesias, J.I.P., Navarro, E., 1996. Differential absorption of biochemical components in the diet of
the cockle Cerastoderma edule: enzymatic responses to variations in seston composition. Can. J. Zool. 74, 1887–1897.
Ibarrola, I., Navarro, E., Iglesias, J.I.P., 1998a. Short-term adaptation of digestive processes in the cockle
Cerastoderma edule exposed to different food quantity and quality. J. Comp. Biochem. Physiol. B. 168,
32–40.
Ibarrola, I., Larretxea, X., Iglesias, J.I.P., Urrutia, M.B., Navarro, E., 1998b. Seasonal variation of digestive enzyme activities in the digestive gland and the crystalline style of the common cockle Cerastoderma edule. Comp. Biochem. Physiol. A. 121, 25–34.
Ibarrola, I., Navarro, E., Iglesias, J.I.P., Urrutia, M.B., 1999. Time-course of digestive enzyme acclimation in the cockle Cerastoderma edule. Mar. Biol. 135, 47–56.
Langton, R.W., 1975. Synchrony in the digestive diverticula of Mytilus edulis L. J. Mar. Biol. Assoc. UK 55, 221–229.
Larretxea, X., 1995. Estudios de crecimiento en Cerastoderma edule L. (Bivalvia, Cardiidae): bases
´ ´
fisiologicas de la reproduccion individual. University of the Basque Country, Ph.D.
Lowe, D.M., Moore, M.N., Clarke, K.R., 1981. Effects of oil on digestive cells on mussels: quantitative alterations in cellular and lysosomal structure. Aquat. Toxicol. 1, 213–226.
´ ´ ´ ´
Marigomez, I., 1989. Aportaciones cito-histologicas a la evaluacion ecotoxicologica de niveles subletales de ´
cadmio en el medio marino: estudios en el laboratorio del gasteropodo prosobranquio Littorina littorea (L). University of the Basque Country, Ph.D.
´
Marigomez, I., Orbea, A., Olabarrieta, I., Etxebarria, M., Cajaraville, M.P., 1996. Structural changes in the digestive lysosomal system of sentinel mussels as biomarkers of environmental stress in mussel-watch programmes. Comp. Biochem. Physiol. 113C (2), 291–297.
Mathers, N.F., 1973. A comparative histochemical survey of enzymes associated with the processes of digestion in Ostrea edulis and Crassostrea angulata (Mollusca: Bivalvia). J. Zool. Lond. 169, 169–179. Moore, M.N., 1976. Cytochemical demonstration of latency of lysosomal hydrolases in digestive cells of the
common mussel, Mytilus edulis, and changes induced by thermal stress. Cell Tiss. Res. 175, 279–287. Morton, B.S., 1973. A new theory of feeding and digestion in the filter feeding Lamellibranchia. Malacologia
14, 63–79.
Morton, B.S., 1983. Feeding and digestion in bivalvia. In: Saleuddin, A.S.M., Wilbur, K.M. (Eds.), Physiology. The Mollusca, Vol. V. Academic Press, New York, pp. 65–147, Part 2.
Navarro, E., Iglesias, J.I.P., 1993. Infaunal filter-feeding bivalves and the physiological response to short-term fluctuations in food availability and composition. In: Dame, R.F. (Ed.), Bivalve Filter Feeders in Estuarine and Coastal Ecosystem Processes. NATO ASI Series, Vol. G33. Springer-Verlag, pp. 25–56.
Navarro, E., Iglesias, J.I.P., Ortega, M.M., Larretxea, X., 1994. The basis for a functional response to variable food quantity and quality in cockels Cerastoderma edule (Bivalvia, Cardiidae). Physiol. Zool. 67, 468–498. Owen, G., 1970. The fine structure of the digestive tubules of the marine bivalve Cardium edule. Phil. Trans.
R. Soc. Lond. Ser. B 258, 245–260.
Owen, G., 1974. Feeding and digestion in the bivalvia. Adv. Comp. Physiol. Biochem. 5, 1–35.
Pal, S.G., 1971. The fine structure of the digestive tubules of Mya arenaria L. I. Basophil cell. Proc. Malacol. Soc. Lond. 39, 303–309.
Palmer, R.E., 1979. A histological and histochemical study of digestion in the bivalve Arctica islandica L. Biol. Bull. 156, 115–129.
Pearse, A.G.E., 1976. In: Histochemistry, Theoretical and Applied. Churchill Livingstone, Edinburgh, p. 759. Reid, R.G.B., Rauchert, K., 1972. Protein digestion in members of the genus Macoma (Mollusca: Bivalvia).
Comp. Biochem. Physiol. 41A, 887–895.
Robinson, W.E., 1983. Assessment of bivalve intracellular digestion based on direct measurements. J. Moll. Stud. 49, 1–8.
Robinson, W.E., Langton, R.W., 1980. Digestion in a subtidal population of Mercenaria mercenaria (Bivalvia). Mar. Biol. 58, 173–179.
Robinson, W.E., Michael, R.P., Langton, R.W., 1981. Variability of tubule types the digestive glands of
Mercenaria mercenaria (L), Ostrea edulis L., and Mytilus edulis L. J. Exp. Mar. Biol. Ecol. 54, 265–276.
Thompson, R.J., Bayne, C.J., Moore, M.N., Carefeot, T.J., 1978. Haemolymph volume, changes in a biochemical composition of the blood, cytological responses of the digestive cells in ‘‘Mytilus califor-nianus’’ Conrad induced by nutritional, thermal and exposure stress. J. Comp. Physiol. 127, 287–298. Wilson, J.H., La Touche, R.W., 1978. Intracellular digestion in two sublittoral populations of Ostrea edulis
(Lamellibranchia). Mar. Biol. 47, 71–77.